A Comprehensive Review of Cardiac Tumors: Imaging, Pathology, Treatment, and Challenges in the Third Millennium
Abstract
1. Introduction
2. Primary Benign Cardiac Tumors
3. Primary Malignant Cardiac Tumors
4. Secondary Cardiac Tumors: Cardiac Metastases
5. Clinical Presentation and Diagnosis of Cardiac Tumors
- heart failure, caused by obstruction, valve dysfunction, or direct invasion of the myocardium;
- embolic events: tumors like myxomas can fragment and cause embolism, leading to stroke, organ infarction, limb ischemia, or pulmonary obstruction (right-sided);
- arrhythmias and ECG abnormalities, due to the disruption of normal electrical conduction, especially in case of intramural masses; patients may present ventricular tachycardia, left or right ventricular hypertrophy or atrioventricular block;
- chest pain that may occur due to direct tumor invasion or associated pericardial inflammation;
- syncope, often related to obstruction of blood flow or arrhythmias;
- symptoms related to pericardial effusion.
6. Biopsy of Cardiac Tumors
7. Treatment and Prognosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| FDG-PET | Fluorodeoxyglucose Positron emission tomography |
| CNC | Carney’s complex |
| MEN | Multiple endocrine neoplasia |
| PPDAP | Primary pigmented nodular adrenocortical disease |
| LASH | Lipomatous atrial septal hypertrophy |
| TSC | Tuberous sclerosis complex |
| SEGA | Subependymal giant cell astrocytoma |
| IMT | Inflammatory myofibroblastic tumor |
| ALK | Anaplastic lymphoma kinase |
| FISH | Fluorescence in situ hybridization |
| HMCM | Hamartoma of mature cardiomyocytes |
| CAT | Calcified amorphous tumor |
| MICE | Mesothelial/monocytic incidental cardiac excrescence |
| PMCT | Primary malignant cardiac tumor |
| HPF | High-power field |
| TTE | Transthoracic echocardiography |
| TEE | Transesophageal echocardiography |
| DECT | Dual-energy CT |
| LGE | Late gadolinium enhancement |
| SUV | Standardized uptake value |
| HTOC | Hydrazinonicotinamide-3-trypsin-octreotide |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| NGS | Next-generation sequencing |
References
- Bussani, R.; Castrichini, M.; Restivo, L.; Fabris, E.; Porcari, A.; Ferro, F.; Pivetta, A.; Korcova, R.; Cappelletto, C.; Manca, P.; et al. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr. Cardiol. Rep. 2020, 22, 169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campisi, A.; Ciarrocchi, A.P.; Asadi, N.; Dell’Amore, A. Primary and secondary cardiac tumors: Clinical presentation, diagnosis, surgical treatment, and results. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 107–115. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Del Re, F.; Barzaghi, C.; Bortolotti, U.; Papi, L.; Pucci, A. Occult primary cardiac lymphomas causing unexpected/sudden death or acute heart failure. Virchows Arch. 2020, 477, 603–607. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Thoracic tumours [Internet]. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2021; Volume 5, Available online: https://tumourclassification.iarc.who.int/chapters/35 (accessed on 31 March 2025).
- Kurmann, R.; El-Am, E.; Ahmad, A.; Abbasi, M.A.; Mazur, P.; Akiki, E.; Anand, V.; Herrmann, J.; Casanegra, A.I.; Young, P.; et al. Cardiac Masses Discovered by Echocardiogram; What to Do Next? Struct. Heart. 2023, 7, 100154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corradi, D.; Moreno, P.R.; Rahouma, M.; Abascal, V.M.; Guareschi, D.; Tafuni, A.; Grazioli, V.; Palumbo, A.; Niccoli, G.; Lorusso, R. Cardiac tumors: Updated classifications and main clinico-pathologic findings. Trends Cardiovasc Med. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Murzi, B.; Pucci, A.; Aquaro, G.D.; Barison, A.; Clemente, A.; Spini, V.; Benedetti, G.; Emdin, M. What Is Hidden Behind Inferior Negative T Waves: Multiple Cardiac Glomangiomas. JACC Case Rep. 2019, 1, 657–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- La Malfa, G.; Spallarossa, P.; Tini, G.; Sarocchi, M.; Salsano, A.; Parolari, G.; Guadagno, A.; Sciallero, S.; Porto, I.; Santini, F. Cancer Patient with Unusual Dyspnea in the COVID-19 Era: Challenging Management of a Rare Cardiac Tumor. JACC Case Rep. 2021, 3, 823–828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, L.F.; Steffen, S.P.; Gaspar, S.F.D.; Gaiotto, F.A.; Bacal, F.; Jatene, F.B. Recurrent Cardiac Myxoma Treated by Heart Transplantation. Ann. Thorac. Surg. 2021, 111, e387. [Google Scholar] [CrossRef] [PubMed]
