Comparison of Clinical Outcomes Between Fluoroscopic and Computer Tomographic Guidance in Concurrent Use of Radiofrequency Ablation and Vertebral Augmentation in Spinal Metastases: A Scoping Review
Abstract
1. Introduction
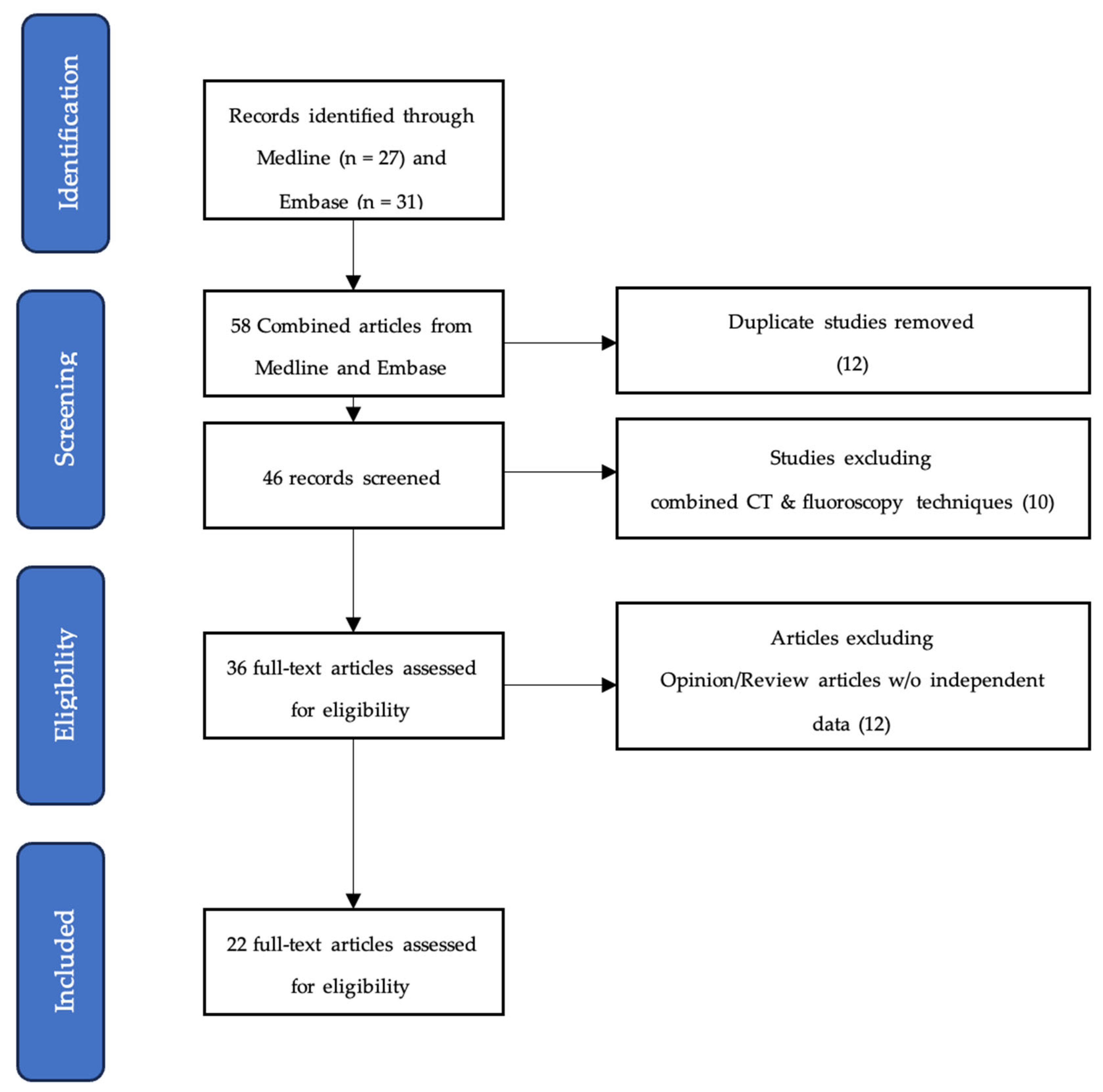
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
3.1. Pain Control
3.1.1. CT Guidance Only
3.1.2. Fluoroscopy Guidance Only
3.2. Quality of Life (QoL)/Functional Capacity
3.2.1. CT Guidance Only
3.2.2. Fluoroscopy Guidance Only
3.3. Analgesia Use
3.3.1. CT Guidance Only
3.3.2. Fluoroscopy Guidance Only
3.4. Complications
3.4.1. CT Guidance Only
3.4.2. Fluoroscopy Guidance Only
4. Discussion
4.1. Precision
4.2. Radiation Dose
Novel Imaging Modalities
5. Limitations
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Portenoy, R.K.; Payne, D.; Jacobsen, P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain 1999, 81, 129–134. [Google Scholar] [CrossRef]
- Jing, D.; Zhao, Q.; Zhao, Y.; Lu, X.; Feng, Y.; Zhao, B.; Zhao, X. Management of pain in patients with bone metastases. Front. Oncol. 2023, 13, 1156618. [Google Scholar] [CrossRef]
- Bollen, L.; Dijkstra, S.P.D.; Bartels, R.H.M.A.; de Graeff, A.; Poelma, D.L.; Brouwer, T.; Algra, P.R.; Kuijlen, J.M.A.; Minnema, M.C.; Nijboer, C.; et al. Clinical management of spinal metastases—The Dutch national guideline. Eur. J. Cancer 2018, 104, 81–90. [Google Scholar] [CrossRef]
- Colonna, S.; Bianconi, A.; Cofano, F.; Prior, A.; Di Perna, G.; Palmieri, G.; Zona, G.; Garbossa, D.; Fiaschi, P. Radiofrequency Ablation in Vertebral Body Metastasis with and without Percutaneous Cement Augmentation: A Systematic Review Addressing the Need for SPINE Stability Evaluation. Diagnostics 2023, 13, 1164. [Google Scholar] [CrossRef]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Bulter, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: An open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021, 22, 1023–1033. [Google Scholar] [CrossRef]
- Guckenberger, M.; Dahele, M.; Ong, W.L.; Sahgal, A. Stereotactic Body Radiation Therapy for Spinal Metastases: Benefits and Limitations. Semin. Radiat. Oncol. 2023, 33, 159–171. [Google Scholar] [CrossRef]
- Mossa-Basha, M.; Gerszten, P.C.; Myrehaug, S.; Mayr, N.A.; Yuh, W.T.; Jabehdar Maralani, P.; Sahgal, A.; Lo, S.S. Spinal metastasis: Diagnosis, management and follow-up. Br. J. Radiol. 2019, 92, 20190211. [Google Scholar] [CrossRef]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef]
- Wray, J.K.; Dixon, B.; Przkora, R. Radiofrequency Ablation; StatPearls Publishing LLC.: Florida, FL, USA, 2024. [Google Scholar]
- Wallace, A.N.; Tomasian, A.; Vaswani, D.; Vyhmeister, R.; Chang, R.O.; Jennings, J.W. Radiographic Local Control of Spinal Metastases with Percutaneous Radiofrequency Ablation and Vertebral Augmentation. Am. J. Neuroradiol. 2016, 37, 759–765. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Costanzo, R.; Paolini, F.; Benigno, U.E.; Porzio, M.; Brunasso, L.; Basile, L.; Guli, C.; Pino, M.A.; Gerardi, R.M.; et al. Management of Spinal Bone Metastases with Radiofrequency Ablation, Vertebral Reinforcement and Transpedicular Fixation: A Retrospective Single-Center Case Series. Front. Oncol. 2022, 11, 818760. [Google Scholar] [CrossRef]
