Fetal Death Due to an Unusual Coexistence of Two Umbilical Cord Anomalies: Analysis in a Forensic Perspective
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical History
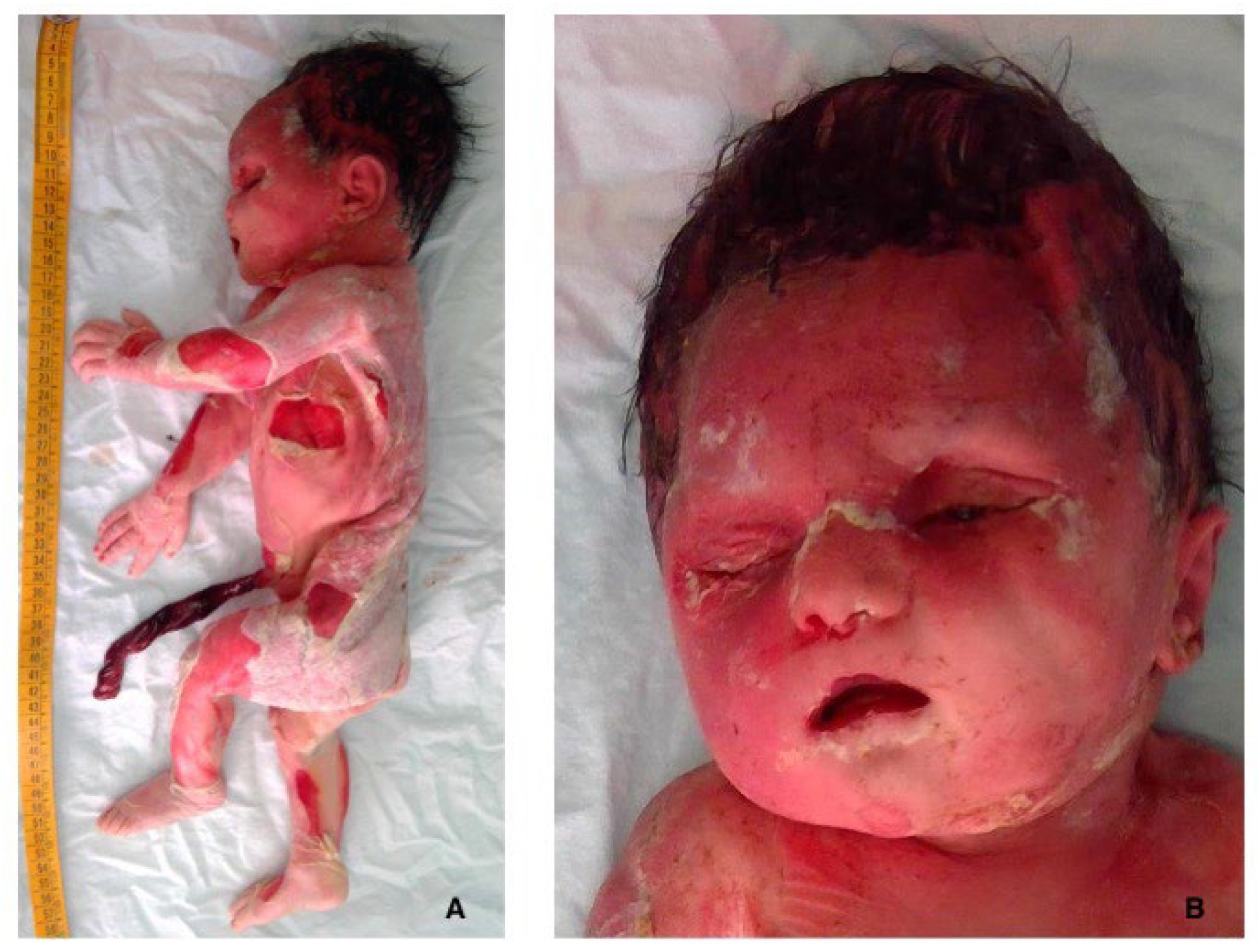
2.2. Fetal Post-Mortem Examination
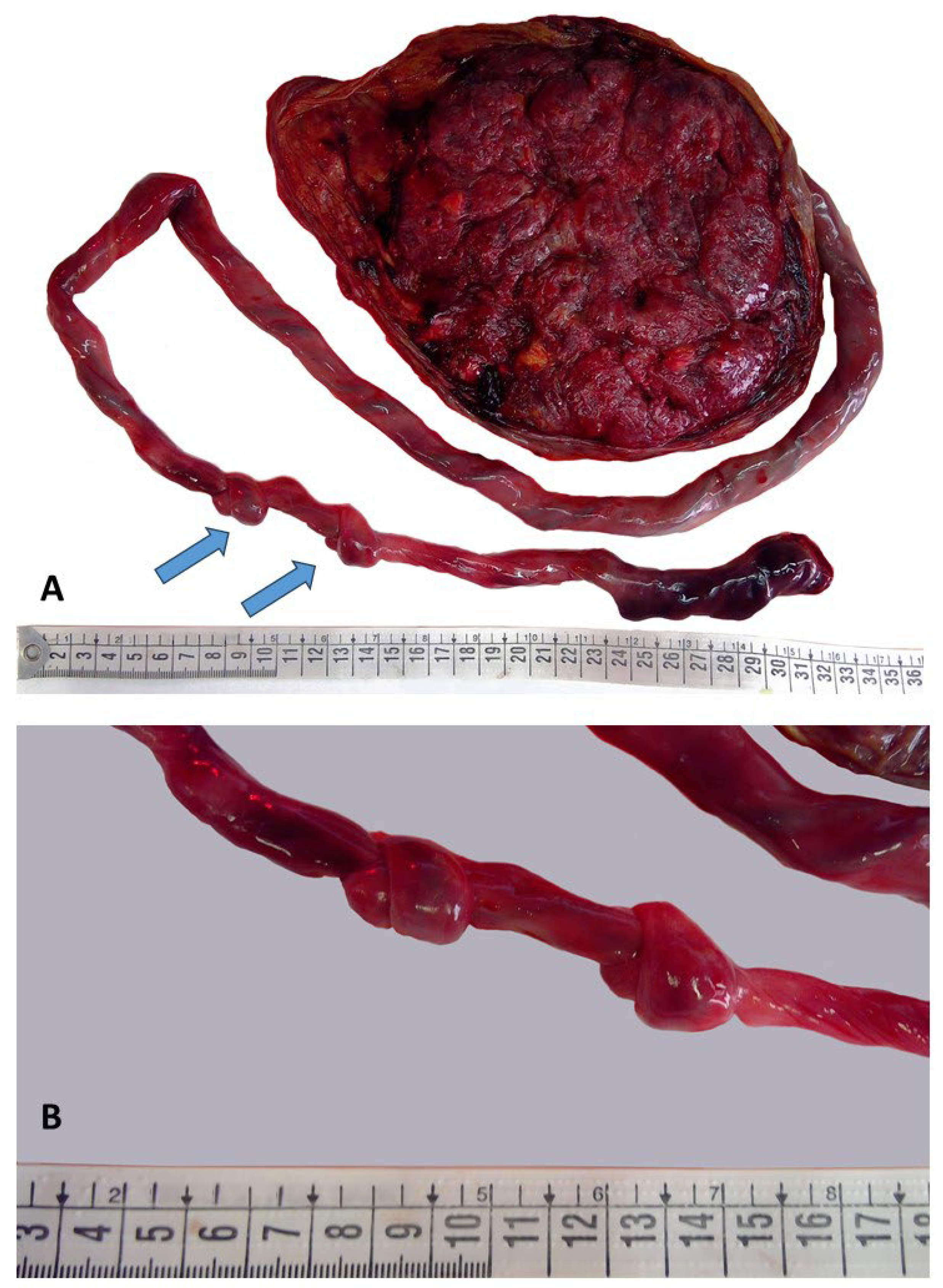
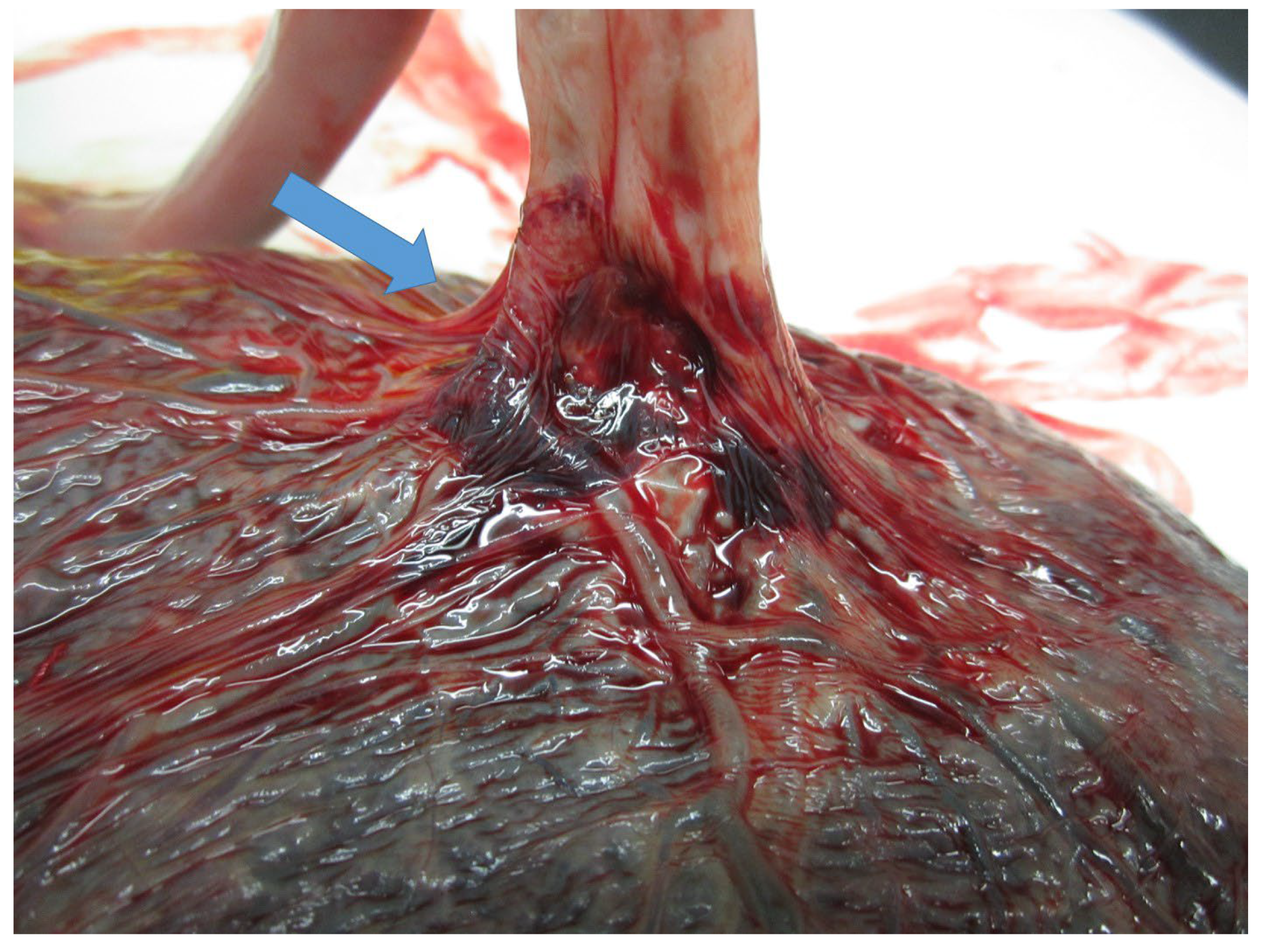
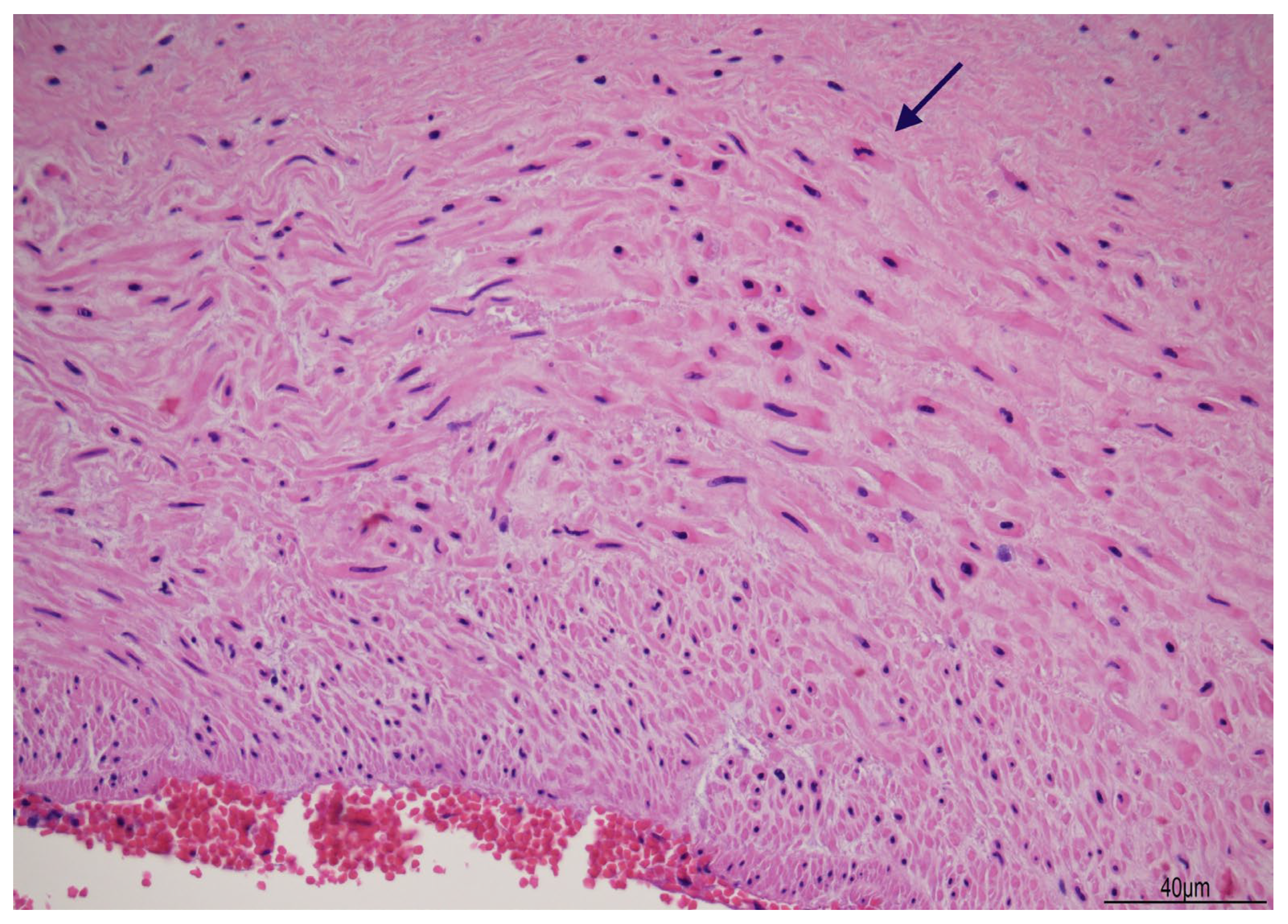
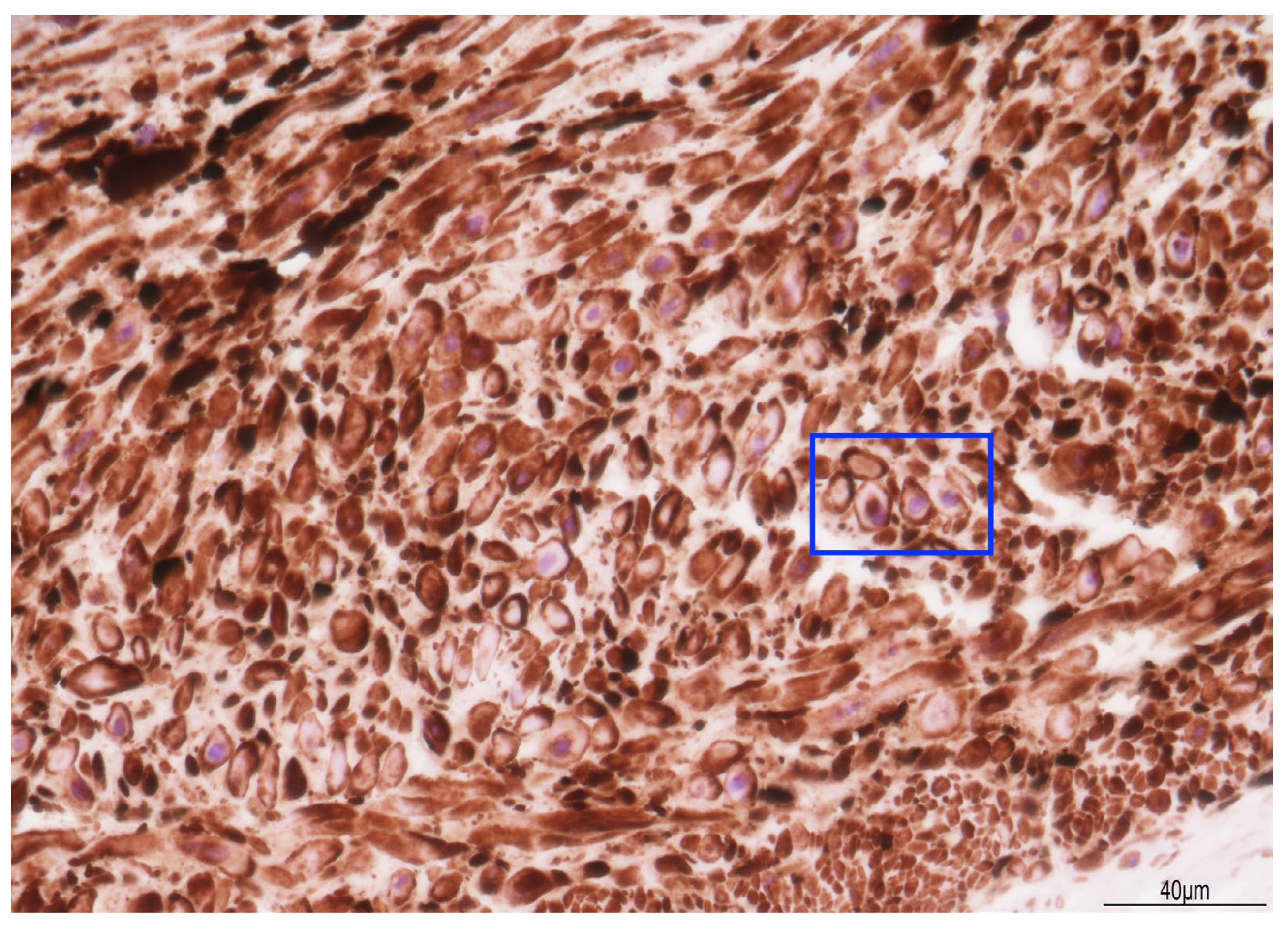
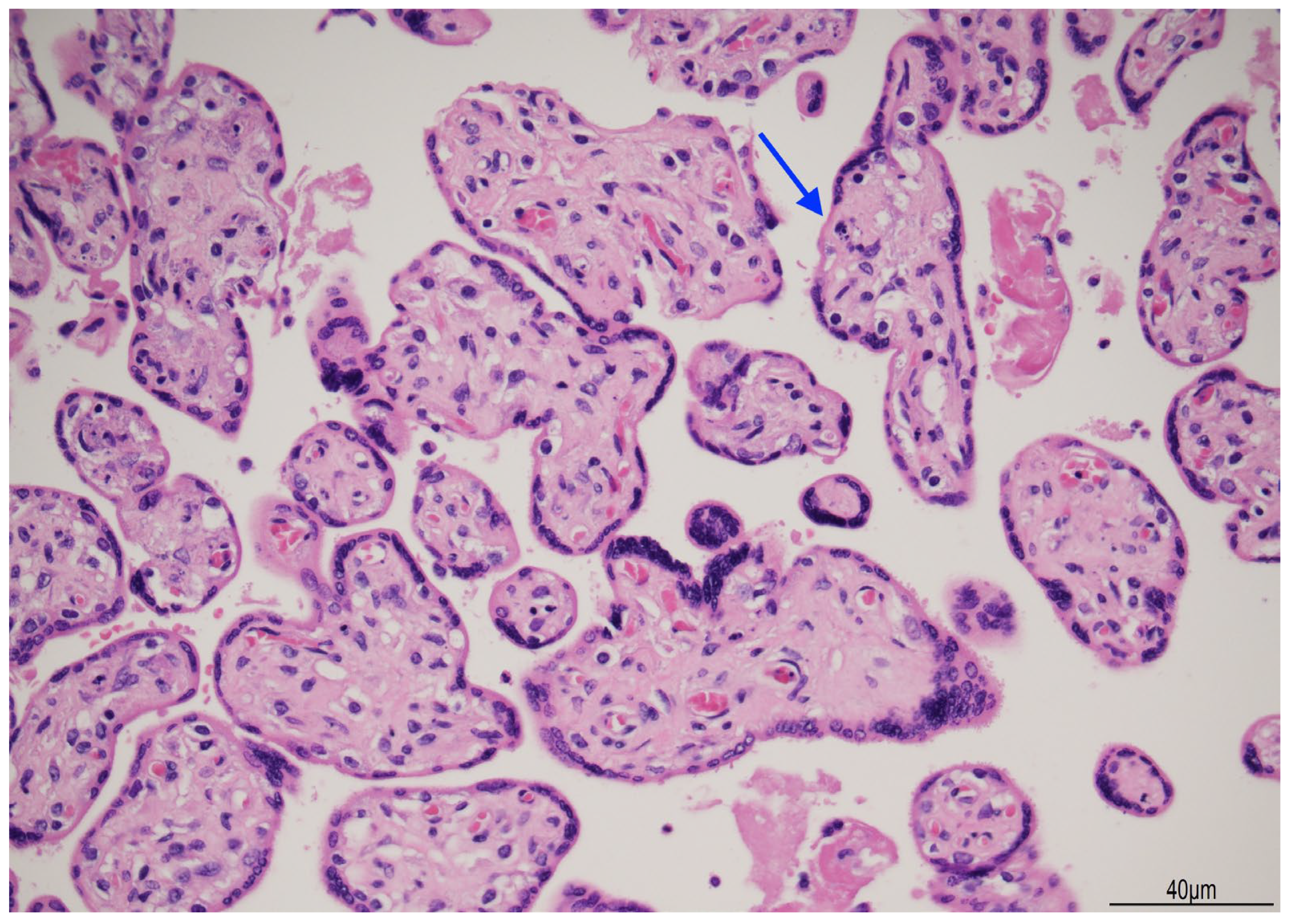
2.3. Placental Examination
3. Discussion
3.1. The Cause of Fetal Death
3.2. The Time of Fetal Death
3.3. The Type of Death
3.4. Minimally Invasive Tissue Sampling in a Forensic Context
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linde, L.E.; Rasmussen, S.; Kessler, J.; Ebbing, C. Extreme Umbilical Cord Lengths, Cord Knot and Entanglement: Risk Factors and Risk of Adverse Outcomes, a Population-Based Study. PLoS ONE 2018, 13, e0194814. [Google Scholar] [CrossRef] [PubMed]
- Al Qasem, M.; Meyyazhagan, A.; Tsibizova, V.; Clerici, G.; Arduini, M.; Khader, M.; Alkarabsheh, A.M.; Di Renzo, G.C. Knots of the Umbilical Cord: Incidence, Diagnosis, and Management. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2024, 166, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Houri, O.; Bercovich, O.; Wertheimer, A.; Pardo, A.; Berezowsky, A.; Hadar, E.; Hochberg, A. Clinical Significance of True Umbilical Cord Knot: A Propensity Score Matching Study. BMC Pregnancy Childbirth 2024, 24, 59. [Google Scholar] [CrossRef]
