Pessary for Prevention of Preterm Birth and Perinatal Mortality in Pregnancies with a Short Cervix: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Study Selection and Data Extraction
2.4. Data Management and Statistical Analyses
2.5. Assessment of Risk of Bias
2.6. Protocol Registration
3. Results
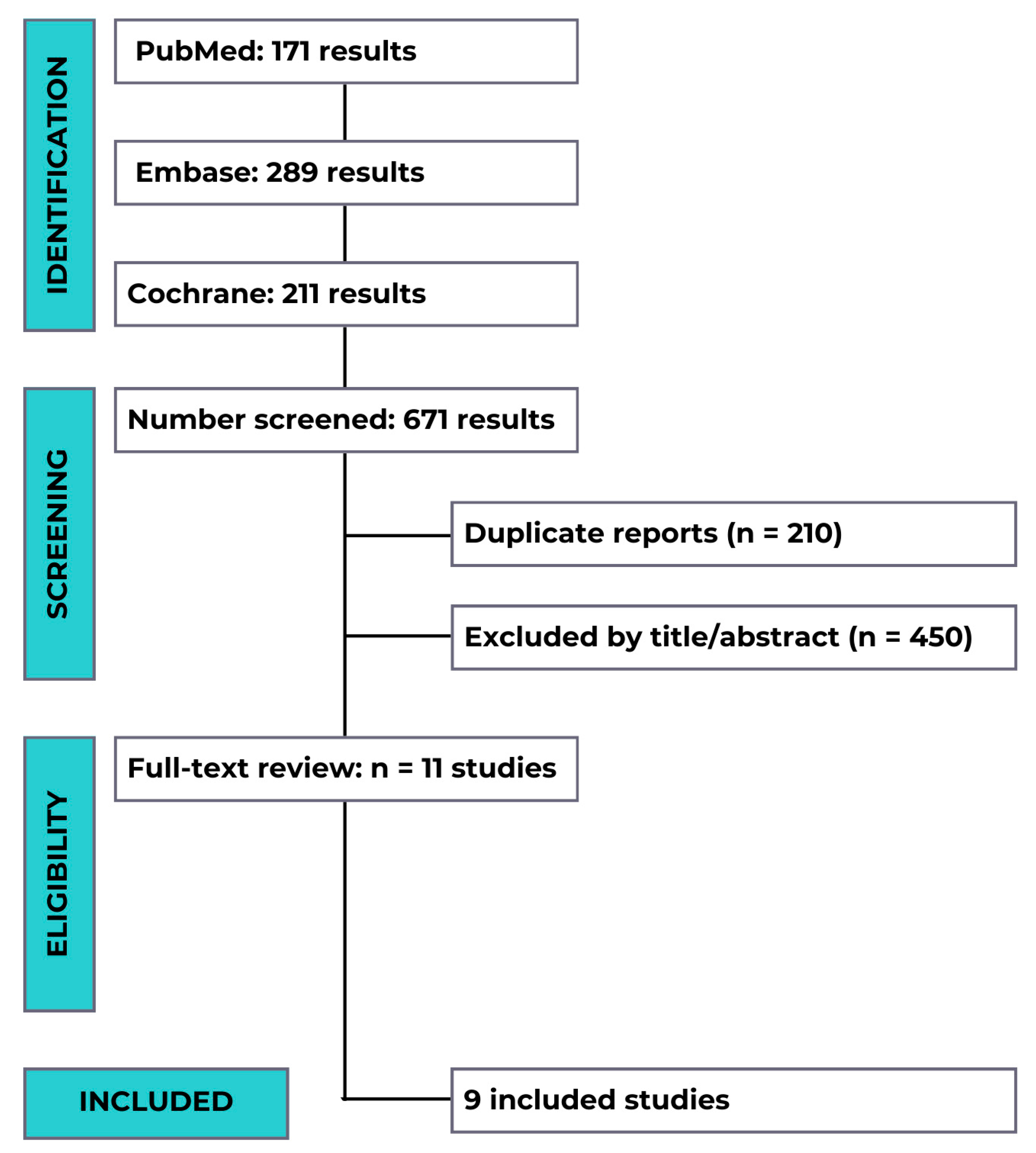
3.1. Studies Triage