- Jha, R.M.; Hirsch, A.E.; Yoo, A.J.; Ozonoff, A.; Growney, M.; Hirsch, J.A. Palliation of compression fractures in cancer patients by vertebral augmentation: A retrospective analysis. J. Neurointerv. Surg. 2010, 2, 221–228. [Google Scholar] [CrossRef]
- Hirsch, J.A.; Hirsch, A.E.; Jha, R.; Growney, M.; Rabinov, J.D.; Nogueira, R.G.; Pryor, J.C.; Yoo, A.J. Practical management of malignant compression fractures. J. Neurointerv. Surg. 2010, 2, 219–220. [Google Scholar] [CrossRef]
- Lu, M.; Lei, Z.; Dai, S.; Hou, C.; Du, S.; Chen, W.; Li, H. A narrative review of the application of radiofrequency ablation in the surgery of spinal metastases. Ann. Jt. 2021, 6, 30. [Google Scholar] [CrossRef]
- Patel, A.; Petrone, B.; Carter, K.R. Percutaneous Vertebroplasty and Kyphoplasty; StatPearls Publishing LLC.: Florida, FL, USA, 2024. [Google Scholar]
- Greenwood, T.J.; Wallace, A.; Friedman, M.V.; Hillen, T.J.; Robinson, C.G.; Jennings, J.W. Combined Ablation and Radiation Therapy of Spinal Metastases: A Novel Multimodality Treatment Approach. Pain Physician 2015, 18, 573–581. [Google Scholar] [CrossRef]
- Levy, J.; Hopkins, T.; Morris, J.; Tran, N.D.; David, E.; Massari, F.; Farid, H.; Vogel, A.; O’Connell, W.G.; Sunenshine, P.; et al. Radiofrequency Ablation for the Palliative Treatment of Bone Metastases: Outcomes from the Multicenter OsteoCool Tumor Ablation Post-Market Study (OPuS One Study) in 100 Patients. J. Vasc. Interv. Radiol. 2020, 31, 1745–1752. [Google Scholar] [CrossRef]
- Levy, J.; David, E.; Hopkins, T.; Morris, J.; Tran, N.D.; Farid, H.; Massari, F.; O’Connell, W.G.; Vogel, A.; Gangi, A.; et al. Radiofrequency Ablation Provides Rapid and Durable Pain Relief for the Palliative Treatment of Lytic Bone Metastases Independent of Radiation Therapy: Final Results from the OsteoCool Tumor Ablation Post-Market Study. Cardiovasc. Interv. Radiol. 2023, 46, 600–609. [Google Scholar] [CrossRef]
- Pusceddu, C.; Marsico, S.; Derudas, D.; Ballicu, N.; Melis, L.; Zedda, S.; De Felice, C.; Calabrese, A.; Santucci, D.; Faiella, E. Clinical Rationale of Using Steerable Technologies for Radiofrequency Ablation Followed by Cavity Creation and Cement Augmentation in the Treatment of Painful Spinal Metastases. Curr. Oncol. 2023, 30, 4257–4268. [Google Scholar] [CrossRef]
- Ragheb, A.; Vanood, A.; Fahim, D.K. The Addition of Radiofrequency Tumor Ablation to Kyphoplasty May Reduce the Rate of Local Recurrence in Spinal Metastases Secondary to Breast Cancer. World Neurosurg 2022, 161, e500–e507. [Google Scholar] [CrossRef]
- Sayed, D.; Jacobs, D.; Sowder, T.; Haines, D.; Orr, W. Spinal Radiofrequency Ablation Combined with Cement Augmentation for Painful Spinal Vertebral Metastasis: A Single-Center Prospective Study. Pain Physician 2019, 22, E441–E449. [Google Scholar] [CrossRef]
- Shawky Abdelgawaad, A.; Ezzati, A.; Krajnovic, B.; Seyed-Emadaldin, S.; Abdelrahman, H. Radiofrequency ablation and balloon kyphoplasty for palliation of painful spinal metastases. Eur. Spine J. 2021, 30, 2874–2880. [Google Scholar] [CrossRef]
- Georgy, B.A. Bone cement deposition patterns with plasma-mediated radio-frequency ablation and cement augmentation for advanced metastatic spine lesions. Am. J. Neuroradiol. 2009, 30, 1197–1202. [Google Scholar] [CrossRef]
