Predicting Early Outcomes of Prostatic Artery Embolization Using n-Butyl Cyanoacrylate Liquid Embolic Agent: A Machine Learning Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Data Preprocessing
2.4. Model Development and Assessment
3. Results
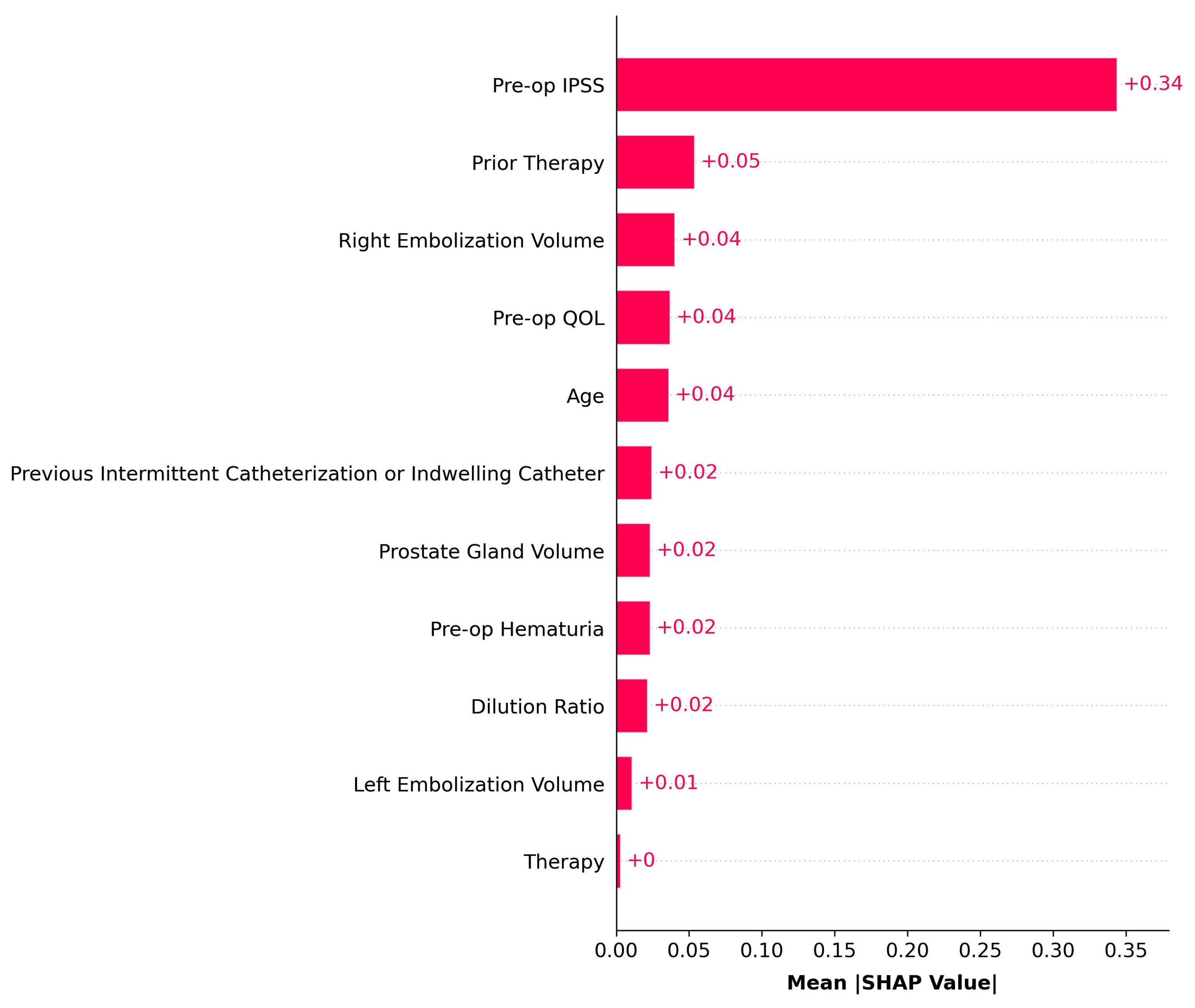
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAE | Prostatic artery embolization |
| nBCA | n-butyl cyanoacrylate |
| IPSS | International Prostate Symptom Score |
| QoL | Quality of life |
| ML | Machine learning |
| AI | Artificial intelligence |
| PFN | Prior-data fitted network |
| Qmax | Maximum flow rate |
| Qavg | Average flow rate |
| PGV | Prostate gland volume |
| PVR | Post-void residual |
| kNN | K-nearest neighbor |
| ROC | Receiver operating characteristic |
| PRC | Precision–recall curve |
| AUROC | Area under the ROC curve |
| AUPRC | Area under the PRC |
| MCC | Matthews Correlation Coefficient |
| CI | Confidence interval |
| SHAP | SHapley Additive ExPlanations |
| IQR | Interquartile range |
| SD | Standard deviation |
References
- Checcucci, E.; Veccia, A.; De Cillis, S.; Piramide, F.; Volpi, G.; Amparore, D.; Pecoraro, A.; Piana, A.; Granato, S.; Verri, P.; et al. New Ultra-minimally Invasive Surgical Treatment for Benign Prostatic Hyperplasia: A Systematic Review and Analysis of Comparative Outcomes. Eur. Urol. Open Sci. 2021, 33, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, G.; Doshi, N.; Maclean, D.; Bryant, T.; Harris, M.; Hacking, N.; Farrahi, K.; Niranjan, M.; Modi, S. Machine Learning to Predict Prostate Artery Embolization Outcomes. Cardiovasc. Interv. Radiol. 2024, 47, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, F.C.; Moreira, A.M.; de Assis, A.M.; Antunes, A.A.; Cristina de Paula Rodrigues, V.; Srougi, M.; Cerri, G.G. Prostatic Artery Embolization for the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: 10 Years’ Experience. Radiology 2020, 296, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-W.; Zhou, C.-G.; Tian, W.; Shi, H.-B.; Liu, S. Long-Term Efficacy and Recurrence Prediction of Prostatic Artery Embolization for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Cardiovasc. Interv. Radiol. 2022, 45, 1801–1809. [Google Scholar] [CrossRef]