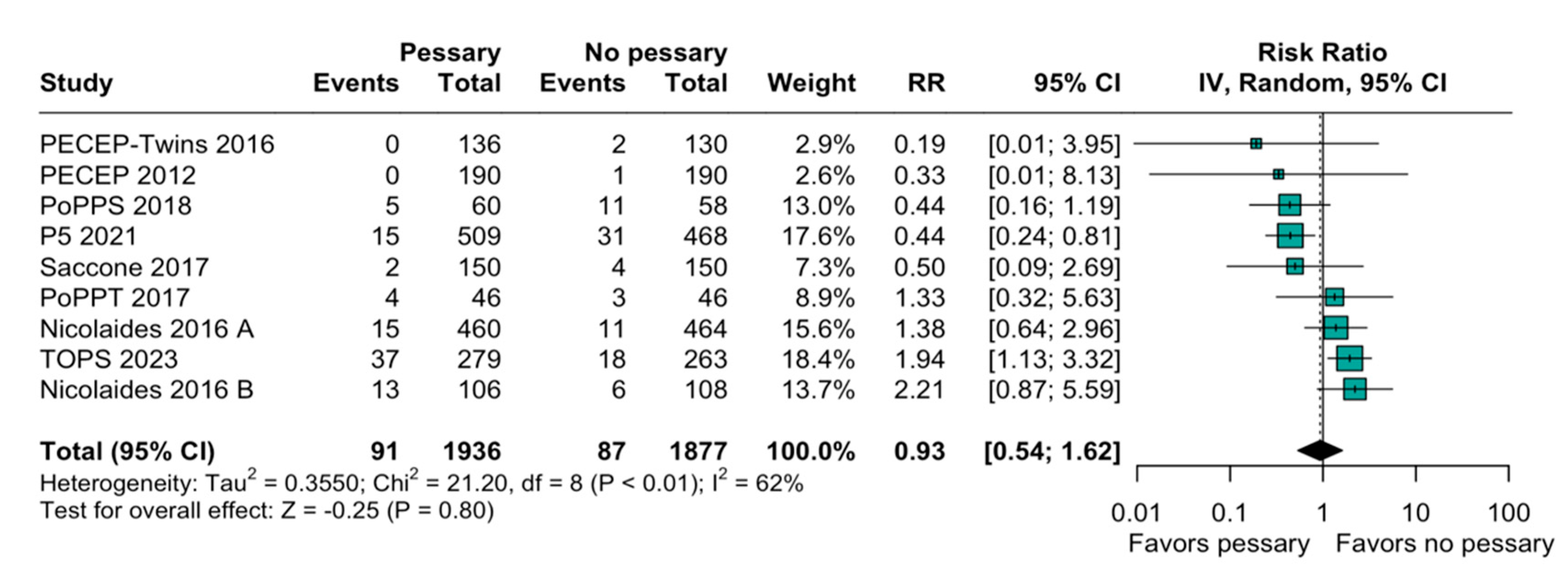
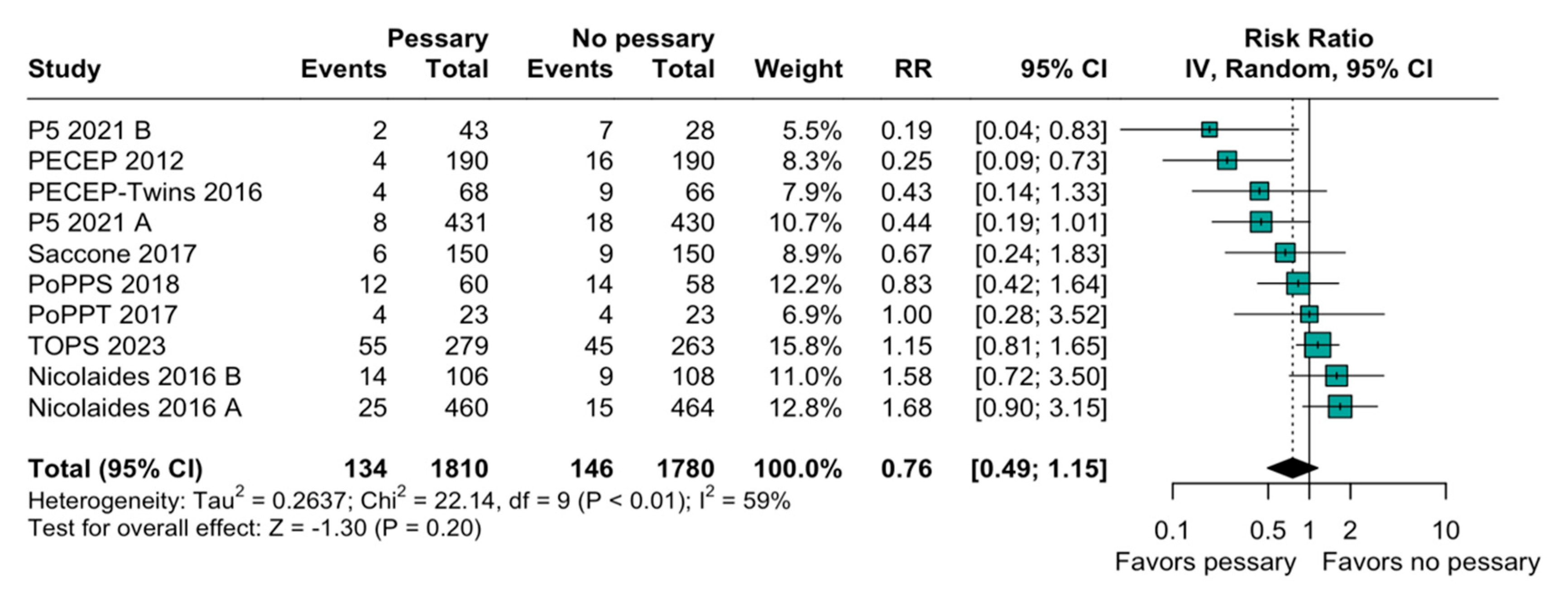
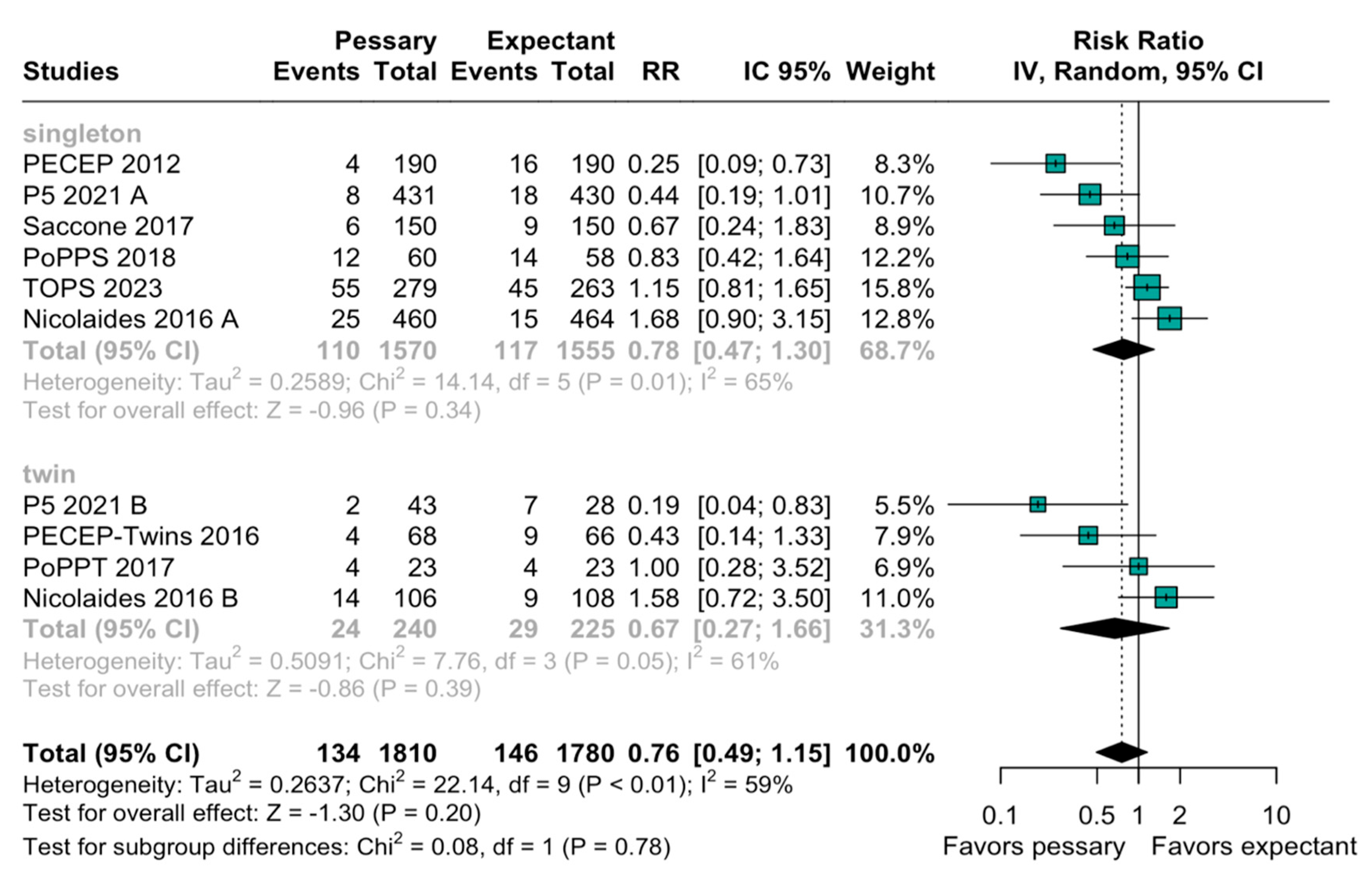
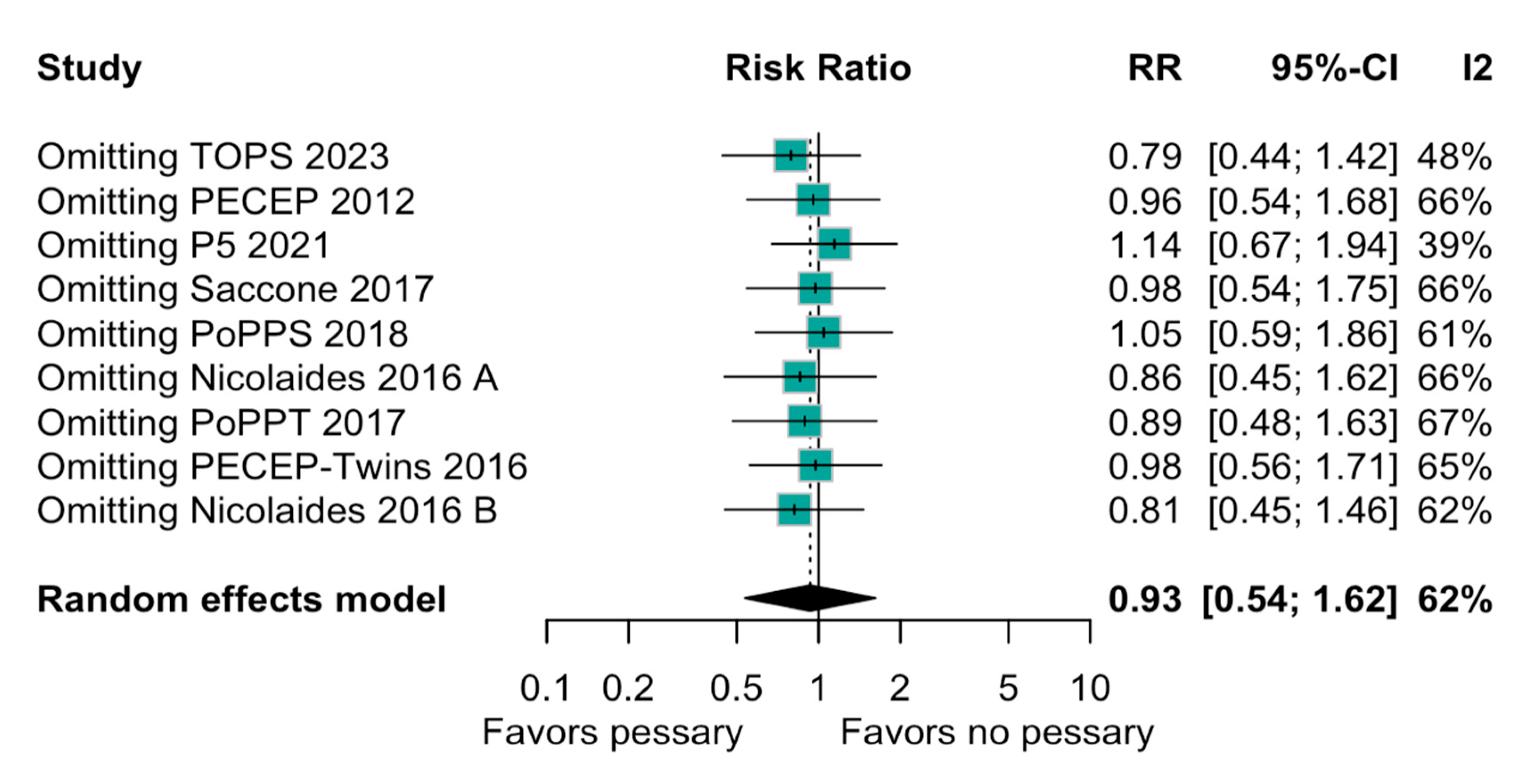
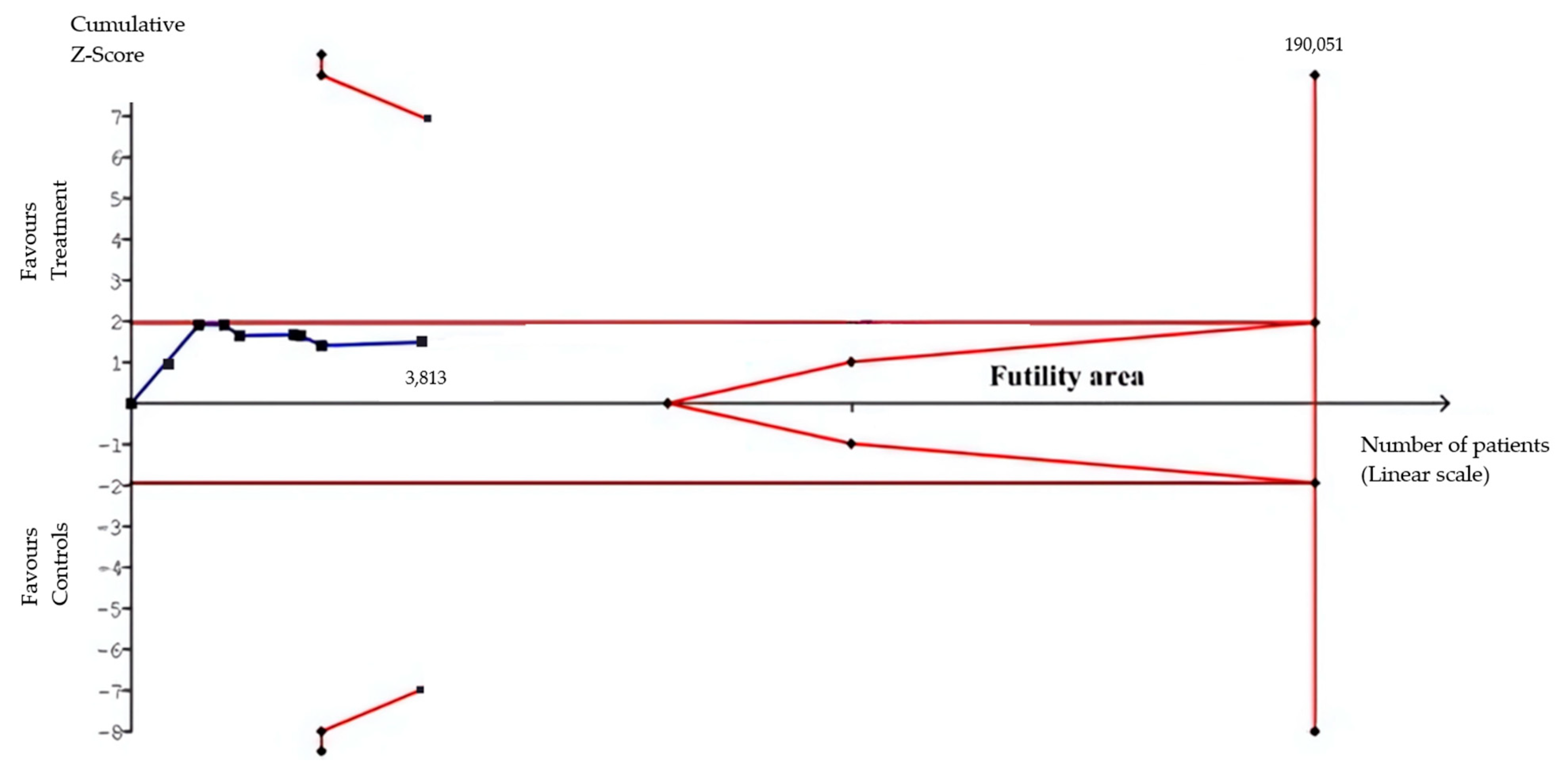
3.2. Statistical Results
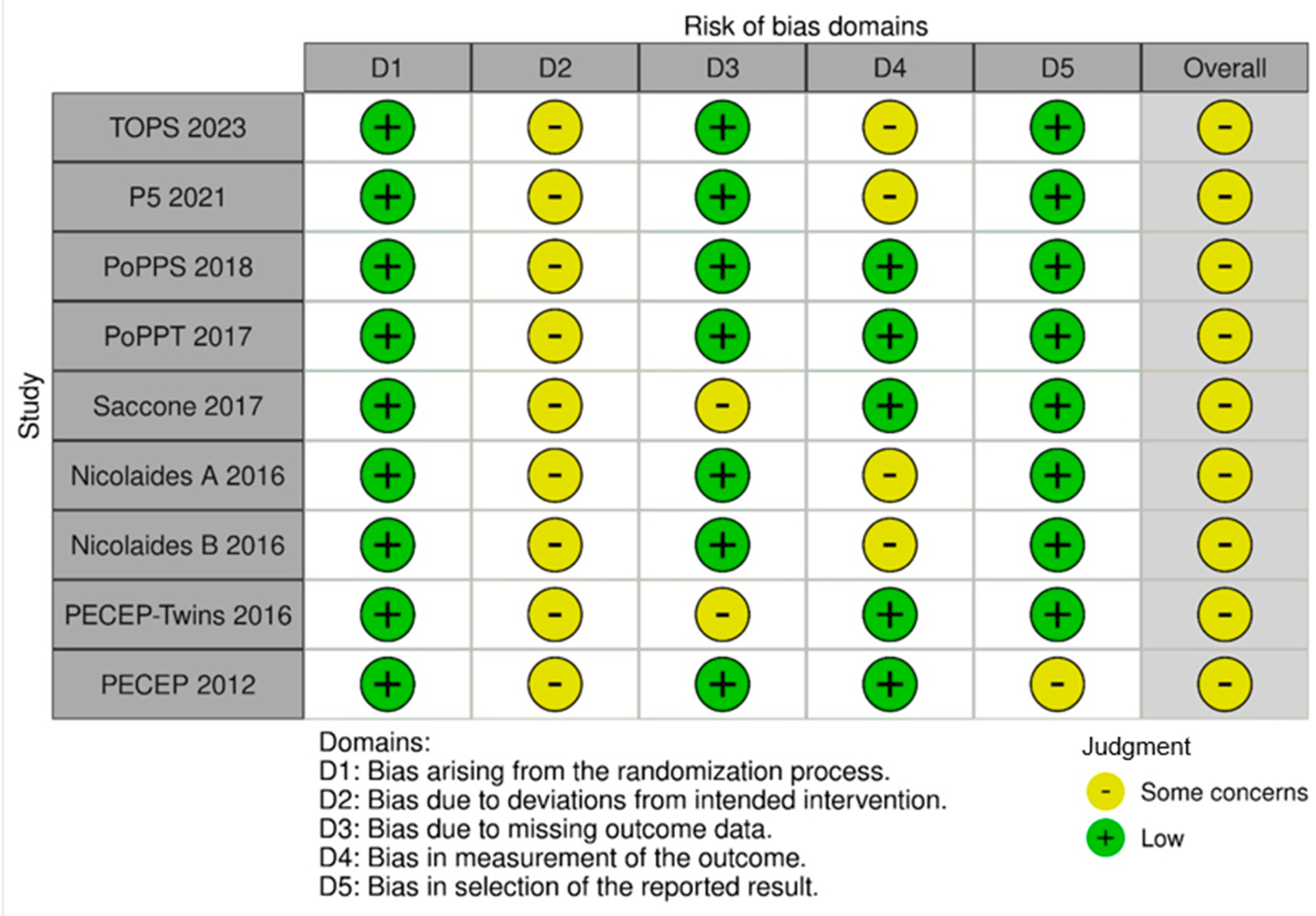
3.3. Quality Assessment
4. Discussion
4.1. Summary of Findings
4.2. Strengths and Limitations
4.2.1. Strengths
- Inclusion of only RCTs: Utilizing only randomized controlled trials (RCTs) allows for a more precise and reliable analysis, enabling adjustments for baseline characteristics and the exploration of subgroup effects.
- Comprehensive Search Strategy: The extensive search strategy across multiple databases and the inclusion of unpublished data ensure a thorough collection of relevant studies.
4.2.2. Limitations
- Heterogeneity: Prematurity research is inherently heterogeneous due to the global variability in clinical thresholds and practices. Additional factors contributing to the heterogeneity in this meta-analysis include the use of different pessary models, the variability in cervical length thresholds (e.g., <20 mm vs. <30 mm), and the inconsistent reporting of progesterone co-administration. Different pessary types were used across the included trials, which may have influenced the clinical outcomes. However, the absence of stratified outcome data by pessary model limited our ability to assess this effect. Unfortunately, due to the insufficient stratified data in the primary studies, a further subgroup analysis was not feasible.
