Effects of Stroke Volume Maximization Before One-Lung Ventilation on Video-Assisted Thoracic Surgery: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients
2.3. Anesthetic Management
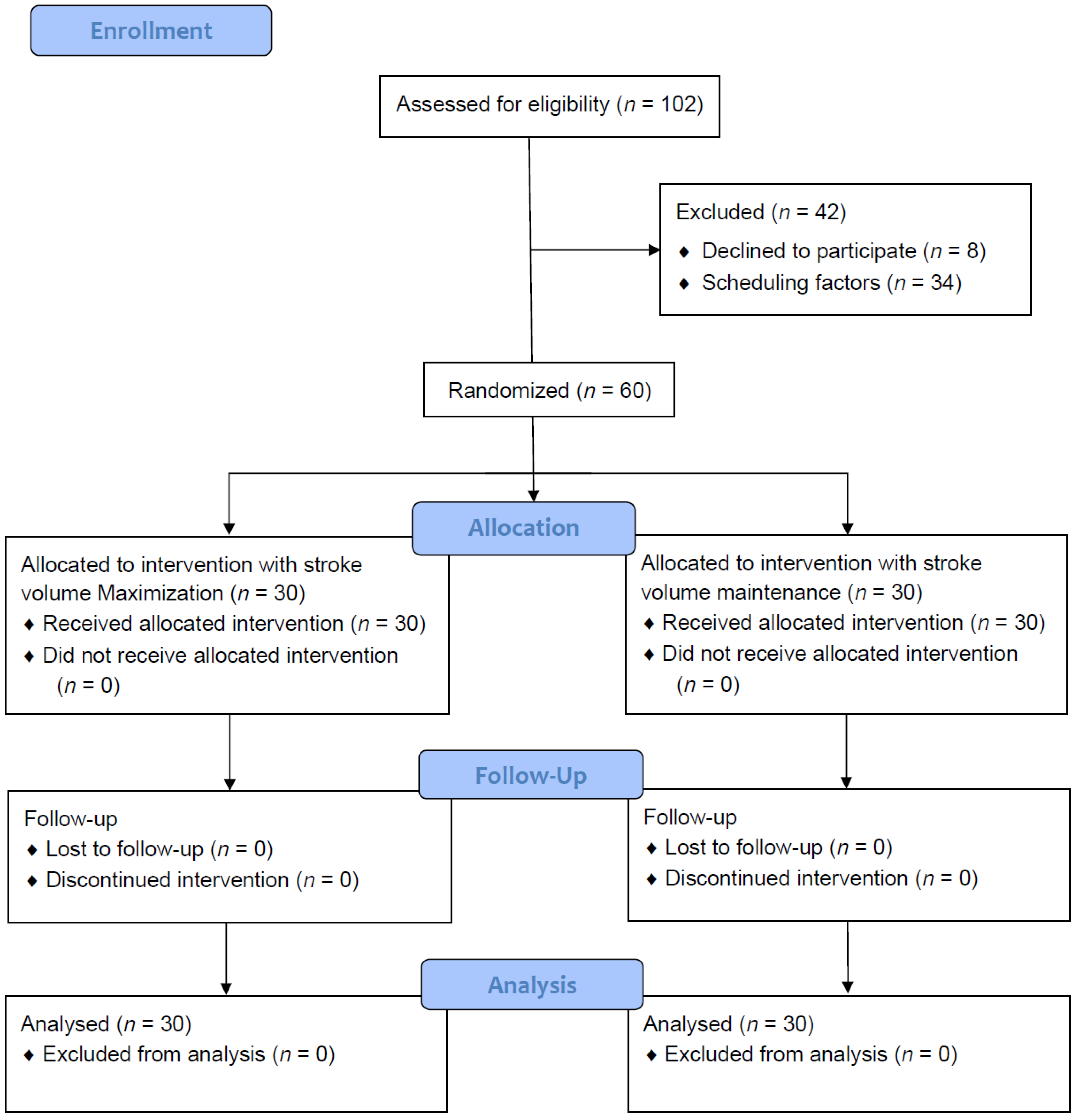
2.4. Randomization and Group Assignments
2.5. Fluid Management Protocols
2.6. Outcome Measurement
2.7. Statistical Analysis
3. Results
3.1. Demographic, Anesthetic, and Operative Results
3.2. Primary Outcome
3.3. Secondary Outcome
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ASA | American Society of Anesthesiologists |
| BB | Bronchial blocker |
| CC16 | Clara cell secretory protein |
| DLT | Double-lumen endo-bronchial tube |
| ETCO2 | End-tidal carbon dioxide |
| FEV1 | Forced expiratory volume in 1 s |
| FVC | Forced vital capacity |
| GDFT | Goal-directed fluid therapy |
| IL-6 | Interleukin 6 |
| NGAL | Neutrophil gelatinase–associated lipocalin |
| OLV | One-lung ventilation |
| PPV | Pulse pressure variation |
| SPD | Surfactant protein D |
| SV | Stroke volume |
| SVM | Stroke volume maximization |
| SVV | Stroke volume variation |
| TBARS | Thiobarbituric acid-reactive substances |
| VATS | Video-assisted thoracoscopic surgery |
References
- Arslantas, M.K.; Kara, H.V.; Tuncer, B.B.; Yildizeli, B.; Yuksel, M.; Bostanci, K.; Bekiroglu, N.; Kararmaz, A.; Cinel, I.; Batirel, H.F. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J. Thorac. Cardiovasc. Surg. 2015, 149, 314–321.e1. [Google Scholar] [CrossRef] [PubMed]
- Brandstrup, B.; Tønnesen, H.; Beier-Holgersen, R.; Hjortsø, E.; Ørding, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.B.; Loop, T.; Heinrich, S. Working Group of the German Thorax Registry. Risk factors for postoperative pulmonary complications in lung cancer patients after video-assisted thoracoscopic lung resection: Results of the German Thorax Registry. Acta Anaesthesiol. Scand. 2019, 63, 1009–1018. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, H.J.; Oh, A.R.; Choi, J. Restrictive intraoperative fluid management was associated with higher incidence of composite complications compared to less restrictive strategies in open thoracotomy: A retrospective cohort study. Sci. Rep. 2020, 10, 8449. [Google Scholar] [CrossRef]