- Bilhim, T.; Costa, N.V.; Torres, D.; Pinheiro, L.C.; Spaepen, E. Long-Term Outcome of Prostatic Artery Embolization for Patients with Benign Prostatic Hyperplasia: Single-Centre Retrospective Study in 1072 Patients Over a 10-Year Period. Cardiovasc. Interv. Radiol. 2022, 45, 1324–1336. [Google Scholar] [CrossRef]
- Loffroy, R.; Guillen, K.; Salet, E.; Marcelin, C.; Comby, P.-O.; Midulla, M.; Grenier, N.; Chevallier, O.; Petitpierre, F. Prostate Artery Embolization Using N-Butyl Cyanoacrylate Glue for Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: A Valid Alternative to Microparticles? J. Clin. Med. 2021, 10, 3161. [Google Scholar] [CrossRef]
- Loffroy, R.; Quirantes, A.; Guillen, K.; Mazit, A.; Comby, P.-O.; Aho-Glélé, L.S.; Chevallier, O. Prostate artery embolization using n-butyl cyanoacrylate glue for symptomatic benign prostatic hyperplasia: A six-month outcome analysis in 103 patients. Diagn. Interv. Imaging 2024, 105, 129–136. [Google Scholar] [CrossRef]
- Bamshad, D.; Sanghvi, J.; Galla, N.; Geffner, A.; Menon, K.; Bishay, V.; Shilo, D.; Garcia-Reyes, K.; Lookstein, R.; Rastinehad, A.; et al. Early Outcomes of Prostatic Artery Embolization using n-Butyl Cyanoacrylate Liquid Embolic Agent: A Safety and Feasibility Study. J. Vasc. Interv. Radiol. 2024, 35, 1855–1861. [Google Scholar] [CrossRef]
- Boeken, T. Commentary on Machine Learning to Predict Prostate Artery Embolization Outcomes? Patient Selection for Prostatic Artery Embolization: Why it Matters. Cardiovasc. Interv. Radiol. 2024, 47, 1255–1256. [Google Scholar] [CrossRef]