- Limited Data on Specific Subgroups: Some subgroups, such as those based on the presence of amniotic sludge, had limited data, making it challenging to draw robust conclusions for these groups.
- Potential for Bias: Although all of the included studies were RCTs, several exhibited methodological limitations that may affect the reliability of outcome estimates. The open-label design of these trials may have introduced detection bias, particularly for outcomes such as NICU admission or diagnosis timing. This, coupled with concerns regarding the outcome measurements, may have influenced how the outcomes were ascertained and reported. Furthermore, the potential for publication bias and selective reporting cannot be entirely ruled out despite efforts to include unpublished data and perform a comprehensive search strategy.
4.3. Evaluation of Pessary Use and Perinatal Mortality
4.4. Interpretation of Findings
4.5. Clinical Implications and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J.; the Born Too Soon Preterm Birth Action Group. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Odibo, A.O.; Tolosa, J.E. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: A randomized trial. Am. J. Obstet. Gynecol. 2004, 191, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.B.; Celik, E.; Parra, M.; Singh, M.; Nicolaides, K.H.; Fonseca, E.B.; Celik, E.; Parra, M.; Singh, M.N.K. Progesterone and the Risk of Preterm Birth among Women with a Short Cervix. N. Engl. J. Med. 2007, 357, 462–469. [Google Scholar] [CrossRef] [PubMed]
- França, M.S.; Hatanaka, A.R.; Cruz, J.D.J.; Júnior, L.D.A.; Emy, T.; Hamamoto, K.; Sarmento, S.G.P.; Júnior, J.E.; Pares, D.B.d.S.; Mattar, R.; et al. Cervical pessary plus vaginal progesterone in a singleton pregnancy with a short cervix: An experience-based analysis of cervical pessary’s efficacy. J. Matern.-Fetal Neonatal Med. 2021, 35, 6670–6680. [Google Scholar] [CrossRef]
- Franca, M.S.; Hatanaka, A.R.; Andrade, V.L., Jr.; Elito, J., Jr.; Pares, D.B.S.; Hamamoto, T.E.N.; Sarmento, S.G.P.; Mattar, R.; Moron, A.F. Cervical pessary plus progesterone for twin pregnancy with short cervix compared to unselected and non-treated twin pregnancy: A historical equivalence cohort study (EPM Twin Pessary Study). Rev. Bras. Ginecol. Obs. 2020, 42, 621–629. [Google Scholar] [CrossRef]
- Biggio, J. SMFM Consult Series #70: Management of short cervix in individuals without a history of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2024, 231, B2–B13. [Google Scholar]
- Coutinho, C.M.; Sotiriadis, A.; Odibo, A.; Khalil, A.; D’Antonio, F.; Feltovich, H.; da Silva Costa, F. ISUOG Practice Guidelines: Role of ultrasound in the prediction of spontaneous preterm birth. Ultrasound Obstet. Gynecol. 2022, 60, 435–456. [Google Scholar] [CrossRef]
- Roman, A.; Suhag, A.; Berghella, V. Overview of Cervical Insufficiency: Diagnosis, Etiologies, and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 237–240. [Google Scholar] [CrossRef]
- Bortoletto, T.G.; Silva, T.V.; Borovac-Pinheiro, A.; Pereira, C.M.; Silva, A.D.; Franca, M.S.; Hatanaka, A.R.; Argenton, J.P.; Passini, R.; Mol, B.W.; et al. Cervical length varies considering different populations and gestational outcomes: Results from a systematic review and meta- analysis. PLoS ONE 2021, 16, e0245746. [Google Scholar] [CrossRef]
- Silva, T.V.; Borovac-Pinheiro, A.; Cecatti, J.G.; Mol, B.W.; Silva Costa, F.; França, M.S.; Souza, R.T.; Devlieger, R.; Passini, R.; Pacagnella, R.C. Association between cervical length and gestational age at birth in singleton pregnancies: A multicentric prospective cohort study in the Brazilian population. Reprod. Health 2023, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Thom, E.; McNellis, D.; Copper, R.L.; Johnson, F.; et al. The length of the cervix and the risk of spontaneous premature delivery. N. Engl. J. Med. 1996, 334, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Santucci França, M.; Lacerda de Andrade, V., Jr.; Roberto Hatanaka, A.; Santos, R.; Herlanio Costa Carvalho, F.; Laura Costa, M.; França, G.U.S.; Mattar, R.; Mol, B.W.; Moron, A.F.; et al. A new screening of preterm birth in gestation with short cervix after pessary plus progesterone. Rev. Bras. Ginecol. Obstet. 2024, 46, 39. [Google Scholar] [CrossRef] [PubMed]
- Costantine, M.M.; Ugwu, L.; Grobman, W.A.; Mercer, B.M.; Tita, A.T.N.; Rouse, D.J.; Sorokin, Y.; Wapner, R.J.; Blackwell, S.C.; Tolosa, J.E.; et al. Cervical length distribution and other sonographic ancillary findings of singleton nulliparous patients at midgestation. Am. J. Obstet. Gynecol. 2021, 225, 181.e1–181.e11. [Google Scholar] [CrossRef]