- Hayes, D.J.L.; Warland, J.; Parast, M.M.; Bendon, R.W.; Hasegawa, J.; Banks, J.; Clapham, L.; Heazell, A.E.P. Umbilical Cord Characteristics and Their Association with Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0239630. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, C.; Rasmussen, S.; Kessler, J.; Moster, D. Association of Placental and Umbilical Cord Characteristics with Cerebral Palsy: National Cohort Study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2023, 61, 224–230. [Google Scholar] [CrossRef]
- Laranjo, M.; Neves, B.M.; Peixinho, C. True Double Umbilical Cord Knot. BMJ Case Rep. 2022, 15, e251388. [Google Scholar] [CrossRef]
- Razavi, B.; Kasraeian, M.; Hashemi, A.; Moradi Alamdarloo, S.; Najib, F.S. Complex Twisted Knots of Umbilical Cord in a Monochorionic-Diamniotic Twin Gestation: A Case Report. Galen Med. J. 2020, 9, e1878. [Google Scholar] [CrossRef]
- Weller, M.A. Two Knots in the Umbilical Cord. J. R. Coll. Gen. Pract. 1976, 26, 704–705. [Google Scholar]
- McLennan, H.; Price, E.; Urbanska, M.; Craig, N.; Fraser, M. Umbilical Cord Knots And Encirclements. Aust. N. Z. J. Obstet. Gynaecol. 1988, 28, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, L.; Jahanshahi, F.; Dini, P. Two Knots in an Umbilical Cord with Seventy Centimeter Length: A Case Report. Clin. Case Rep. 2020, 8, 1579–1581. [Google Scholar] [CrossRef]