- Wingo, M.; de Biasi, A.R.; Shudo, Y.; Bharathi, V.; Blackburn, A.; Gaudino, M.; Girardi, L.N.; Woo, Y.J. Cardiac transplantation for cancer involving the heart. J. Heart Lung Transplant. 2020, 39, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Aranda-Michel, E.; Habertheuer, A.; Kilic, A.; Arnaoutakis, G.; Bianco, V.; Okusanya, O. Long-Term Outcomes of Primary Cardiac Lymphoma. Circulation 2020, 142, 2194–2195. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, J.; Liu, B.; Meng, H.; Zhao, L.; Wang, P.; Sun, J.; Wang, J.; Liu, N. Simultaneous integrated dose reduction intensity-modulated radiotherapy improves survival in patients with locally advanced non-small cell lung cancer by reducing cardiac irradiation exposure. Discov. Oncol. 2025, 16, 300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia Brás, P.; Branco, L.M.; Galrinho, A.; Timóteo, A.T.; Branco Mano, T.; Ferreira, V.; Cardoso, I.; Castelo, A.; Pinto, E.; Coelho, P.; et al. Malignant Primary and Metastatic Cardiac Tumors: A Single-Center 27-Year Case Review. Oncology 2023, 101, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Mariño-Enríquez, A.; Bovée, J.V. Molecular Pathogenesis and Diagnostic, Prognostic and Predictive Molecular Markers in Sarcoma. Surg. Pathol. Clin. 2016, 9, 457–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klener, P.; Klanova, M. Drug Resistance in Non-Hodgkin Lymphomas. Int. J. Mol. Sci. 2020, 21, 2081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pucci, A.; Gagliardotto, P.; Zanini, C.; Pansini, S.; di Summa, M.; Mollo, F. Histopathologic and clinical characterization of cardiac myxoma: Review of 53 cases from a single institution. Am. Heart J. 2000, 140, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Okongwu, C.C.; Olaofe, O.O. Cardiac myxoma: A comprehensive review. J. Cardiothorac. Surg. 2025, 20, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kacar, P.; Pavsic, N.; Bervar, M.; Strazar, Z.D.; Zadnik, V.; Jelenc, M.; Prokselj, K. Cardiac myxoma: Single tertiary centre experience. Radiol. Oncol. 2022, 56, 535–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shams, S.; Kyavar, M.; Sadeghipour, P.; Khesali, H.; Mozaffari, K.; Mahdieh, N.; Zanganehfar, M.E. Carney Complex syndrome. Cardiovasc. Pathol. 2020, 49, 107231. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.A.; Gordon, H.; Carpenter, P.C.; Shenoy, B.V.; Go, V.L. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine 1985, 64, 270–283. [Google Scholar] [CrossRef] [PubMed]
- AlRasheed, M.M. Genetics of Cardiac Tumours: A Narrative Review. Heart Lung Circ. 2024, 33, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Griborio-Guzman, A.G.; Aseyev, O.I.; Shah, H.; Sadreddini, M. Cardiac myxomas: Clinical presentation, diagnosis and management. Heart 2022, 108, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; Mattioli, C.; Matteucci, M.; Lorenzini, D.; Panvini, F.; Pacini, S.; Ippolito, C.; Celiento, M.; De Martino, A.; Dolfi, A.; et al. Cell differentiation in cardiac myxomas: Confocal microscopy and gene expression analysis after laser capture microdissection. Heart Vessels. 2018, 33, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; Bartoloni, G.; Tessitore, E.; Carney, J.A.; Papotti, M. Cytokeratin profile and neuroendocrine cells in the glandular component of cardiac myxoma. Virchows Arch. 2003, 443, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Maleszewski, J.J.; Halushka, M.K. Benign cardiac tumors and tumorlike conditions. Ann. Diagn. Pathol. 2010, 14, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Mrvic, S.; Zdravkovic, R.; Preveden, A.; Kalinic, N.; Bjeljac, I.; Milosavljevic, A.M.; Todic, M. Papillary Fibroelastomas: 16-Year Single-Center Experience. Braz. J. Cardiovasc. Surg. 2025, 40, e20230431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pucci, A.; Botta, G.; Sina, N.; Tibaldi, M.; Valori, A.; Grosso, E.; Zonta, A.; Giudici, M.; Agnoletti, G.; Bergamasco, L.; et al. Life-threatening tumors of the heart in fetal and postnatal age. J. Pediatr. 2013, 162, 964–969.e1. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Blasi, S.; Lorenzini, D.; Fornaro, M.; Basolo, F.; Bortolotti, U.; Pucci, A. Lipomatous hamartoma-like lesion of a bicuspid aortic valve: An incidental surgical finding. Cardiovasc. Pathol. 2016, 25, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Mordi, I.; Radjenovic, A.; Stanton, T.; Gardner, R.S.; McPhaden, A.; Carrick, D.; Berry, C.; Tzemos, N. Prevalence and Prognostic Significance of Lipomatous Metaplasia in Patients with Prior Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; Valori, A.; Muscio, M.; Garofalo, L.; Ferroni, F.; Abbruzzese, P.A. Asymptomatic inflammatory myofibroblastic tumor of the heart: Immunohistochemical profile, differential diagnosis, and review of the literature. Cardiovasc. Pathol. 2009, 18, 187–190. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours [Internet]. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2020; Volume 3, Available online: https://tumourclassification.iarc.who.int/chapters/33 (accessed on 31 March 2025).