- Gu, Y.F.; Tian, Q.H.; Li, Y.D.; Wu, C.-G.; Su, Y.; Song, H.-M.; He, C.-J.; Chen, D. Percutaneous vertebroplasty and interventional tumor removal for malignant vertebral compression fractures and/or spinal metastatic tumor with epidural involvement: A prospective pilot study. J. Pain Res. 2017, 10, 211–218. [Google Scholar] [CrossRef][Green Version]
- Jain, S.; Kinch, L.; Rana, M.; Anitescu, M. Comparison of post-operative pain scores and opioid use between kyphoplasty and radiofrequency ablation (RFA) systems combined with cement augmentation. Skelet. Radiol. 2020, 49, 1789–1794. [Google Scholar] [CrossRef]
- Lv, N.; Geng, R.; Ling, F.; Zhou, Z.; Liu, M. Clinical efficacy and safety of bone cement combined with radiofrequency ablation in the treatment of spinal metastases. BMC Neurol. 2020, 20, 418. [Google Scholar] [CrossRef]
- Masala, S.; Roselli, M.; Massari, F.; Fiori, R.; Ursone, A.; Fossile, E.; Laudisi, A.; Simonetti, G. Radiofrequency Heat Ablation and Vertebroplasty in the treatment of neoplastic vertebral body fractures. Anticancer Res. 2004, 24, 3129–3133. [Google Scholar]
- Munk, P.L.; Rashid, F.; Heran, M.K.; Papirny, M.; Liu, D.M.; Malfair, D.; Badii, M.; Clarkson, P.W. Combined Cementoplasty and Radiofrequency Ablation in the Treatment of Painful Neoplastic Lesions of Bone. J. Vasc. Interv. Radiol. 2009, 20, 903–911. [Google Scholar] [CrossRef]
- Senol, N.; Oguzoglu, A.S.; Goksel, H.M. Radiofrequency Ablation and Augmentation in the Management of Spinal Metastases: Clinical Experience in 41 Patients. World Neurosurg. 2022, 163, e420–e425. [Google Scholar] [CrossRef]
- Reyes, M.; Georgy, M.; Brook, L.; Ortiz, O.; Brook, A.; Agarwal, V.; Muto, M.; Manfre, L.; Marcia, S.; Georgy, B.A. Multicenter clinical and imaging evaluation of targeted radiofrequency ablation (t-RFA) and cement augmentation of neoplastic vertebral lesions. J. Neurointerv. Surg. 2018, 10, 176–182. [Google Scholar] [CrossRef]
- Sandri, A.; Carbognin, G.; Regis, D.; Gaspari, D.; Calciolari, C.; Girardi, V.; Masueto, G.; Bartolozzi, P. Combined radiofrequency and kyphoplasty in painful osteolytic metastases to vertebral bodies. Radiol. Med. 2010, 115, 261–271. [Google Scholar] [CrossRef]
- Tomasian, A.; Hillen, T.J.; Chang, R.O.; Jennings, J.W. Simultaneous bipedicular radiofrequency ablation combined with vertebral augmentation for local tumor control of spinal metastases. Am. J. Neuroradiol. 2018, 39, 1768–1773. [Google Scholar] [CrossRef]
- Yildizhan, S.; Boyaci, M.G.; Rakip, U.; Aslan, A.; Canbek, I. Role of radiofrequency ablation and cement injection for pain control in patients with spinal metastasis. BMC Musculoskelet Disord. 2021, 22, 912. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Z.; Sun, M.; Zeng, H.; Zuo, D.; Hua, Y.; Cai, Z. A preliminary study of the safety and efficacy of radiofrequency ablation with percutaneous kyphoplasty for thoracolumbar vertebral metastatic tumor treatment. Med. Sci. Monit. 2014, 20, 556–563. [Google Scholar] [CrossRef]
- Wang, L.; Lu, M.; Zhang, X.; Zhao, Z.; Li, X.; Liu, T.; Xu, L.; Yu, S. Risk factors for pulmonary cement embolism after percutaneous vertebroplasty and radiofrequency ablation for spinal metastases. Front. Oncol. 2023, 13, 1129658. [Google Scholar] [CrossRef]