- Myles, P.S.; Bellomo, R.; Corcoran, T.; Forbes, A.; Peyton, P.; Story, D.; Christophi, C.; Leslie, K.; McGuinness, S.; Parke, R.; et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N. Engl. J. Med. 2018, 378, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, R.; Xu, J.; Rusidanmu, A.; Zhang, X.; Hu, J. Effects of Intraoperative Fluid Management on Postoperative Outcomes After Lobectomy. Ann. Thorac. Surg. 2019, 107, 1663–1669. [Google Scholar] [CrossRef]
- Zhang, J.C.C.; Lei, X.Z.; Feng, Z.Y.; Zhu, S.M. Goal-directed fluid optimization based on stroke volume variation and cardiac index during one-lung ventilation in patients undergoing thoracoscopy lobectomy operations: A pilot study. Clinics 2013, 68, 1065–1070. [Google Scholar] [CrossRef]
- Xu, H.; Shu, S.H.; Wang, D.; Chai, X.Q.; Xie, Y.H.; Zhou, W.D. Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: A randomized controlled trial. J. Thorac. Dis. 2017, 9, 2992–3004. [Google Scholar] [CrossRef]
- Sahutoglu, C.; Turksal, E.; Kocabas, S.; Askar, F.Z. Influence of stroke volume variation on fluid treatment and postoperative complications in thoracic surgery. Ther. Clin. Risk. Manag. 2018, 14, 575–581. [Google Scholar] [CrossRef]
- Lema Tome, M.; De la Gala, F.A.; Piñeiro, P.; Olmedilla, L.; Garutti, I. Behavior of stroke volume variation in hemodynamic stable patients during thoracic surgery with one-lung ventilation periods. Rev. Bras. Anestesiol. 2018, 68, 225–230. [Google Scholar] [CrossRef]
- Jeong, D.M.; Ahn, H.J.; Park, H.W.; Yang, M.; Kim, J.A.; Park, J. Stroke Volume Variation and Pulse Pressure Variation Are Not Useful for Predicting Fluid Responsiveness in Thoracic Surgery. Anesth. Analg. 2017, 125, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.-S.; Oh, C.-S.; Park, C.; Shin, B.M.; Yoon, T.-G.; Rhee, K.-Y.; Woo, N.-S.; Kim, S.-H. Diagnosis Accuracy of Mean Arterial Pressure Variation during a Lung Recruitment Maneuver to Predict Fluid Responsiveness in Thoracic Surgery with One-Lung Ventilation. Biomed. Res. Int. 2016, 2016, 3623710. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Eichhorn, V.; Hasbach, T.; Trepte, C.; Kutup, A.; Goetz, A.E.; Reuter, D.A. Goal-directed fluid therapy using stroke volume variation does not result in pulmonary fluid overload in thoracic surgery requiring one-lung ventilation. Crit. Care Res. Pract. 2012, 2012, 687018. [Google Scholar] [CrossRef][Green Version]
- Myatra, S.N.; Prabu, N.R.; Divatia, J.V.; Monnet, X.; Kulkarni, A.P.; Teboul, J.L. The Changes in Pulse Pressure Variation or Stroke Volume Variation After a “Tidal Volume Challenge” Reliably Predict Fluid Responsiveness During Low Tidal Volume Ventilation. Crit. Care Med. 2017, 45, 415–421. [Google Scholar] [CrossRef]