- Stabile, G.; Carlucci, S.; De Bonis, L.; Sorrentino, F.; Nappi, L.; Ricci, G. Umbilical Cord Knots: Is the Number Related to Fetal Risk? Med. Kaunas Lith. 2022, 58, 703. [Google Scholar] [CrossRef] [PubMed]
- Waldron, J.E.; Muir, S.M.; Hubbard, J. Double and Single True Knot of an Umbilical Cord: A Case Report. Cureus 2023, 15, e36393. [Google Scholar] [CrossRef] [PubMed]
- Eleje, G.U.; Nwammuo, C.B.; Nnamani, K.O.; Igbodike, E.P.; Nwankwo, E.U.; Okafor, C.G.; Njoku, T.K.; Ekwebene, O.C.; Egwuatu, E.C.; Malachy, D.E.; et al. Double True Umbilical Cord Knots Coexisting with a Nuchal Cord with Successful Fetal Outcome: A Case Report. SAGE Open Med. Case Rep. 2024, 12, 2050313X241239524. [Google Scholar] [CrossRef] [PubMed]
- Archie, J.G.; Collins, J.S.; Lebel, R.R. Quantitative Standards for Fetal and Neonatal Autopsy. Am. J. Clin. Pathol. 2006, 126, 256–265. [Google Scholar] [CrossRef]
- Pinar, H.; Koch, M.; Hawkins, H.; Heim-Hall, J.; Abramowsky, C.; Thorsten, V.; Carpenter, M.; Zhou, H.; Reddy, U. The Stillbirth Collaborative Research Network Postmortem Examination Protocol. Am. J. Perinatol. 2012, 29, 187–202. [Google Scholar] [CrossRef]