- Wang, F.; Gu, J.; Xu, C.; Li, G.; Lv, P. The combination of radiofrequency ablation and vertebroplasty shows advantages over single vertebroplasty in treating vertebral neoplastic lesions. Skelet. Radiol. 2022, 51, 565–571. [Google Scholar] [CrossRef]
- Proschek, D.; Kurth, A.; Proschek, P.; Vogl, T.J.; Mack, M.G. Prospective Pilot-study of Combined Bipolar Radiofrequency Ablation and Application of Bone Cement in Bone Metastases. Anticancer Res. 2009, 29, 2787–2792. [Google Scholar]
- Madaelil, T.P.; Wallace, A.N.; Jennings, J.W. Radiofrequency ablation alone or in combination with cementoplasty for local control and pain palliation of sacral metastases: Preliminary results in 11 patients. Skeletal Radiol. 2016, 45, 1213–1219. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Hu, J.-H.; Peng, Z.-H.; Chen, J.-Z.; Huang, J.-Q.; Jiang, Y.-N.; Luo, G.; Yi, G.-F.; Shen, J.; et al. Palliative pain relief and safety of percutaneous radiofrequency ablation combined with cement injection for bone metastasis. Jpn. J. Clin. Oncol. 2018, 48, 753–759. [Google Scholar] [CrossRef]
- Kastler, A.; Barbé, D.A.; Alemann, G.; Hadjidekov, G.; Cornelis, F.H.; Kastler, B. Bipolar radiofrequency ablation of painful spinal bone metastases performed under local anesthesia: Feasibility regarding patient’s experience and pain outcome. Medicina 2021, 57, 966. [Google Scholar] [CrossRef]
- Chen, A.L.; Sagoo, N.S.; Vannabouathong, C.; Reddy, Y.; Deme, S.; Patibandla, S.; Passias, P.G.; Vira, S. Combination radiofrequency ablation and vertebral cement augmentation for spinal metastatic tumors: A systematic review and meta-analysis of safety and treatment outcomes. N. Am. Spine Soc. J. 2024, 17, 100317. [Google Scholar] [CrossRef]
- Feigl, G.C.; Dreu, M.; Kastner, M.; Rosmarin, W.; Ulz, H.; Kniesel, B.; Likar, R. Thermocoagulation of the Medial Branch of the Dorsal Branch of the Lumbal Spinal Nerve: Flouroscopy Versus CT. Pain Med. 2017, 18, 36–40. [Google Scholar] [CrossRef]
- Lee, S.A.; Chiu, C.K.; Chan, C.Y.W.; Yaakup, N.A.; Wong, J.H.D.; Kadir, K.A.A.; Kwan, M.K. The clinical utility of fluoroscopic versus CT guided percutaneous transpedicular core needle biopsy for spinal infections and tumours: A randomized trial. Spine J. 2020, 20, 1114–1124. [Google Scholar] [CrossRef]
- Amoretti, N.; Marcy, P.Y.; Lesbats-Jacquot, V.; Hovorka, I.; Fonquerne, M.-E.; Roux, C.; Hericord, O.; Maratos, Y.; Euller-Ziegler, L. Combined CT and fluoroscopic guidance of balloon kyphoplasty versus fluoroscopy-only procedures. Skelet. Radiol. 2009, 38, 703–707. [Google Scholar] [CrossRef]
- Wieschhoff, G.G.; Miskin, N.P.; Kim, J.S.; Hamberg, L.M.; Mandell, J.C. Radiation dose of fluoroscopy-guided versus ultralow-dose CT-fluoroscopy-guided lumbar spine epidural steroid injections. Skelet. Radiol. 2022, 51, 1055–1062. [Google Scholar] [CrossRef]
- Sag, A.A.; Zuchowski, A.; Ronald, J.; Goodwin, C.R.; Enterline, D.S. Augmented reality overlay fluoroscopic guidance versus CT-fluoroscopic guidance for sacroplasty. Clin. Imaging 2022, 85, 14–21. [Google Scholar] [CrossRef]