- Calvo-Vecino, J.M.; Ripolles-Melchor, J.; Mythen, M.G.; Casans-Francés, R.; Balik, A.; Artacho, J.P.; Martínez-Hurtado, E.; Serrano Romero, A.; Fernández Pérez, C.; Asuero de Lis, S.; et al. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: A multicentre randomised controlled trial (FEDORA trial). Br. J. Anaesth. 2018, 120, 734–744. [Google Scholar] [CrossRef]
- Bastin, A.J.; Davies, N.; Lim, E.; Quinlan, G.J.; Griffiths, M.J. Systemic inflammation and oxidative stress post-lung resection: Effect of pretreatment with N-acetylcysteine. Respirology 2016, 21, 180–187. [Google Scholar] [CrossRef]
- de la Gala, F.; Pineiro, P.; Reyes, A.; Vara, E.; Olmedilla, L.; Cruz, P.; Garutti, I. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br. J. Anaesth. 2017, 119, 655–663. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Chan, K.C.; Chien, C.T.; Sun, W.Z.; Lin, C.J. Oxidative stress during 1-lung ventilation. J. Thorac. Cardiovasc. Surg. 2006, 132, 513–518. [Google Scholar] [CrossRef]
- García-De-La-Asunción, J.; García-Del-Olmo, E.; Perez-Griera, J.; Martí, F.; Galan, G.; Morcillo, A.; Wins, R.; Guijarro, R.; Arnau, A.; Sarriá, B.; et al. Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: A study of exhaled breath condensate and blood. Eur. J. Cardiothorac. Surg. 2015, 48, e37–e44. [Google Scholar] [CrossRef]
- Bijker, J.B.; van Klei, W.A.; Kappen, T.H.; van Wolfswinkel, L.; Moons, K.G.; Kalkman, C.J. Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007, 107, 213–220. [Google Scholar] [CrossRef]
- De Backer, D.; Heenen, S.; Piagnerelli, M.; Koch, M.; Vincent, J.-L. Pulse pressure variations to predict fluid responsiveness: Influence of tidal volume. Intensive Care Med. 2005, 31, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Louart, G.; Bousquet, P.-J.; Candela, D.; Zoric, L.; de La Coussaye, J.-E.; Jaber, S.; Lefrant, J.-Y. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010, 36, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jeon, Y.; Bahk, J.-H.; Gil, N.-S.; Hong, D.M.; Kim, J.H.; Kim, H.J. Pulse pressure variation as a predictor of fluid responsiveness during one-lung ventilation for lung surgery using thoracotomy: Randomised controlled study. Eur. J. Anaesthesiol. 2011, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, K.; Okutani, R. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing one-lung ventilation. J. Cardiothorac. Vasc. Anesth. 2010, 24, 772–775. [Google Scholar] [CrossRef]