- Genest, D.R.; Williams, M.A.; Greene, M.F. Estimating the Time of Death in Stillborn Fetuses: I. Histologic Evaluation of Fetal Organs; an Autopsy Study of 150 Stillborns. Obstet. Gynecol. 1992, 80, 575–584. [Google Scholar]
- Roberts, D.J.; Polizzano, C. AFIP Atlases of Tumor and Non-Tumor Pathology. In AFIP-ARP. Atlas of Placental Pathology; American Registry of Pathology (ARP Press): Arlington, VA, USA, 2021; Volume 6, p. 134. [Google Scholar]
- Srinivasan, A.; Graves, L. Four True Umbilical Cord Knots. J. Obstet. Gynaecol. Can. JOGC J. Obstet. Gynecol. Can. JOGC 2006, 28, 32–35. [Google Scholar] [CrossRef]
- Weissmann-Brenner, A.; Domniz, N.; Weissbach, T.; Mazaki-Tovi, S.; Achiron, R.; Weisz, B.; Kassif, E. Antenatal Detection of True Knot in the Umbilical Cord—How Accurate Can We Be? Ultraschall Med. Stuttg. Ger. 1980 2022, 43, 298–303. [Google Scholar] [CrossRef]
- AIUM-ACR-ACOG-SMFM-SRU Practice Parameter for the Performance of Standard Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2018, 37, E13–E24. [CrossRef]
- Predisposing Factors in the Formation of True Knots of the Umbilical Cord-analysis of Morphometric and Perinatal Data—Blickstein—1987—International Journal of Gynecology & Obstetrics—Wiley Online Library. Available online: https://obgyn.onlinelibrary.wiley.com/doi/abs/10.1016/0020-7292%2887%2990346-8 (accessed on 24 May 2025).