| Articles (Year) | Study Country | Study Type | Patient No. | Age (Range) | Male–Female Number | Tumor Location | Treatment Group(s) | Mean Cement Volume | Supplemental Treatment | Pain Improvement | Analgesia Use | Functional/QoL Improvement | Recurrence/Progression | Follow-Up Duration | Complications | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proschek (2009) [37] | Germany | Prospective | 16 | Mean: 59.5 years (52–69) | 0:16 | Thoracic and lumbar spine | RFA alone vs. RFA with VA | Unreported | Unreported | Pain reduction (~50%) in both groups | Unreported | Improved QoL (ODI) | No local recurrence | Mean: 20.4 months | None reported | Safe, no additional benefit of cement in pain/QoL |
| Madaelil (2016) [38] | USA | Retrospective | 11 | Median: 58 years (37–79) | 3:8 | Sacrum | RFA alone vs. RFA with VA | 9.1 mL | 45% received pre-procedural or post-procedural chemotherapy | NRS reduced from 8 to 3 at 1 month | Increased in 40% | Not measured | 75% local control | Median: 4.7 months (0.9–28.7) | None reported | RFA safe and effective for sacral metastases |
| Zhao (2018) [39] | China | Retrospective | 16 | Mean: 66.8 years (54–84) | 4:12 | Sternum, scapula, ribs, thoracic spine, lumbar spine, and sacrum | RFA alone vs. RFA with VA | Unreported | Unreported | VAS reduced from 8.1 to 1.4 over 6 months | Stopped within 2 months | Improved QoL (via EORTC QLQ-C30) | No recurrence (6–12 months) | 6–12 months | 1 case (6.25%) of cement leakage | Safe and effective for palliative treatment |
| Kastler (2021) [40] | France | Prospective | 25 | Mean: 60 years | 18:7 | Thoracic spine, lumbar spine, sacrum, and coccyx | RFA alone vs. RFA with VA | 4 mL | Unreported | VAS improvement up to 79% | Unreported | Not measured | Unreported | Up to 12 months | Minor cement leakage (11/16) without clinical symptoms | Effective, well-tolerated under local anesthesia |
| Pusceddu (2023) [19] | Italy | Retrospective | 16 | Mean: 67 years (41–84) | 8:8 | Thoracolumbar spine | RFA, cavity creation, vertebral augmentation | Unreported | Unreported | Significant reduction in VAS (p < 0.001) | Unreported | Improved mobility (FMS) | No tumor recurrence | 6 months | None reported | Safe and effective for pain and QoL improvement |
| Articles (Year) | Study Country | Study Type | Patient No. | Age (Range) | Male–Female Number | Tumor Location | Treatment Group(s) | Mean Cement Volume | Supplementary Treatment | Pain Improvement | Analgesia Use | Functional/QoL Improvement | Recurrence/Progression | Follow-Up Duration | Complications | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdelgawaad (2021) [22] | Germany | Retrospective | 60 | (35–80) | 35:25 | Thoracic and lumbar spine | Cooled RFA, VA | Unreported | Radiotherapy and chemotherapy | Mean VAS reduced from 7.2/10 ± 2.3 (4–9) to 2.7/10 ± 1.9 (1–5) on day 3 and 3/10 ± 2.1 (1–6) at 6 months, p = 0.0001 | Unreported | Unreported | No recurrence | Mean 13.2 ± 6.3 months; minimum 6 months; maximum 36 months | 2 leaks into needle tracks, 2 leaks into veins, and 1 into disc spaces—all asymptomatic | Combined RFA and VA appears to be a safe, practical, effective and reproducible palliative treatment |