- Bianchi, G.; Zancanaro, E.; Pucci, A.; Solinas, M. Hamartoma of mature cardiomyocytes presenting with atypical angina, 18F-fluorodeoxyglucose positron emission tomography uptake, and myocardial bridging: A case report. Eur. Heart J. Case Rep. 2023, 7, ytad077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pucci, A.; Bacca, A.; Barravecchia, I.; Di Stefano, I.; Belgio, B.; Lorenzini, D.; Torregrossa, L.; Chiacchio, S.; Congregati, C.; Materazzi, G.; et al. Sclerosing Paragangliomas: Correlations of Histological Features with Patients’ Genotype and Vesicular Monoamine Transporter Expression. Head. Neck Pathol. 2022, 16, 998–1011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2025; Volume 10, Available online: https://tumourclassification.iarc.who.int/chapters/53 (accessed on 31 March 2025).
- Formelli, B.; Farina, A.; Pescini, F.; Palumbo, V.; Grazia D’Alfonso, M.; Oddo, A.; Mori, F.; Poggesi, A. Cardiac Calcified Amorphous Tumor as a Rare Cause of Ischemic Stroke: Clinical Case. Circ. Cardiovasc. Imaging 2020, 13, e009623. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.L.; Lü, H.; Yin, H.L.; Wen, J.F.; Jin, O. A case of mesothelial/monocytic incidental cardiac excrescence and literature review. Diagn. Pathol. 2010, 5, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bortolotti, U.; Vendramin, I.; Lechiancole, A.; Sponga, S.; Pucci, A.; Milano, A.D.; Livi, U. Blood cysts of the cardiac valves in adults: Review and analysis of published cases. J. Card. Surg. 2021, 36, 4690–4698. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Oliva, S.; Ferraù, F. (Eds.) Manual of Cardio-Oncology: Cardiovascular Care in the Cancer Patient; Springer: Berlin/Heidelberg, Germany, 2017; pp. 339–365. [Google Scholar]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meijs, T.A.; Heidendael, J.F.; Schurink, B.; Bugiani, M.; van Boven, W.J.P.; Boekholdt, S.M.; Robbers, L.F.H.J. Constrictive Pericarditis Caused by Primary Pericardial Mesothelioma: A Case Series. Circ. Cardiovasc. Imaging 2024, 17, e016847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet]. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2024; Volume 11, Available online: https://tumourclassification.iarc.who.int/chapters/63 (accessed on 31 March 2025).
- Maleszewski, J.J.; Anavekar, N.S.; Moynihan, T.J.; Klarich, K.W. Pathology, imaging, and treatment of cardiac tumours. Nat. Rev. Cardiol. 2017, 14, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Carsote, M.; Cucu, A.P.; Stanciu, M.; Popa, F.L.; Ciuche, A.; Ciobica, M.L. Primary Cardiac Intimal Sarcoma: Multi-Layered Strategy and Core Role of MDM2 Amplification/Co-Amplification and MDM2 Immunostaining. Diagnostics 2024, 14, 919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pucci, A.; De Martino, A.; Levantino, M.; Berchiolli, R.; Basolo, F.; Bortolotti, U. Intimal Sarcoma of the Descending Aorta Mimicking Aortitis. Aorta 2016, 4, 142–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bianchi, G.; Ferrarini, M.; Matteucci, M.; Monteleone, A.; Aquaro, G.D.; Passino, C.; Pucci, A.; Glauber, M. Giant solitary fibrous tumor of the epicardium causing reversible heart failure. Ann. Thorac. Surg. 2013, 96, e49–e51. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Chang, L.; Feng, X.; Hu, W.; Yang, Z.; Zhang, Y. Primary cardiac lymphoma: A clinicopathological study of 121 cases. Front. Oncol. 2025, 14, 1509100. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Bearz, A.; Lafaras, C.; Gralec, R.; Cervesato, E.; Tomkowski, W.; DeBiasio, M.; Viel, E.; Bishiniotis, T.; Platogiannis, D.N.; et al. Neoplastic pericardial disease in lung cancer: Impact on outcomes of different treatment strategies. A multicenter study. Lung Cancer 2011, 72, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, B.; Mazine, A.; Chan, E.Y.; Barker, C.M.; Gritti, M.; Reul, R.M.; Ravi, V.; Ibarra, S.; Shapira, O.M.; Cusimano, R.J.; et al. Surgery for Tumors of the Heart. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Carabello, B.A.; Lang, R.M.; Lopez, L.; Pellikka, P.A.; Picard, M.H.; Thomas, J.D.; Varghese, P.; Wang, T.Y.; Weissman, N.J.; et al. 2019 ACC/AHA/ASE Key Data Elements and Definitions for Transthoracic Echocardiography: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Transthoracic Echocardiography) and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1161–1248. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Shimoi, T.; Kawai, A.; Yonemori, K. Diagnosis and treatment of cardiac tumors. Med. Oncol. 2025, 42, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- L’Angiocola, P.D.; Donati, R. Cardiac Masses in Echocardiography: A Pragmatic Review. J. Cardiovasc. Echogr. 2020, 30, 5–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kassop, D.; Donovan, M.S.; Cheezum, M.K.; Nguyen, B.T.; Gambill, N.B.; Blankstein, R.; Villines, T.C. Cardiac Masses on Cardiac CT: A Review. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D.; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation 2010, 122, e525–e555. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mattei, J.; Yang, E.H.; Baldassarre, L.A.; Agha, A.; Blankstein, R.; Choi, A.D.; Chen, M.Y.; Meyersohn, N.; Daly, R.; Slim, A.; et al. Cardiac computed tomographic imaging in cardio-oncology: An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). Endorsed by the International Cardio-Oncology Society (ICOS). J. Cardiovasc. Comput. Tomogr. 2023, 17, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.S.; François, C.J.; Leiner, T. Cardiac MRI: State of the Art. Radiology 2023, 307, e223008. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Bodega, F.; Bergamaschi, L.; Armillotta, M.