- Berghella, V.; Kuhlman, K.; Weiner, S.; Texeira, L.; Wapner, R.J. Cervical funneling: Sonographic criteria predictive of preterm delivery. Ultrasound Obstet. Gynecol. 1997, 10, 161–166. [Google Scholar] [CrossRef]
- Hatanaka, A.R.; Mattar, R.; Kawanami, T.E.N.; França, M.S.; Rolo, L.C.; Nomura, R.M.Y.; Araujo Junior, E.; Nardozza, L.M.M.; Moron, A.F. Amniotic fluid “sludge” is an independent risk factor for preterm delivery. J. Matern. -Fetal Neonatal Med. 2016, 29, 120–125. [Google Scholar] [CrossRef]
- Hatanaka, A.R.; Franca, M.S.; Hamamoto, T.E.N.K.; Rolo, L.C.; Mattar, R.; Moron, A.F. Antibiotic treatment for patients with amniotic fluid “sludge” to prevent spontaneous preterm birth: A historically controlled observational study. Acta Obstet. Gynecol. Scand. 2019, 98, 1157–1163. [Google Scholar] [CrossRef]
- Pacagnella, R.C.; Silva, T.V.; Cecatti, J.G.; Passini, R.; Fanton, T.F.; Borovac-Pinheiro, A.; Pereira, C.M.; Fernandes, K.G.; França, M.S.; Li, W.; et al. Pessary Plus Progesterone to Prevent Preterm Birth in Women With Short Cervixes: A Randomized Controlled Trial. Obstet. Gynecol. 2022, 139, 41–51. [Google Scholar] [CrossRef]
- Schnettler, W.; Manoharan, S.; Smith, K. Transvaginal Sonographic Assessment Following Cervical Pessary Placement for Preterm Birth Prevention. Am. J. Perinatol. Rep. 2022, 12, e80–e88. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; Poon, L.C.; de Paco Matallana, C.; Plasencia, W.; Molina, F.S.; Conturso, R. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 214, e1–e3. [Google Scholar] [CrossRef]
- Thom, E.A.; Saade, G.R.; Askie, L.M.; Ugwu, L.G.; Mol, B.W.J.; Vayssiere, C.; Norman, J.E.; Pajkrt, E.; Clifton, R.G.; Biggio, J.R.; et al. PROMPT: Prospective Meta-analysis for Pessary Trials Study Protocol. Am. J. Perinatol. 2024, 41, e2230–e2237. [Google Scholar] [CrossRef] [PubMed]
- Wennerholm, U.B.; Bergman, L.; Kuusela, P.; Ljungström, E.; Möller, A.C.; Hongslo Vala, C.; Jacobsson, B. Progesterone, cerclage, pessary, or acetylsalicylic acid for prevention of preterm birth in singleton and multifetal pregnancies—A systematic review and meta-analyses. Front. Med. 2023, 10, 1111315. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.K.; Clifton, R.G.; Biggio, J.R.; Saade, G.R.; Ugwu, L.G.; Longo, M.; Bousleiman, S.Z.; Clark, K.; Grobman, W.A.; Frey, H.A.; et al. Cervical Pessary for Prevention of Preterm Birth in Individuals with a Short Cervix: The TOPS Randomized Clinical Trial. JAMA 2023, 330, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Provinciatto, H.G.; Barbalho, M.E.; Crosara, L.F.; Orsini, P.V.B.; Provinciatto, A.; Philip, C.E.; Ruano, R.; Júnior, E.A. Prevention of preterm birth in twin-to-twin transfusion syndrome: A systematic review and network meta-analysis. J. Perinat. Med. 2024, 52, 712–721. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Wetterslev, J.; Winkel, P.; Lange, T.; Gluud, C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med. Res. Methodol. 2014, 14, 120. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.J.H.W. Cochrane Handbook for Systematic Reviews of Interventions. In Cochrane Handbook for Systematic Reviews of Interventions, 1st ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hui, S.; Chor, C.; Lau, T.; Lao, T.; Leung, T. Cerclage Pessary for Preventing Preterm Birth in Women with a Singleton Pregnancy and a Short Cervix at 20 to 24 Weeks: A Randomized Controlled Trial. Am. J. Perinatol. 2012, 30, 283–288. [Google Scholar]
- Karbasian, N.; Sheikh, M.; Pirjani, R.; Hazrati, S.; Tara, F.; Hantoushzadeh, S. Combined treatment with cervical pessary and vaginal progesterone for the prevention of preterm birth: A randomized clinical trial. J. Obstet. Gynaecol. Res. 2016, 42, 1673–1679. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; Poon, L.C.; Picciarelli, G.; Tul, N.; Zamprakou, A.; Rodriguez Calvo, J. A Randomized Trial of a Cervical Pessary to Prevent Preterm Singleton Birth. Obstet. Gynecol. Surv. 2016, 71, 392–393. [Google Scholar] [CrossRef]
- Saccone, G.; Ciardulli, A.; Xodo, S.; Dugoff, L.; Ludmir, J.; D’Antonio, F.; Boito, S.; Olearo, E.; Votino, C.; Maruotti, G.M.; et al. Cervical pessary for preventing preterm birth in twin pregnancies with short cervical length: A systematic review and meta-analysis. J. Matern. -Fetal Neonatal Med. 2017, 30, 2918–2925. [Google Scholar] [CrossRef]