| Georgy (2009) [23] | USA | Retrospective | 37 | (45–75) | 16:21 | Thoracic and lumbar spine | RFA with VA | Unreported | Unreported | VAS improved in 89.5% of patients, mean VAS reduced from 7.9 to 4.2 (p < 0.0001) | Unreported | Unreported | Unreported | Median 6 months | Unreported | Effective alternative for advanced metastases |
| Greenwood (2015) [16] | USA | Retrospective | 21 | Mean: 61.8 (30–84) | 13:9 | Undefined spinal location | RFA/cryoablation, VA, radiation therapy | Unreported | Radiation therapy | Pain scores reduced from (8.0, SD = 2.3) pre-operatively to 2.9, SD = 3.3; p < 0.0003 | Opioid use was decreased in 62% (13/21), remained unchanged in 19% (4/21), and increased in 19% (4/21) at 4 weeks | General activity level at 4 weeks after ablation treatments was increased in 81% (17/21) and decreased in 19% (4/21) | Disease stable in 12/13 patients at 3 months and 10/10 patients at 6 months | 6 months | No complication other than undefined post-procedural infection | Safe and effective for radiation-resistant tumors for control of pain and local control of tumor |
| Gu (2017) [24] | China | Prospective | 124 | Mean: 58.2 (37–76) vs. 59.5 (33–90) | 75:49 | Thoracic and lumbar spine | Interventional tumor removal, RFA, and VA | Unreported | Unreported | Significant reduction in VAS at 1, 3, and 6 months, and >1 year (p = 0.05) | Not reported | Significant improvement in ODI at 1, 3, and 6 months, and >1 year (p = 0.03) | Minimal | 6–12 months | 29 and 29 cement leaks in 70 and 53 vertebral bodies in groups A and B, respectively; 1 severe complication of paraplegia | Effective for spinal stability and pain relief |
| Jain (2020) [25] | USA | Retrospective | 64 | Mean: 62.3 | Undefined | Thoracic and lumbar spine | RFA (Osteocool) and VA vs. RFA (SpineSTAR) and VA vs. VA only | Unreported | Radiation and chemotherapy | VAS improvement in all groups; re-operative pain scores were on average 6.9/10 for SpineSTAR, 6.3/10 for kyphoplasty alone, and 6/10 for OsteoCool; post-operative pain scores were on average 2.7/10 for SpineSTAR, 2.3/10 for kyphoplasty alone, and 1.7/10 for OsteoCool | Narcotic usage within post-operative month one was seen in 11/22 (50%) of SpineSTAR cases, 9/30 (30%) of kyphoplasty cases, and 5/12 (41.7%) of OsteoCool cases | Unreported | Unreported | 1 month | Unreported | Comparable outcomes for both methods; overall decrease in pain scores for all treatment groups; however, there was no substantial difference in pain scores between patients who received RFA with vertebral augmentation vs. those who received kyphoplasty alone |
| Lv (2020) [26] | China | Retrospective | 87 | Mean: 64 | 53:34 | Thoracic and lumbar spine | VA alone vs. RFA with VA | Unreported | Unreported | VAS for groups A and B improved from 7.52 ± 1.44 and 7.63 ± 1.52 to 2.23 ± 0.46 and 3.15 ± 0.52, respectively, at 6 months | Unreported | Improved QoL; ODI for groups A and B improved from 77.52 ± 8.84 and 76.65 ± 8.12 to 46.46 ± 6.46 and 52.15 ± 7.52, respectively, at 6 months (p = 0.04) | 11.4% recurrence in group A and 30.8% in group B | 6 months | 6.4% cement leak in group A and 20.5% cement leak in group B, all asymptomatic | Bone cement combined with RFA in the treatment of spinal metastatic tumor can effectively relieve patients’ pain, improve their ability for daily activities, and enhance the spinal stability |