- Rudin, C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Karabacak, M.; Ozkara, B.B.; Faizy, T.D.; Hardigan, T.; Heit, J.J.; Lakhani, D.A.; Margetis, K.; Mocco, J.; Nael, K.; Wintermark, M.; et al. Data-Driven Prognostication in Distal Medium Vessel Occlusions Using Explainable Machine Learning. AJNR Am. J. Neuroradiol. 2025, 46, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, G.; Maclean, D.; Doshi, N.; Harris, M.; Bryant, T.J.C.; Hacking, N.C.; Somani, B.; Modi, S. Cardiovascular Comorbidities Do Not Impact Prostate Artery Embolisation (PAE) Outcomes: Retrospective Analysis of the National UK-ROPE Registry. Cardiovasc. Interv. Radiol. 2024, 47, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Fu, H.; Tapping, C.R. Prostate volume: Does it predict patient outcomes following prostate artery embolisation? A retrospective cohort study. CVIR Endovasc. 2024, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Bilhim, T.; Pisco, J.; Pereira, J.A.; Costa, N.V.; Fernandes, L.; Campos Pinheiro, L.; Duarte, M.; Oliveira, A.G. Predictors of Clinical Outcome after Prostate Artery Embolization with Spherical and Nonspherical Polyvinyl Alcohol Particles in Patients with Benign Prostatic Hyperplasia. Radiology 2016, 281, 289–300. [Google Scholar] [CrossRef]
- Martin, A.; Marcelin, C.; Petitpierre, F.; Jambon, E.; Maaloum, R.; Grenier, N.; Le Bras, Y.; Crombé, A. Clinical, Technical, and MRI Features Associated with Patients’ Outcome at 3 Months and 2 Years following Prostate Artery Embolization: Is There an Added Value of Radiomics? J. Pers. Med. 2024, 14, 67. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models That Use Regression or Machine Learning Methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- AUA PRACTICE GUIDELINES COMMITTEE. AUA Guideline on Management of Benign Prostatic Hyperplasia (2003). Chapter 1: Diagnosis and Treatment Recommendations. J. Urol. 2003, 170, 530–547. [Google Scholar] [CrossRef]
- Beretta, L.; Santaniello, A. Nearest neighbor imputation algorithms: A critical evaluation. BMC Med. Inform. Decis. Mak. 2016, 16, 74. [Google Scholar] [CrossRef]