- Airas, U.; Heinonen, S. Clinical Significance of True Umbilical Knots: A Population-Based Analysis. Am. J. Perinatol. 2002, 19, 127–132. [Google Scholar] [CrossRef]
- Tantbirojn, P.; Saleemuddin, A.; Sirois, K.; Crum, C.P.; Boyd, T.K.; Tworoger, S.; Parast, M.M. Gross Abnormalities of the Umbilical Cord: Related Placental Histology and Clinical Significance. Placenta 2009, 30, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.; Georgiadis, L.; Harju, M.; Keski-Nisula, L.; Heinonen, S. True Umbilical Cord Knot and Obstetric Outcome. Int. J. Gynecol. Obstet. 2013, 122, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.; Yalla, S.; Salvi, P. True Knot of the Umbilical Cord and Associated Adverse Perinatal Outcomes: A Case Series. Cureus 2023, 15, e35377. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Ito, H.; Ishise, J.; Yamamoto, S. Fine Structure of Myonecrosis Following Adrenalin-Induced Cerebral Vasospasm. Neurochirurgia 1987, 30, 29–34. [Google Scholar] [CrossRef]
- Guo, H.; Huang, R.; Semba, S.; Kordowska, J.; Huh, Y.H.; Khalina-Stackpole, Y.; Mabuchi, K.; Kitazawa, T.; Wang, C.-L.A. Ablation of Smooth Muscle Caldesmon Affects the Relaxation Kinetics of Arterial Muscle. Pflugers Arch. 2013, 465, 283–294. [Google Scholar] [CrossRef][Green Version]