| Masala (2004) [27] | Italy | Prospective | 3 | Mean: 72.3 (63–82) | 1:2 | Spine (undefined) | RFA with VA | Unreported | Unreported | VAS improved significantly; reduction from average of 8.6 points of VAS pre-procedure to 2.6 post-procedure | Not reported | Unreported | Unreported | 6 months | Unreported | Safe and effective for VCF |
| Munk (2009) [28] | Canada | Retrospective | 19 | Mean: 58.9 (42–82) | 5:14 | Spine, pelvis, and long bones | RFA with VA | 6.1 mL | Unreported | VAS reduced from 7.90 (range, 7.0 –9.0) to 3.82 (range, 0.0–6.2) (p < 0.0001) | Analgesia use reduction achieved in 18 patients, with complete cessation in 1 patient | 18 patients had improvement in mobility | Unreported | Median 9 months | 7 minor complications; 6 cement extravasations, 1 thermal nerve injury | Effective for pain relief in neoplastic lesions |
| Nilgun (2022) [29] | Turkey | Retrospective | 41 | Mean: 67 (45–87) | 22:19 | Thoracic and lumbar spine | RFA with VA | Unreported | Unreported | VAS reduced significantly; mean VAS score was 7.4 at the preprocedural assessment and 3.2 at 6 months (p < 0.0001) | Unreported | Improved QoL; mean ODI was 71.04 at the preprocedural assessment and 34 at 6 months | Unreported | 6 months | 1 patient had pulmonary embolism (mild symptoms), 2 patients had transient motor deficits without cement leak | Safe and effective for metastatic spinal pain |
| Reyes (2017) [30] | USA/Europe | Multicenter | 49 | 64.3 ± 12.6 | 15:34 | Thoracic and lumbar spine | RFA with VA | Unreported | 5 patients had radiotherapy and 3 had chemotherapy | VAS improved from 7.9 ± 2.5 (range 2–10) to 3.5 ± 2.6 (range 0–10) (p < 0.0001) | Unreported | ODI improvement; pre-procedure ODI 34.9 ± 18.3 (range 13–50) vs. post-procedure ODI 21.6 ± 13.8 (range 0–45) (p < 0.0001) | 1 case of recurrence | 2–4 weeks | None reported | Effective for pain reduction and functional improvement |
| Sandri (2010) [31] | Italy | Retrospective | 11 | Mean: 68 (58–82) | 2:9 | Cervical, thoracic, and lumbar spine | RFA with VA | Unreported | Unreported | Mean VAS reduced from 8.0 to 1.8 at 72 h, and 1.9 at 6 weeks (p < 0.001) | Reduction in use in all patients | Unreported | No complications | 6 weeks | 1 case of asymptomatic cement leakage | Safe and effective for osteolytic metastases |
| Sayed (2019) [21] | USA | Prospective | 30 | 18+ | 19:11 | Thoracic and lumbar spine | RFA with VA | Unreported | Unreported | NRS-11 improved from 5.77 to 2.61 at 3 months, p < 0.01 | Unreported | Improved QoL; FACT-G7 improved from 13.0 at baseline to 15.11 at 3 months, p = 0.07 | Unreported | 3 months | Non reported complication | Safe and effective for metastatic spinal lesions |
| Tomasian (2018) [32] | USA | Retrospective | 27 | (23–86) | 17:10 | Thoracic and lumbar spine, and sacrum | RFA with VA | Unreported | Unreported | VAS not reported, but local tumor control achieved in 96% of cases | Unreported | Unreported | Local tumor control for 96% patients | 16 weeks | None reported | Safe and effective for tumor control |