- Hollmann, N.; Müller, S.; Eggensperger, K.; Hutter, F. TabPFN: A Transformer That Solves Small Tabular Classification Problems in a Second. arXiv 2022, arXiv:2207.01848. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Vela-Navarrete, R.; Alfaro, V.; Badiella, L.L.; Fernández-Hernando, N. Age-stratified analysis of I-PSS and QoL values in spanish patients with symptoms potentially related to BPH. Eur. Urol. 2000, 38, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, M.; Duan, F.; Yuan, K.; Li, K.; Yan, J.; Chang, Z.; Wang, Y. Radiological Findings of Prostatic Arterial Anatomy for Prostatic Arterial Embolization: Preliminary Study in 55 Chinese Patients with Benign Prostatic Hyperplasia. PLoS ONE 2015, 10, e0132678. [Google Scholar] [CrossRef] [PubMed]
- Uflacker, A.B.; Haskal, Z.J.; Baerlocher, M.O.; Bhatia, S.S.; Carnevale, F.C.; Dariushnia, S.R.; Faintuch, S.; Gaba, R.C.; Golzarian, J.; Midia, M.; et al. Society of Interventional Radiology Research Reporting Standards for Prostatic Artery Embolization. J. Vasc. Interv. Radiol. 2020, 31, 891–898.e1. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.M.; Adams, W.G.; Bernstam, E.V.; Bickel, J.P.; Fox, K.P.; Marsolo, K.; Raghavan, V.A.; Turchin, A.; Zhou, X.; Murphy, S.N.; et al. Biases introduced by filtering electronic health records for patients with “complete data”. J. Am. Med. Inform. Assoc. 2017, 24, 1134–1141. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, W.; Liang, C.; Pang, L.; Zou, Z. Tokenize features, enhancing tables: The FT-TABPFN model for tabular classification. arXiv 2024, arXiv:2406.06891. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Bixler, B.R.; Dahm, P.; Goueli, R.; Kirkby, E.; Stoffel, J.T.; Wilt, T.J. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia (BPH): AUA Guideline Amendment 2023. J. Urol. 2024, 211, 11–19. [Google Scholar] [CrossRef]
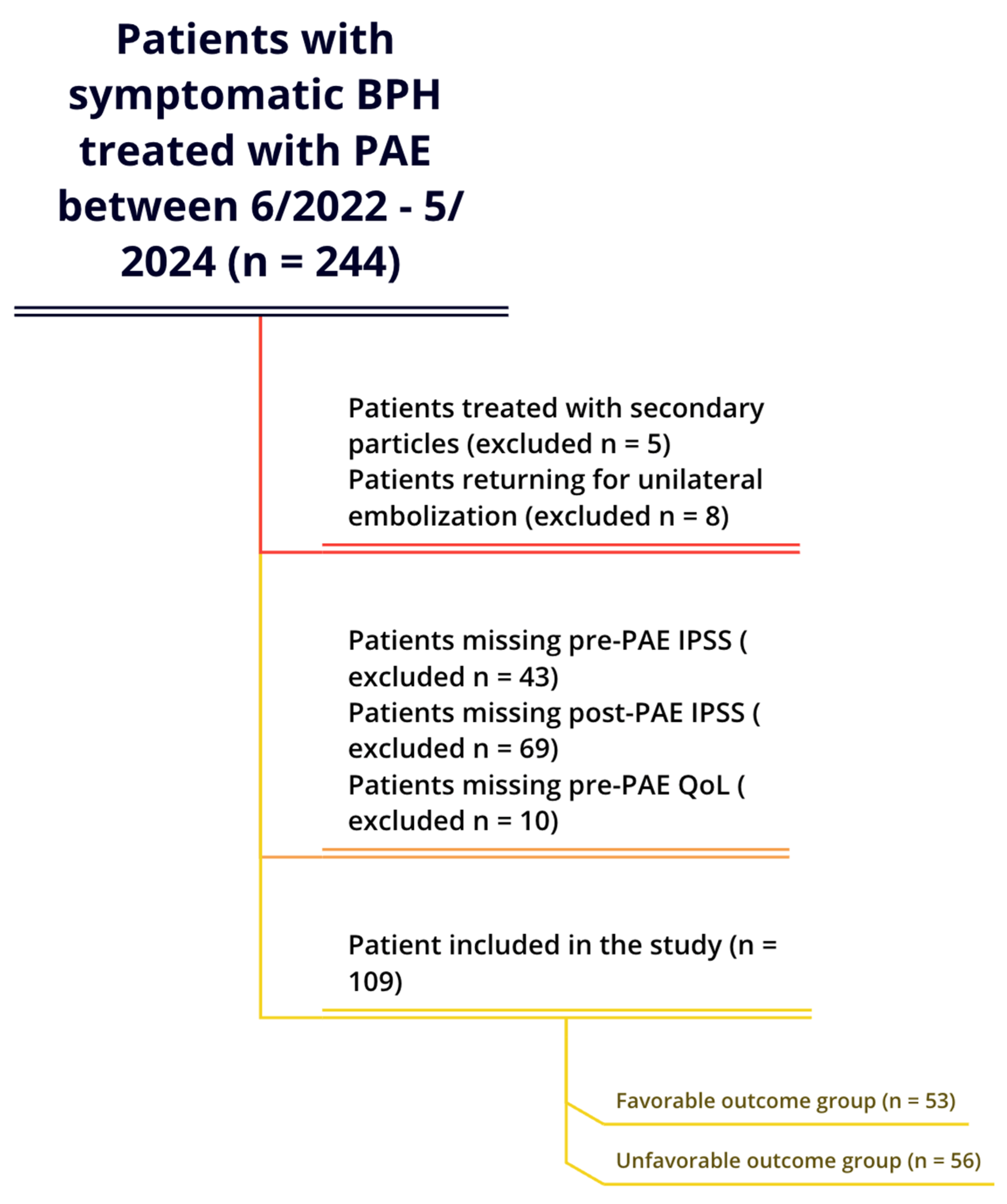
| Variables | Unfavorable Outcome Group (n = 56) | Favorable Outcome Group (n = 53) | Combined (n = 109) | |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Age | Median (IQR), n | 73.5 (13.5), 56 | 70 (12.5), 53 | 72 (13), 109 |
| Mean ± SD, n | 73.7 ± 9.7, 56 | 70 ± 8, 53 | 71.9 ± 9.1, 109 | |