- Michard, F.; Giglio, M.T.; Brienza, N. Perioperative goal-directed therapy with uncalibrated pulse contour methods: Impact on fluid management and postoperative outcome. Br. J. Anaesth. 2017, 119, 22–30. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Eisner, M.D.; Parsons, P.; Matthay, M.A.; Ware, L.; Greene, K. Acute Respiratory Distress Syndrome N: Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003, 58, 983–988. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lu, Y.-F.; Wang, M.-L.; Chen, J.-S.; Hsu, Y.-C.; Yang, F.-S.; Cheng, Y.-J. Effects of Dexmedetomidine Infusion on Inflammatory Responses and Injury of Lung Tidal Volume Changes during One-Lung Ventilation in Thoracoscopic Surgery: A Randomized Controlled Trial. Mediat. Inflamm. 2018, 2018, 2575910. [Google Scholar] [CrossRef]
| Variable | SV Maximization (n = 30) | SV Maintenance (n = 30) | p Value |
|---|---|---|---|
| Age, yr | 57.9 ± 9 | 55 ± 8.2 | 0.086 |
| Female, n (%) | 19 (63) | 23 (77) | 1 |
| Height, cm | 160.2 ± 8.1 | 158.8 ± 23.2 | 0.412 |
| Weight, kg | 62.7 ± 11.7 | 64.4 ± 13.6 | 0.641 |
| Preoperative cardiac index, L/min/m2 | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.279 |
| Preoperative creatinine, mg/dL | 0.81 ± 0.15 | 0.76 ± 0.16 | 0.108 |
| Preoperative lactate, mg/dL | 1.16 ± 0.44 | 0.94 ± 0.34 | 0.064 |
| Preoperative pulmonary function test | |||
| FVC (%) | 113.2 ± 15.5 | 108.3 ± 9.9 | 0.176 |
| FEV1 (%) | 111.9 ± 16.5 | 107 ± 10 | 0.403 |
| History of smoking, n | 1 | 0 | 0.336 |
| Coexisting disease | |||
| Hypertension, n | 8 | 5 | 0.3 |
| Diabetes mellitus, n | 3 | 5 | 1 |
| Asthma, n | 1 | 1 | 1 |
| Variable | SV Maximization (n = 30) | SV Maintenance (n = 30) | p Value | |
|---|---|---|---|---|
| Lung separation device, n | DLT | 8 | 6 | 1 |
| Left BB | 11 | 13 | 1 | |
| Right BB | 11 | 11 | 1 | |
| Duration, min | Induction | 16.6 ± 7.8 | 15.6 ± 9.2 | 0.384 |
| Anesthesia | 175.8 ± 52.7 | 181.9 ± 71.6 | 0.935 | |
| Operation | 122.1 ± 45.3 | 119.1 ± 44.2 | 0.399 | |
| One-lung ventilation | 105 ± 43 | 104 ± 41 | 0.872 | |
| Operative method, n | 0.748 | |||
| Lobectomy | 17 | 21 | ||
| Segmentectomy | 1 | 1 | ||
| Wedge resection | 9 | 6 | ||
| Mediastinal surgery | 3 | 2 | ||