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; Cavallo, D.; Foà, A.; et al. Multimodality Imaging in the Diagnostic Work-Up of Patients with Cardiac Masses: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 847–862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzo, A.; Viccaro, V.; Pavon, A.G.; Leo, L.A.; Treglia, G. [18F]FDG PET imaging in the differentiation of cardiac masses: An updated systematic review and dual Meta-Analysis of diagnostic performance and parameter variability. Eur. J. Nucl. Med. Mol. Imaging 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Shao, F.; Hu, F.; Song, W.; Song, Y.; Guo, J.; Lan, X. 18F-FDG PET/CT in diagnostic and prognostic evaluation of patients with cardiac masses: A retrospective study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Saponara, M.; Ambrosini, V.; Nannini, M.; Gatto, L.; Astolfi, A.; Urbini, M.; Indio, V.; Fanti, S.; Pantaleo, M.A. 18F-FDG-PET/CT imaging in cardiac tumors: Illustrative clinical cases and review of the literature. Ther. Adv. Med. Oncol. 2018, 10, 1758835918793569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Punzo, B.; Tramontano, L.; Clemente, A.; Seitun, S.; Maffei, E.; Saba, L.; Nicola De Cecco, C.; Bossone, E.; Narula, J.; Cavaliere, C.; et al. Advanced imaging of cardiac Paraganglioma: A systematic review. Int. J. Cardiol. Heart Vasc. 2024, 53, 101437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poterucha, T.J.; Kochav, J.; O’Connor, D.S.; Rosner, G.F. Cardiac Tumors: Clinical Presentation, Diagnosis, and Management. Curr. Treat. Options Oncol. 2019, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Margaryan, R.; Kallushi, E.; Cerillo, A.G.; Farneti, P.A.; Pucci, A.; Solinas, M. Outcomes of Video-assisted Minimally Invasive Cardiac Myxoma Resection. Heart Lung Circ. 2019, 28, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.L.; Yost, C.C.; Wong, D.H.; Mandel, J.L.; Prochno, K.W.; Komlo, C.M.; Ott, N.; Guy, T.S. Routine endoscopic robotic cardiac tumor resection using an 8-mm working port and percutaneous cannulation. J. Card. Surg. 2022, 37, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, A.; Abdelbar, A.; Zacharias, J. Minimally invasive resection of benign cardiac tumors. J. Thorac. Dis. 2021, 13, 1993–1999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dang, H.Q.; Le, H.T.; Dinh, L.N. Endoscopic port access resection of left atrial myxoma: Clinical outcomes and a single surgeon’s learning curve experience. JTCVS Tech. 2023, 23, 52–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aboelnazar, N.S.; Loshusan, B.R.; Chu, M.W.A. Long-Term Outcomes of Minimally Invasive Endoscopic Versus Sternotomy Surgical Resection of Primary Cardiac Tumors. Innovations 2024, 19, 550–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Liu, Z.; Li, X.; Jiang, Y.; Lu, C.; Zhang, C.; Ge, S. A comparison of total thoracoscopic versus robotic approach for cardiac myxoma resection: A single-center retrospective study. J. Robot. Surg. 2023, 17, 1393–1400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, A.; El-Am, E.A.; Mazur, P.; Akiki, E.; Sorour, A.A.; Kurmann, R.D.; Klarich, K.W.; Arghami, A.; Rowse, P.G.; Daly, R.C.; et al. A Case Series of Minimally Invasive Robotic-Assisted Resection of Cardiac Papillary Fibroelastoma: The Mayo Clinic Experience. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 143–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Martino, A.; Milano, A.D.; Bortolotti, U. Use of Pericardium for Cardiac Reconstruction Procedures in Acquired Heart Diseases-A Comprehensive Review. Thorac. Cardiovasc. Surg. 2021, 69, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A.; Reardon, M.J.; Frazier, O.H.; Angelini, P. Human cardiac explantation and autotransplantation: Application in a patient with a large cardiac pheochromocytoma. Tex. Heart Inst. J. 1985, 12, 171–176. [Google Scholar] [PubMed] [PubMed Central]
- Randhawa, J.S.; Budd, G.T.; Randhawa, M.; Ahluwalia, M.; Jia, X.; Daw, H.; Spiro, T.; Haddad, A. Primary Cardiac Sarcoma: 25-Year Cleveland Clinic Experience. Am. J. Clin. Oncol. 2016, 39, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Chahine, J.; Shekhar, S.; Mahalwar, G.; Imazio, M.; Collier, P.; Klein, A. Pericardial Involvement in Cancer. Am. J. Cardiol. 2021, 145, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Callese, T.E.; O’Brien, D.P.; Wilhalme, H.; Yang, E.H.; Moriarty, J.M. AngioVac Aspiration Thrombectomy of Right Atrial Thrombus is Safe and Effective in Cancer Patients. Ann. Vasc. Surg. 2021, 77, 243–254. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Pascarella, C.; Angelillis, M.; Picoi, M.E.; Scioti, G.; Bortolotti, U. Novel use of the AngioVac system. Indian J. Thorac. Cardiovasc. Surg. 2019, 35, 208–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, E.Y.; Ali, A.; Umana, J.P.; Nguyen, D.T.; Hamilton, D.J.; Graviss, E.A.; Ravi, V.; MacGillivray, T.E.; Reardon, M.J. Management of primary cardiac paraganglioma. J. Thorac. Cardiovasc. Surg. 2022, 164, 158–166.e1. [Google Scholar] [CrossRef] [PubMed]
- Mulliken, J.B.; Glowacki, J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Yang, W.; Zhou, M.; Zhu, Q.; Jiang, Z. Atrial Hemangioma: A Case Report and Review of the Literature. Ann. Thorac. Cardiovasc. Surg. 2019, 25, 71–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pozzi, M.; Deux, J.F.; Kirsch, M. Conservative management of left ventricle cardiac fibroma in an adult asymptomatic patient. Int. J. Cardiol. 2012, 161, e61–e62. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lei, C.; Hsi, D.H.; Zheng, M.; Ma, H.; Ta, S.; Hu, R.; Han, C.; Li, W.; Li, J.; et al. Echocardiography-Guided Radiofrequency Ablation for Cardiac Tumors. JACC CardioOncol. 