| Pre-PAE IPSS | Median (IQR), n | 16 (7), 56 | 25 (8.5), 53 | 20 (9.5), 109 |
| Mean ± SD, n | 16.1 ± 5, 56 | 24.5 ± 5.2, 53 | 20.2 ± 6.6, 109 | |
| Pre-PAE QoL | Median (IQR), n | 4 (1), 56 | 5 (1.5), 53 | 4 (2), 109 |
| Mean ± SD, n | 3.6 ± 1.2, 56 | 4.2 ± 1.1, 53 | 3.9 ± 1.2, 109 | |
| Pre-PAE Qmax | Median (IQR), n | 7 (5.5), 33 | 6.9 (4.4), 29 | 7 (4), 62 |
| Mean ± SD, n | 8.7 ± 6.9, 33 | 12.7 ± 33.5, 29 | 10.6 ± 23.3, 62 | |
| Pre-PAE Qavg | Median (IQR), n | 4.05 (4), 6 | 4.5 (1.55), 12 | 4.4 (1.9), 18 |
| Mean ± SD, n | 5 ± 2, 6 | 4.5 ± 1.8, 12 | 4.6 ± 1.8, 18 | |
| Pre-PAE PGV | Median (IQR), n | 86.5 (94), 38 | 135 (106.85), 40 | 109 (117.6), 78 |
| Mean ± SD, n | 118.9 ± 85.6, 38 | 143.8 ± 82.1, 40 | 131.7 ± 84.2, 78 | |
| Pre-PAE PVR | Median (IQR), n | 133.2 (181.5), 20 | 100 (257.5), 21 | 120 (235.5), 41 |
| Mean ± SD, n | 217 ± 266, 20 | 167.5 ± 166.7, 21 | 191.6 ± 219.4, 41 | |
| Pre-PAE Hematuria | Yes | 20 (35.7%) | 21 (39.6%) | 41 (37.6%) |
| No | 27 (48.2%) | 29 (54.7%) | 56 (51.4%) | |
| N/A | 9 (16.1%) | 3 (5.7%) | 12 (11.0%) | |
| Prior Therapy | Yes | 14 (25%) | 4 (7.5%) | 18 (16.5%) |
| No | 42 (75%) | 49 (92.5%) | 91 (83.5%) | |
| N/A | 0 (0%) | 0 (0%) | 0 (0%) | |
| Prior Catheterization | Yes | 10 (17.9%) | 11 (20.8%) | 21 (19.3%) |
| No | 45 (80.3%) | 42 (79.2%) | 87 (79.8%) | |
| N/A | 1 (1.8%) | 0 (0%) | 1 (0.9%) | |
| Procedural Characteristics | ||||
| Left Embolization Volume | Median (IQR), n | 1 (0.3), 49 | 1 (0.4), 49 | 1 (0.3), 98 |
| Mean ± SD, n | 0.91 ± 0.34, 49 | 0.90 ± 0.37, 49 | 0.90 ± 0.35, 98 | |
| Right Embolization Volume | Median (IQR), n | 1 (0.3), 49 | 1 (0.4), 48 | 1 (0.3), 97 |
| Mean ± SD, n | 0.98 ± 0.47, 49 | 0.91 ± 0.4, 48 | 0.95 ± 0.43, 97 | |
| Dilution Ratio | Median (IQR), n | 0.1 (0), 54 | 0.1 (0), 52 | 0.1 (0), 106 |
| Mean ± SD, n | 0.098 ± 0.006, 54 | 0.097 ± 0.007, 52 | 0.098 ± 0.007, 106 | |
| Treatment Approach | BGE | 52 (92.9%) | 52 (98.1%) | 104 (95.4%) |
| UGE with CCE | 4 (7.1%) | 1 (1.9%) | 5 (4.6%) | |