| Vasopressor | Use of ephedrine or norepinephrine, n (%) | 6 (20) | 12 (40) | 0.091 |
| Ventilation | Highest ETCO2, mmHg | 42.3 ± 4.2 | 44.4 ± 4.3 | 0.07 |
| Lowest SpO2 (%) | 95 ± 6 | 97 ± 3 | 0.145 | |
| Intravenous fluids | Voluven®, mL | 338 ± 116 | 0 | NA |
| Crystalloid rate, mL/kg/h | 4.2 ± 2.4 | 6.1 ± 2.8 | 0.005 | |
| Urine flow rate, mL/kg/h | 1.63 ± 1.25 | 1.66 ± 2.67 | 0.062 | |
| Variable | SV Maximization (n = 30) | SV Maintenance (n = 30) | Difference by Two-Way ANOVA (p Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma level of biomarkers | T1 | T2 | T3 | T1 | T2 | T3 | Time effect | Group effect | Time and group effect |
| CC-16 | 10.4 ± 5.8 | 13.5 ± 7.2 | 21.5 ± 14.9 | 9.6 ± 5.8 | 13.3 ± 7.2 | 19.9 ± 14.9 | <0.001 | 0.535 | 0.930 |
| IL-6 | 3.7 ± 7.5 | 1.7 ± 3.2 | 32.8 ± 37.7 | 6.0 ± 10.4 | 9.6 ± 16.3 | 38.3 ± 46.3 | <0.001 | <0.001 | <0.001 |
| TBARS | 11 ± 4.9 | 11.7 ± 7.9 | 15.0 ± 11.3 | 10.6 ± 9.0 | 11.5 ± 9.3 | 10.9 ± 10.0 | 0.237 | 0.133 | 0.242 |
| NGAL | 132.2 ± 70.1 | 118.3 ± 50.6 | 162.2 ± 114.8 | 142.3 ± 53.3 | 146.0 ± 56.3 | 163.0 ± 72.8 | 0.252 | 0.058 | 0.566 |
| SPD | 100.1 ± 74.8 | 91.8 ± 69.8 | 85.8 ± 63.2 | 81.7 ± 63.9 | 80.5 ± 66.6 | 76.2 ± 61.1 | 0.723 | 0.310 | 0.939 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-L.; Hsiao, P.-N.; Hsu, H.-H.; Chen, J.-S.; Cheng, Y.-J. Effects of Stroke Volume Maximization Before One-Lung Ventilation on Video-Assisted Thoracic Surgery: A Randomized Controlled Trial. Diagnostics 2025, 15, 1405. https://doi.org/10.3390/diagnostics15111405
Wang M-L, Hsiao P-N, Hsu H-H, Chen J-S, Cheng Y-J. Effects of Stroke Volume Maximization Before One-Lung Ventilation on Video-Assisted Thoracic Surgery: A Randomized Controlled Trial. Diagnostics. 2025; 15(11):1405. https://doi.org/10.3390/diagnostics15111405
Chicago/Turabian StyleWang, Man-Ling, Po-Ni Hsiao, Hsao-Hsun Hsu, Jin-Shing Chen, and Ya-Jung Cheng. 2025. "Effects of Stroke Volume Maximization Before One-Lung Ventilation on Video-Assisted Thoracic Surgery: A Randomized Controlled Trial" Diagnostics 15, no. 11: 1405. https://doi.org/10.3390/diagnostics15111405
APA StyleWang, M.-L., Hsiao, P.-N., Hsu, H.-H., Chen, J.-S., & Cheng, Y.-J. (2025). Effects of Stroke Volume Maximization Before One-Lung Ventilation on Video-Assisted Thoracic Surgery: A Randomized Controlled Trial. Diagnostics, 15(11), 1405. https://doi.org/10.3390/diagnostics15111405