2024, 6, 560–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, R.; Sunthankar, K.I.; Yasinzai, A.Q.K.; Tareen, B.; Zarak, M.S.; Khan, J.; Nasir, H.; Nakasaki, M.; Jahangir, E.; Heneidi, S.; et al. Primary cardiac sarcoma: Demographics, genomic study correlation, and survival benefits of surgery with adjuvant therapy in U.S. population. Clin. Res. Cardiol. 2024, 113, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.; Ali, A.; Zubair, M.M.; Nguyen, D.T.; Ibarra-Cortez, S.H.; Graviss, E.A.; Shapira, O.M.; Ravi, V.; MacGillivray, T.E.; Reardon, M.J. Primary cardiac sarcomas: Treatment strategies. J. Thorac. Cardiovasc. Surg. 2023, 166, 828–838.e2. [Google Scholar] [CrossRef] [PubMed]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.; El-Am, E.; Denu, R.; Abou Alaiwi, S.; El Zarif, T.; Macaron, W.; Abdel-Wahab, N.; Desai, A.; Smith, C.; Parikh, K.; et al. Clinical Outcomes Among Immunotherapy-Treated Patients with Primary Cardiac Soft Tissue Sarcomas: A Multicenter Retrospective Study. JACC CardioOncol. 2024, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Electronic address: Clinicalguidelines@esmo.org. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Urbini, M.; Astolfi, A.; Indio, V.; Nannini, M.; Pizzi, C.; Paolisso, P.; Tarantino, G.; Pantaleo, M.A.; Saponara, M. Genetic aberrations and molecular biology of cardiac sarcoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920918492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houmsse, M.; Muskara, A.; Pasca, D.; Roy, A.; Sughra, S.; Ghazi, S.; Addison, D.; Husain, M. Characterizing Cardiotoxicity of FDA-Approved Soft Tissue Sarcoma Targeted Therapies and Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2025, 17, 827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahouma, M.; Arisha, M.J.; Elmously, A.; El-Sayed Ahmed, M.M.; Spadaccio, C.; Mehta, K.; Baudo, M.; Kamel, M.; Mansor, E.; Ruan, Y.; et al. Cardiac tumors prevalence and mortality: A systematic review and meta-analysis. Int. J. Surg. 2020, 76, 178–189. [Google Scholar] [CrossRef] [PubMed]

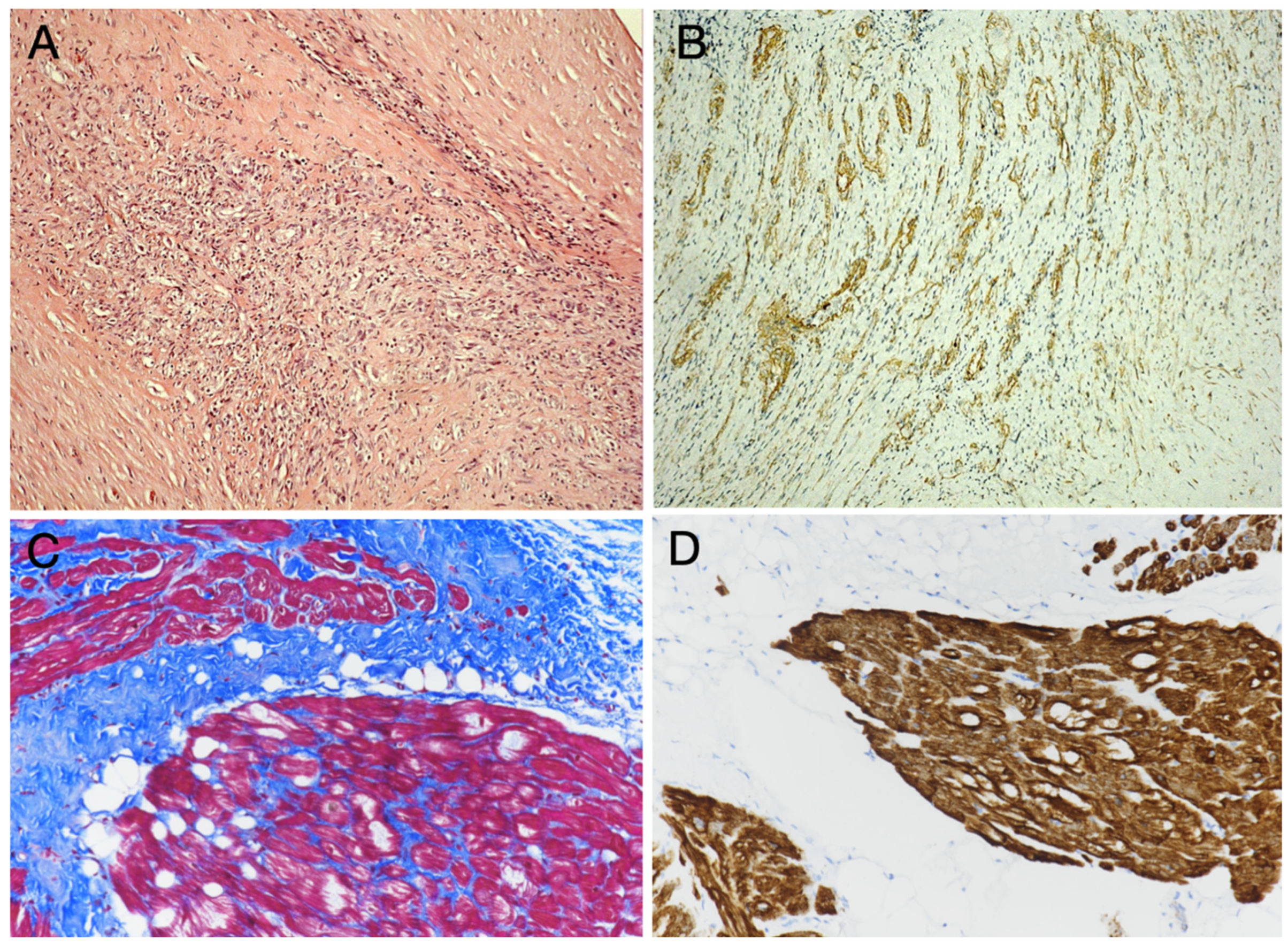
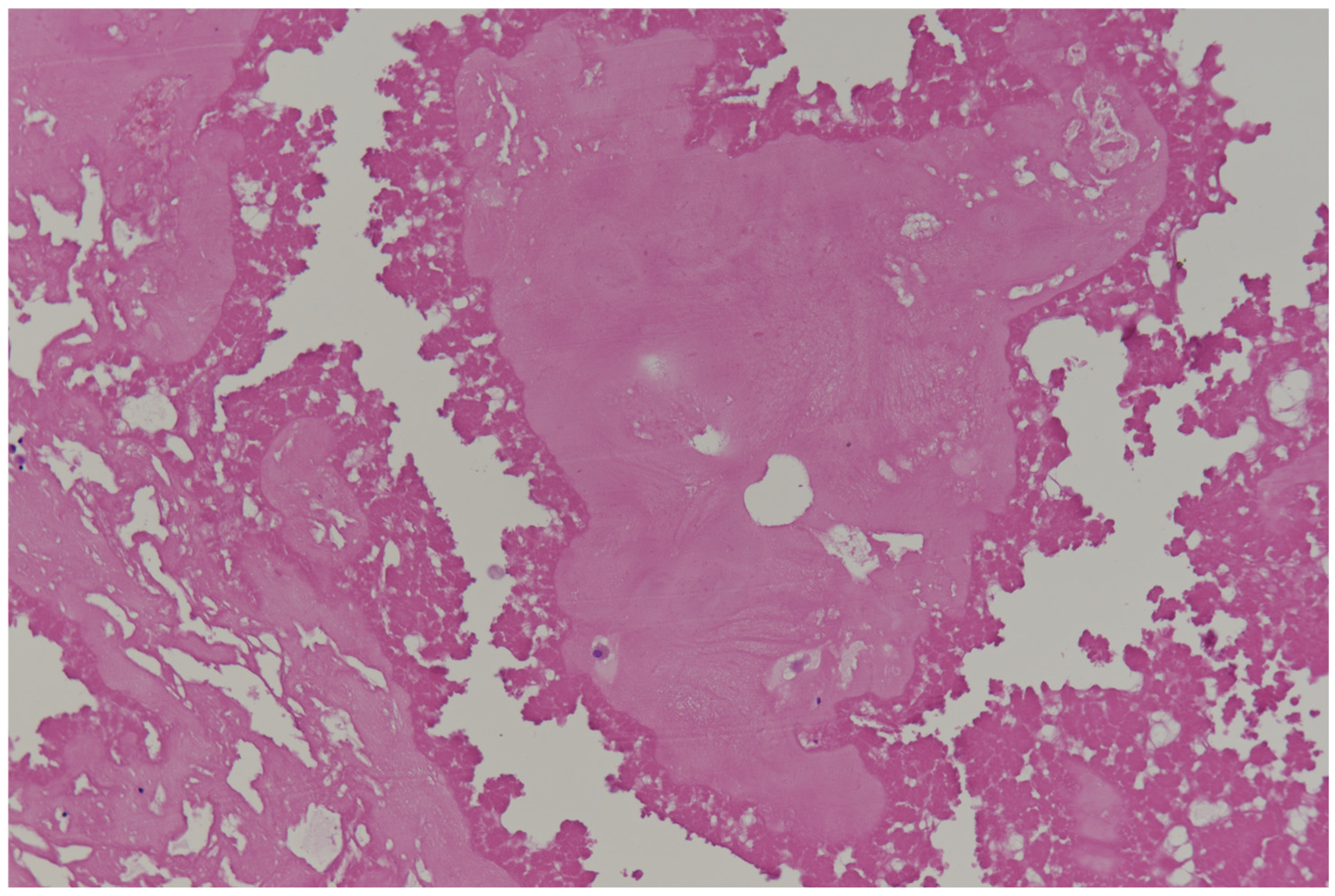


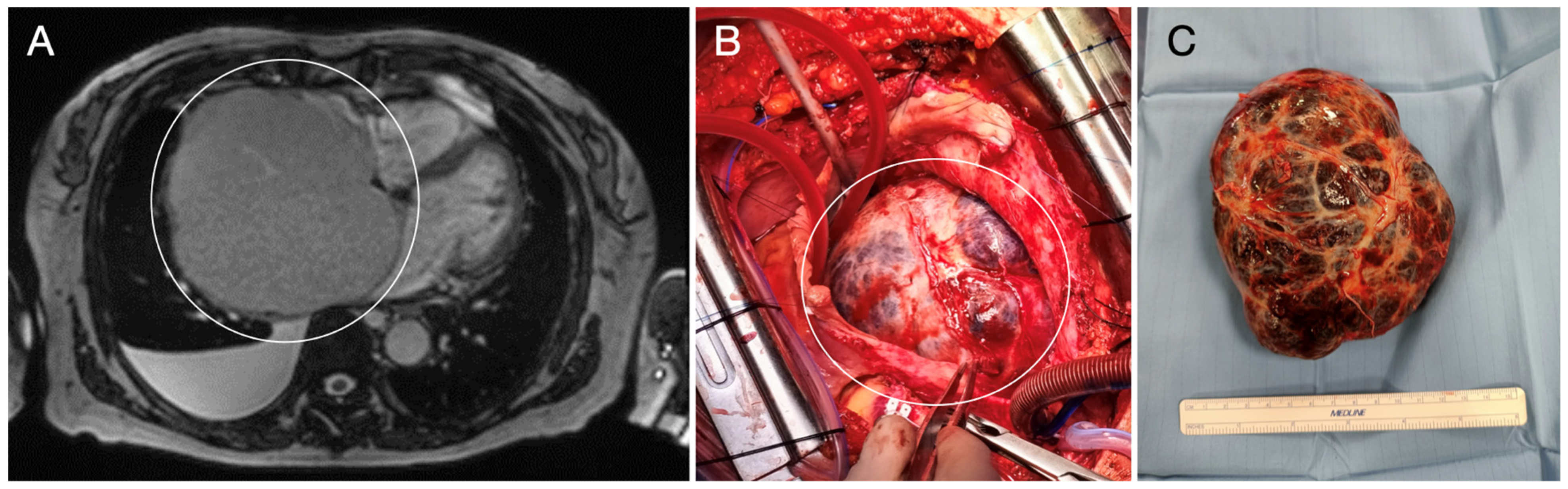

| Benign tumors and tumor-like lesions Papillary fibroelastoma Cardiac myxoma Cardiac fibroma Cardiac rhabdomyoma Adult cellular rhabdomyoma Cardiac lipoma and lipomatous hypertrophy of the atrial septum Lipomatous hamartoma of the atrioventricular valve Hamartoma of mature cardiac myocytes Mesenchymal cardiac hamartoma Cardiac hemangiomas Conduction system hamartoma Cystic tumor of the atrioventricular node | Malignant tumors Cardiac angiosarcoma Cardiac leiomyosarcoma Cardiac undifferentiated pleomorphic sarcoma Other sarcomas that may involve the heart Hematolymphoid tumors Cardiac diffuse large B-cell lymphoma Cardiac fibrin-associated diffuse large B-cell lymphoma |
| Tumor Type | Age | Location | Syndromic Associations | Therapy/Prognosis |
|---|---|---|---|---|
| Cardiac myxoma | Adult | Atria | Carney complex | Surgical excision/good |