- Smet, M.E.; Ormandy, A.; Libbrecht, S.; Arrage, N.; Pesce, A.; Moghimi, A. Furcate Cord Insertion: Under-Reported but Likely Relevant. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2024, 63, 280–281. [Google Scholar] [CrossRef]
- Kosian, P.; Henrich, W.; Entezami, M.; Weichert, A. Furcate Insertion of the Umbilical Cord: Pathological and Clinical Characteristics in 132 Cases. J. Perinat. Med. 2020, 48, 819–824. [Google Scholar] [CrossRef]
- Kraus, F.T. Perinatal Pathology, the Placenta, and Litigation. Hum. Pathol. 2003, 34, 517–521, discussion 522–527. [Google Scholar] [CrossRef]
- Fanaroff, J.M.; Donn, S.M. Medico-Legal Implications of the Fetal Inflammatory Response Syndrome. Semin. Fetal. Neonatal Med. 2020, 25, 101127. [Google Scholar] [CrossRef]
- Genest, D.R. Estimating the Time of Death in Stillborn Fetuses: II. Histologic Evaluation of the Placenta; a Study of 71 Stillborns. Obstet. Gynecol. 1992, 80, 585–592. [Google Scholar]
- Genest, D.R.; Singer, D.B. Estimating the Time of Death in Stillborn Fetuses: III. External Fetal Examination; a Study of 86 Stillborns. Obstet. Gynecol. 1992, 80, 593–600. [Google Scholar] [PubMed]
- Paternoster, M.; Perrino, M.; Travaglino, A.; Raffone, A.; Saccone, G.; Zullo, F.; D’Armiento, F.P.; Buccelli, C.; Niola, M.; D’Armiento, M. Parameters for Estimating the Time of Death at Perinatal Autopsy of Stillborn Fetuses: A Systematic Review. Int. J. Legal Med. 2019, 133, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.M.; Spencer, D.; Al-Jishi, T. Stillbirth Investigations: An Iconographic and Concise Diagnostic Workup in Perinatal Pathology. J. Lab. Physicians 2023, 15, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Tanko, N.M.; Bakytkaly, I.; Issanov, A.; Poddighe, D.; Terzic, M. Validating a Minimally Invasive Tissue Sampling (MITS) Method in Determining Cause of Death in Stillbirths and Neonates. Children 2021, 8, 1095. [Google Scholar] [CrossRef]
- Paganelli, C.R.; Goco, N.J.; McClure, E.M.; Banke, K.K.; Blau, D.M.; Breiman, R.F.; Menéndez, C.; Rakislova, N.; Bassat, Q. The Evolution of Minimally Invasive Tissue Sampling in Postmortem Examination: A Narrative Review. Glob. Health Action 2020, 13, 1792682. [Google Scholar] [CrossRef]
- Menezes, R.G.; Monteiro, F.N. Forensic Autopsy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
| Reference | Stillbirth Cases/Total Number of Cases with UCTK | Percentage % | Incidence |
|---|---|---|---|
| Blickstein et al. [21] | 2/54 | 3.7 | 0.037 |
| Airas et al. [22] | 4/288 | 1.4 | 0.014 |
| Tantbirojn et al. [23] | 2/33 | 6.06 | 0.06 |
| Raisanen et al. [24] | 3/339 | 0.88 | 0.009 |
| Linde et al. [1] | At term: 83/1216 | 6.82 | 0.068 |
| Linde et al. [1] | Pre-term: 58/2046 | 2.83 | 0.028 |