| Wang F (2021) [36] | China | Retrospective | 35 | Mean: 63.1 (45–83) vs. 61.5 (41–81) | 22:13 | Thoracic and lumbar spine | VA alone vs. RFA with VA | 5.95 mL | 26 patients on chemotherapy | VAS improved significantly in both groups; VAS scores of group A (1.86 ± 0.78) were significantly lower than those in group B (4.59 ± 1.06) 6 months after the treatment (p < 0.001) | Unreported | Significant ODI improvement in RFA + vertebroplasty group compared to single vertebroplasty (p < 0.05) | Unreported | 6 months | No major complications; minor pain, cement leakage, pulmonary venous cement leak without symptoms | Better outcomes with combined treatment |
| Wang L (2023) [35] | China | Retrospective | 47 | Mean: 59.9 (61.5–58.3) | 19:28 | Thoracic and lumbar spine | RFA with VA | Unreported | Unreported | VAS score measured pre-operatively only | Unreported | Unreported | Unreported | Unreported | Pulmonary cement embolism was detected in 11 patients (23.4%), and all patients were asymptomatic | Safe with awareness of embolism risk |
| Yildizhan (2021) [33] | Turkey | Retrospective | 66 | Undefined | Undefined | Thoracic and lumbar spine | RFA alone vs. RFA with VA | Unreported | Unreported | Significant pain improvement in both groups; VAS in RFA + VP group improved from 7.44 ± 1.06 to 2.31 ± 1.42, while VAS in RFA group improved from 8.33 ± 1.07 to 4.42 ± 1.08 (p < 0.001) | Unreported | ODI improved from 78.5% to 14.2% post-treatment (p < 0.001) | No recurrence noted | 6 months | None reported | Combined therapy more effective |
| Zheng (2014) [34] | China | Retrospective | 26 | Mean: 59.3 (32–75) | 12:14 | Thoracic and lumbar spine | RFA with VA | 6.73 mL | Unreported | VAS reduced significantly; VAS improved from 7.69 ± 1.12 at baseline to 2.96 ± 0.92 at 6 months (p < 0.01) | Unreported | Unreported | No tumor recurrence | 8.4 months | None reported | Safe and effective for palliation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Q.Z.; Sarrafpour, S.; Hasoon, J.; Yong, R.J.; Robinson, C.L.; Chung, M. Comparison of Clinical Outcomes Between Fluoroscopic and Computer Tomographic Guidance in Concurrent Use of Radiofrequency Ablation and Vertebral Augmentation in Spinal Metastases: A Scoping Review. Diagnostics 2025, 15, 1463. https://doi.org/10.3390/diagnostics15121463
Ruan QZ, Sarrafpour S, Hasoon J, Yong RJ, Robinson CL, Chung M. Comparison of Clinical Outcomes Between Fluoroscopic and Computer Tomographic Guidance in Concurrent Use of Radiofrequency Ablation and Vertebral Augmentation in Spinal Metastases: A Scoping Review. Diagnostics. 2025; 15(12):1463. https://doi.org/10.3390/diagnostics15121463
Chicago/Turabian StyleRuan, Qing Zhao, Syena Sarrafpour, Jamal Hasoon, R. Jason Yong, Christopher L. Robinson, and Matthew Chung. 2025. "Comparison of Clinical Outcomes Between Fluoroscopic and Computer Tomographic Guidance in Concurrent Use of Radiofrequency Ablation and Vertebral Augmentation in Spinal Metastases: A Scoping Review" Diagnostics 15, no. 12: 1463. https://doi.org/10.3390/diagnostics15121463
APA StyleRuan, Q. Z., Sarrafpour, S., Hasoon, J., Yong, R. J., Robinson, C. L., & Chung, M. (2025). Comparison of Clinical Outcomes Between Fluoroscopic and Computer Tomographic Guidance in Concurrent Use of Radiofrequency Ablation and Vertebral Augmentation in Spinal Metastases: A Scoping Review. Diagnostics, 15(12), 1463. https://doi.org/10.3390/diagnostics15121463