| Papillary fibroelastoma | Adult | Valves | – | Surgical excision/good |
| Hemangioma | Adult | Ventricles | – | Surgical excision/good |
| Fibroma | Infant | Ventricles | Gorlin syndrome | Imaging monitoring/good |
| Lipoma | Adult | Pericardium | Tuberous sclerosis | Imaging monitoring/good |
| Lipomatous hypertrophy of the atrial septum | Adult | Atria | – | Imaging monitoring/good |
| Rhabdomyoma | Infant | Ventricles | Tuberous sclerosis | Spontaneous regression/good |
| Inflammatory myofibroblastic tumor | Infant | Valves | – | Imaging monitoring ± surgical excision/variable |
| Paraganglioma | Adult | Atria | – | Surgical excision/variable |
| Hamartoma of mature cardiac myocytes | Adult | Ventricles | – | Imaging monitoring ± surgical excision/good |
| Glomus tumor | Adult | Ventricles/Pericardium | – | Surgical excision/good |
| Angiosarcoma | Adult | Atria | Li–Fraumeni syndrome | Surgical excision ± Chemotherapy/recurrence |
| Rhabdomyosarcoma | Surgical excision ± Chemotherapy/recurrence | |||
| Intimal sarcoma | Surgical excision ± Chemotherapy/recurrence | |||
| Lymphoma | Adult | Pericardium | – | Chemotherapy/variable |
| Metastasis | Adult | Ventricles | – | Chemotherapy/poor |
| Feature/Advantage | Echocardiography | MRI | CT |
|---|---|---|---|
| Calcium | +++ | + | ++++ |
| Extracardiac extension | + | +++ | ++++ |
| Fat | ++ | ++++ | +++ |
| Mobility | ++++ | ++ | ++ |
| Thrombus | ++ | ++++ | +++ |
| Coronary characterization | − | ++ | ++++ |
| Availability | ++++ | ++ | +++ |
| Children | ++++ | ++ | + |
| Radiation | − | − | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Martino, A.; Pattuzzi, C.; Garis, S.; Bosco, F.; Virgone, V.M.; Salsano, A.; Santini, F.; Pucci, A. A Comprehensive Review of Cardiac Tumors: Imaging, Pathology, Treatment, and Challenges in the Third Millennium. Diagnostics 2025, 15, 1390. https://doi.org/10.3390/diagnostics15111390
De Martino A, Pattuzzi C, Garis S, Bosco F, Virgone VM, Salsano A, Santini F, Pucci A. A Comprehensive Review of Cardiac Tumors: Imaging, Pathology, Treatment, and Challenges in the Third Millennium. Diagnostics. 2025; 15(11):1390. https://doi.org/10.3390/diagnostics15111390
Chicago/Turabian StyleDe Martino, Andrea, Claudia Pattuzzi, Sara Garis, Francesca Bosco, Vittorio Maria Virgone, Antonio Salsano, Francesco Santini, and Angela Pucci. 2025. "A Comprehensive Review of Cardiac Tumors: Imaging, Pathology, Treatment, and Challenges in the Third Millennium" Diagnostics 15, no. 11: 1390. https://doi.org/10.3390/diagnostics15111390
APA StyleDe Martino, A., Pattuzzi, C., Garis, S., Bosco, F., Virgone, V. M., Salsano, A., Santini, F., & Pucci, A. (2025). A Comprehensive Review of Cardiac Tumors: Imaging, Pathology, Treatment, and Challenges in the Third Millennium. Diagnostics, 15(11), 1390. https://doi.org/10.3390/diagnostics15111390