| Gaikwad et al. [25] | 2/3 case series | 66.66 | 0.666 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferretti, A.; Bonasoni, M.P.; Petrachi, B.; Comitini, G.; Blasi, I.; Giorgetti, A.; Fais, P.; Pelotti, S.; Aguzzoli, L. Fetal Death Due to an Unusual Coexistence of Two Umbilical Cord Anomalies: Analysis in a Forensic Perspective. Diagnostics 2025, 15, 1423. https://doi.org/10.3390/diagnostics15111423
Ferretti A, Bonasoni MP, Petrachi B, Comitini G, Blasi I, Giorgetti A, Fais P, Pelotti S, Aguzzoli L. Fetal Death Due to an Unusual Coexistence of Two Umbilical Cord Anomalies: Analysis in a Forensic Perspective. Diagnostics. 2025; 15(11):1423. https://doi.org/10.3390/diagnostics15111423
Chicago/Turabian StyleFerretti, Alice, Maria Paola Bonasoni, Benedetta Petrachi, Giuseppina Comitini, Immacolata Blasi, Arianna Giorgetti, Paolo Fais, Susi Pelotti, and Lorenzo Aguzzoli. 2025. "Fetal Death Due to an Unusual Coexistence of Two Umbilical Cord Anomalies: Analysis in a Forensic Perspective" Diagnostics 15, no. 11: 1423. https://doi.org/10.3390/diagnostics15111423
APA StyleFerretti, A., Bonasoni, M. P., Petrachi, B., Comitini, G., Blasi, I., Giorgetti, A., Fais, P., Pelotti, S., & Aguzzoli, L. (2025). Fetal Death Due to an Unusual Coexistence of Two Umbilical Cord Anomalies: Analysis in a Forensic Perspective. Diagnostics, 15(11), 1423. https://doi.org/10.3390/diagnostics15111423