| Outcome | ||||
| IPSS Reduction | Median (IQR), n | 4.5 (5), 56 | 16.5 (7), 53 | 9 (11.8), 109 |
| Mean ± SD, n | 3.7 ± 4.6, 56 | 17.1 ± 5, 53 | 10.2 ± 8.2, 109 | |
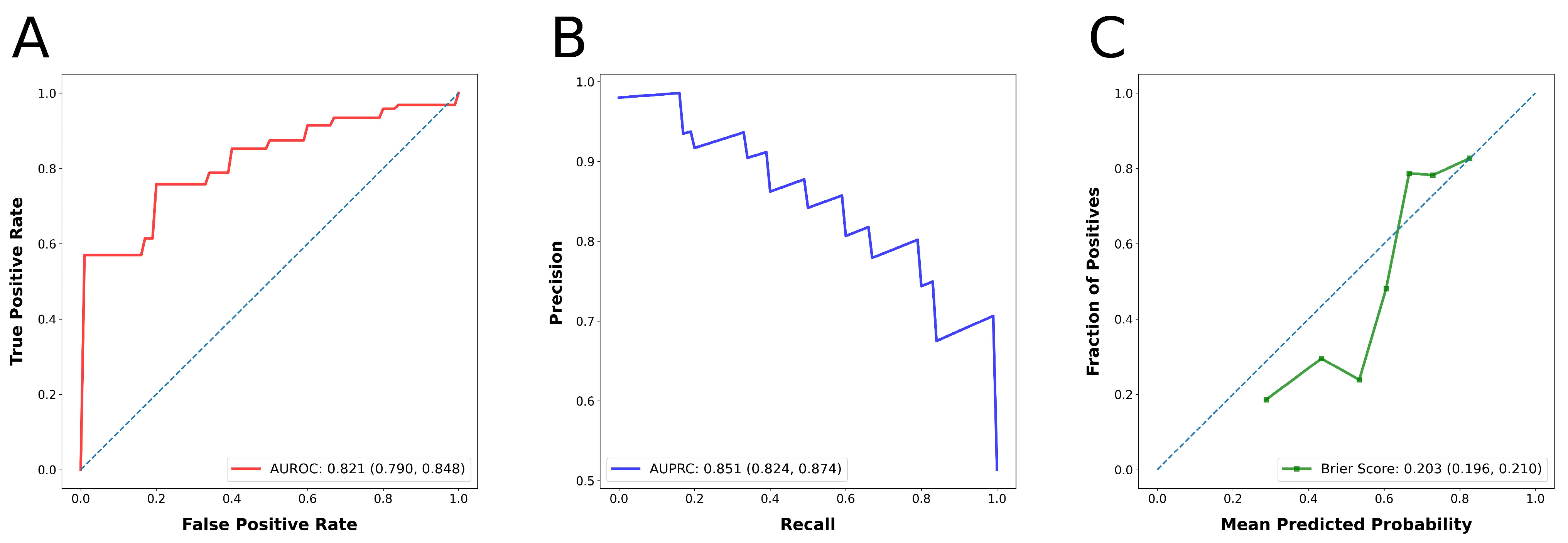
| Performance Metric | Metric Value (95% Confidence Interval) |
|---|---|
| Precision | 0.666 (0.640, 0.698) |
| Recall | 0.856 (0.825, 0.885) |
| F1 Score | 0.731 (0.709, 0.752) |
| Accuracy | 0.676 (0.647, 0.705) |
| Matthews Correlation Coefficient | 0.363 (0.302, 0.423) |
| AUROC | 0.821 (0.790, 0.848) |
| AUPRC | 0.851 (0.824, 0.874) |
| Brier Score | 0.203 (0.196, 0.210) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkara, B.B.; Bamshad, D.; Gowda, R.; Karabacak, M.; Bishay, V.; Garcia-Reyes, K.; Rastinehad, A.R.; Shilo, D.; Fischman, A. Predicting Early Outcomes of Prostatic Artery Embolization Using n-Butyl Cyanoacrylate Liquid Embolic Agent: A Machine Learning Study. Diagnostics 2025, 15, 1351. https://doi.org/10.3390/diagnostics15111351
Ozkara BB, Bamshad D, Gowda R, Karabacak M, Bishay V, Garcia-Reyes K, Rastinehad AR, Shilo D, Fischman A. Predicting Early Outcomes of Prostatic Artery Embolization Using n-Butyl Cyanoacrylate Liquid Embolic Agent: A Machine Learning Study. Diagnostics. 2025; 15(11):1351. https://doi.org/10.3390/diagnostics15111351
Chicago/Turabian StyleOzkara, Burak Berksu, David Bamshad, Ramita Gowda, Mert Karabacak, Vivian Bishay, Kirema Garcia-Reyes, Ardeshir R. Rastinehad, Dan Shilo, and Aaron Fischman. 2025. "Predicting Early Outcomes of Prostatic Artery Embolization Using n-Butyl Cyanoacrylate Liquid Embolic Agent: A Machine Learning Study" Diagnostics 15, no. 11: 1351. https://doi.org/10.3390/diagnostics15111351
APA StyleOzkara, B. B., Bamshad, D., Gowda, R., Karabacak, M., Bishay, V., Garcia-Reyes, K., Rastinehad, A. R., Shilo, D., & Fischman, A. (2025). Predicting Early Outcomes of Prostatic Artery Embolization Using n-Butyl Cyanoacrylate Liquid Embolic Agent: A Machine Learning Study. Diagnostics, 15(11), 1351. https://doi.org/10.3390/diagnostics15111351