- Berghella, V.; Dugoff, L.; Ludmir, J. Prevention of preterm birth with pessary in twins (PoPPT): A randomized controlled trial. Ultrasound Obstet. Gynecol. 2017, 49, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Dugoff, L.; Berghella, V.; Sehdev, H.; Mackeen, A.D.; Goetzl, L.; Ludmir, J. Prevention of preterm birth with pessary in singletons (PoPPS): Randomized controlled trial. Ultrasound Obstet. Gynecol. 2018, 51, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Goya, M.; Pratcorona, L.; Merced, C.; Rodó, C.; Valle, L.; Romero, A.; Juan, M.; Rodríguez, A.; Muñoz, B.; Santacruz, B.; et al. Cervical pessary in pregnant women with a short cervix (PECEP): An open-label randomised controlled trial. Lancet 2012, 379, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Goya, M.; De La Calle, M.; Pratcorona, L.; Merced, C.; Rodó, C.; Muñoz, B.; Juan, M.; Serrano, A.; Llurba, E.; Higueras, T.; et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: A multicenter randomized controlled trial (PECEP-Twins). Am. J. Obstet. Gynecol. 2016, 214, 145–152. [Google Scholar] [CrossRef]
| Study | Inclusion Criteria | Gestational Age at Cervical Screening (Weeks of Pregnancy) | Intervention | Control |
|---|---|---|---|---|
| PECEP 2012 [34] | Singleton pregnancies with a cervical length of 25 millimeters or less | 18 0/7–22 6/7 | Arabin pessary + standard care (n = 190) | Standard care (no pessary) (n = 190) |
| PECEP-Twins 2016 [35] | Twin pregnancies with a cervical length of 25 millimeters or less | 18 0/7–22 6/7 | Arabin pessary + standard care (n = 68) | Standard care (no pessary) (n = 66) |
| Nicolaides 2016 A [30] | Singleton pregnancies with a cervical length of 25 millimeters or less | 20 0/7–24 6/7 | Arabin pessary + standard care (n = 460) | Standard care (no pessary) (n = 464) |
| Nicolaides 2016 B [20] | Twin pregnancies irrespective of cervical length | 20 0/7–24 6/7 | Arabin pessary + standard care (n = 106) | Standard care (no pessary) (n = 108) |
| Saccone 2017 [31] | Singleton pregnancies with a cervical length of 25 millimeters or less | 18 0/7–23 6/7 | Arabin pessary + standard care (n = 150) | Standard care (no pessary) (n = 150) |
| PoPPT 2017 [32] | Twin pregnancies with a cervical length of 30 millimeters or less | 18 0/7–23 6/7 | Bioteque pessary + standard care (n = 23) | Standard care (no pessary) (n = 23) |
| PoPPS 2018 [33] | Singleton pregnancies with a cervical length of 25 millimeters or less | 18 0/7–23 6/7 | Bioteque pessary + standard care (n = 60) | Standard care (no pessary) (n = 58) |
| TOPS 2023 [23] | Singleton pregnancies with a cervical length of 20 millimeters or less | 16 0/7–23 6/7 | Arabin pessary + standard care (n = 279) | Standard care (no pessary) (n = 263) |
| P5 2021 [18] | Singleton or twin pregnancies with a cervical length of 30 millimeters or less | 18 0/7–22 6/7 | Ingamed pessary + vaginal progesterone (200 mg/d) (n = 431) | Vaginal progesterone (200 mg/d) (n = 430) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provinciatto, H.G.; Araujo Júnior, E.; Callado, G.Y.; Hatanaka, A.R.; Santos, R.A.F.; Traina, E.; França, G.U.S.; Coutinho, L.G.; de Amorim, A.L.; das Chagas, L.A.; et al. Pessary for Prevention of Preterm Birth and Perinatal Mortality in Pregnancies with a Short Cervix: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics 2025, 15, 1466. https://doi.org/10.3390/diagnostics15121466
Provinciatto HG, Araujo Júnior E, Callado GY, Hatanaka AR, Santos RAF, Traina E, França GUS, Coutinho LG, de Amorim AL, das Chagas LA, et al. Pessary for Prevention of Preterm Birth and Perinatal Mortality in Pregnancies with a Short Cervix: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics. 2025; 15(12):1466. https://doi.org/10.3390/diagnostics15121466
Chicago/Turabian StyleProvinciatto, Henrique Graf, Edward Araujo Júnior, Gustavo Yano Callado, Alan Roberto Hatanaka, Roberto Angelo Fernandes Santos, Evelyn Traina, Gabriela Ubeda Santucci França, Luiza Graça Coutinho, Alan Lebrão de Amorim, Lucas Almeida das Chagas, and et al. 2025. "Pessary for Prevention of Preterm Birth and Perinatal Mortality in Pregnancies with a Short Cervix: Systematic Review and Meta-Analysis of Randomized Controlled Trials" Diagnostics 15, no. 12: 1466. https://doi.org/10.3390/diagnostics15121466
APA StyleProvinciatto, H. G., Araujo Júnior, E., Callado, G. Y., Hatanaka, A. R., Santos, R. A. F., Traina, E., França, G. U. S., Coutinho, L. G., de Amorim, A. L., das Chagas, L. A., Mattar, R., & França, M. S. (2025). Pessary for Prevention of Preterm Birth and Perinatal Mortality in Pregnancies with a Short Cervix: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics, 15(12), 1466. https://doi.org/10.3390/diagnostics15121466







