Exercise Hemodynamics and Sex-Specific Data in Asymptomatic Adults: An Exploratory Pilot Study
Abstract
1. Introduction
2. Methods
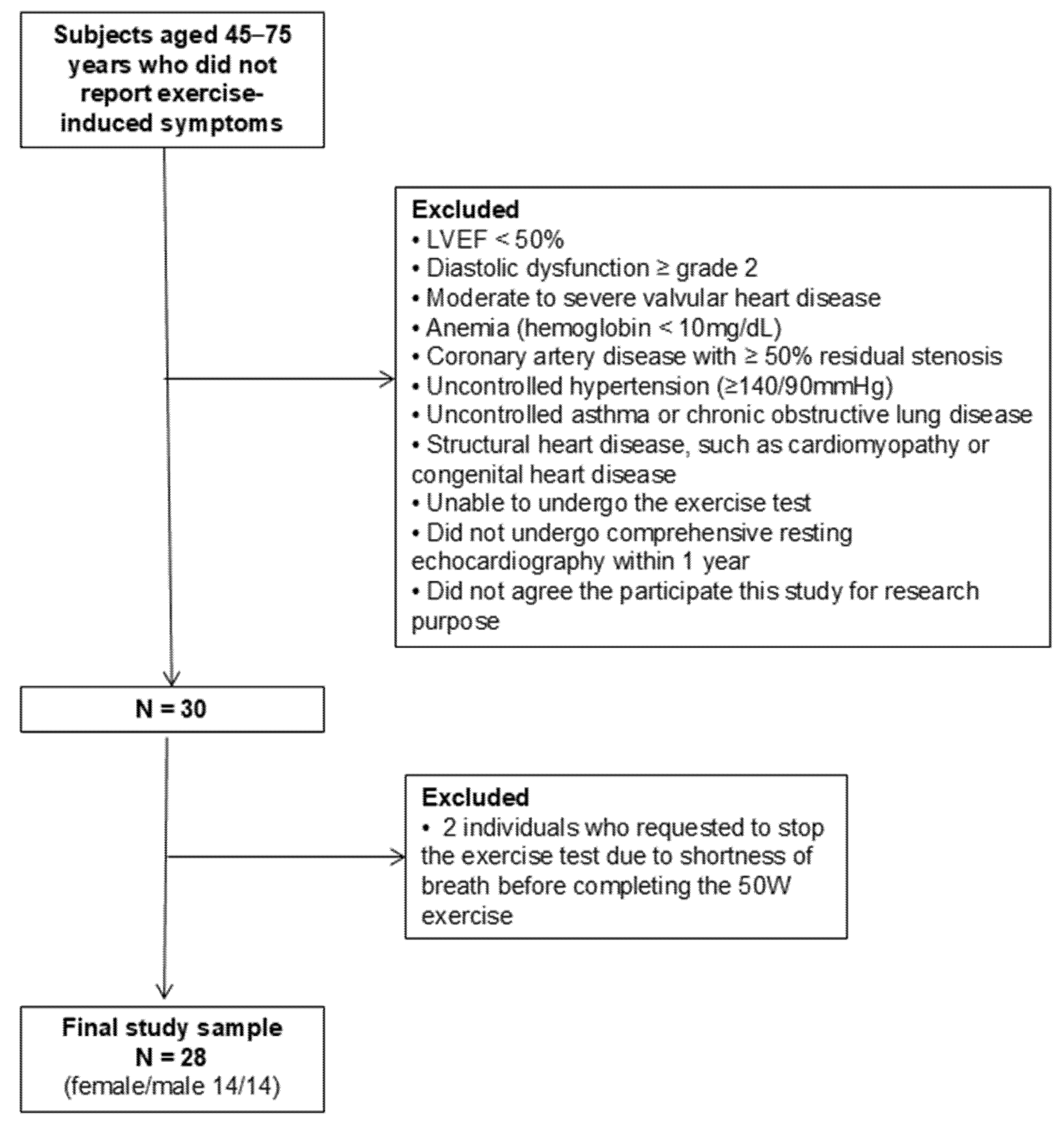
2.1. Study Design and Study Population
2.2. Bicycle Exercise Echocardiography
2.3. Doppler Echocardiographic Measurements During Resting and Exercise
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Exercise-Induced Hemodynamic Changes
3.3. Exercise-Induced Hemodynamic Changes Based on Sex
3.4. Reproducibility of the Exercise Echocardiographic Parameters
4. Discussion
4.1. Normal Exercise Hemodynamic Profiles in Asymptomatic Older Adults
4.2. Sex-Specific Data During Exercise
4.3. Clinical Implications
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFpEF | Heart failure with preserved ejection fraction |
| ICC | Intraclass correlation coefficient |
| LA | Left atrial |
| LV | Left ventricular |
| LVEF | Left ventricular ejection fraction |
| LVFP | Left ventricular filling pressure |
| PA | Pulmonary artery |
| rpm | Rotations per minute |
| RV | Right ventricle |
| SPAP | Systolic pulmonary artery pressure |
| TAPSE | Tricuspid annular plane systolic excursion |
References
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Xanthakis, V.; Lyass, A.; Andersson, C.; Tsao, C.; Cheng, S.; Aragam, J.; Benjamin, E.J.; Larson, M.G. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study: An Echocardiographic Study Over 3 Decades. JACC Cardiovasc. Imaging 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Shin, M.S. Obesity-related heart failure with preserved ejection fraction: Diagnostic and therapeutic challenges. Korean J. Intern. Med. 2023, 38, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, S.Y.; Jung, M.H.; Youn, J.C.; Kim, D.; Cho, J.Y.; Cho, D.H.; Hyun, J.; Cho, H.J.; Park, S.M.; et al. Korean Society of Heart Failure Guidelines for the Management of Heart Failure: Management of the Underlying Etiologies and Comorbidities of Heart Failure. Int. J. Heart Fail. 2023, 5, 127–145. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Oh, J.K.; Kane, G.C. Diastolic stress echocardiography: The time has come for its integration into clinical practice. J. Am. Soc. Echocardiogr. 2014, 27, 1060–1063. [Google Scholar] [CrossRef]
- Ha, J.W.; Andersen, O.S.; Smiseth, O.A. Diastolic Stress Test: Invasive and Noninvasive Testing. JACC Cardiovasc. Imaging 2020, 13 Pt 2, 272–282. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, H.O.; Kim, M.J.; Lee, J.W.; Youn, H.J. Impact of prolapsing leaflet location on exercise pulmonary hypertension in mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 576–583. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, H.O.; Lee, J.W.; Youn, H.J. Decreases in left atrial compliance during early-stage exercise are related to exercise intolerance in asymptomatic significant mitral stenosis. Echocardiography 2017, 34, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Lulic, F.; Bailey, K.R.; Pellikka, P.A.; Seward, J.B.; Tajik, A.J.; Oh, J.K. Effects of treadmill exercise on mitral inflow and annular velocities in healthy adults. Am. J. Cardiol. 2003, 91, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Choi, E.Y.; Choi, D.; Park, S.; Shim, C.Y.; Lee, J.H.; Kim, J.M.; Ahn, J.A.; Lee, S.W.; Oh, J.K.; et al. Time course of recovery of left ventricular filling pressure after exercise in healthy subjects. Circ. J. 2008, 72, 186–188. [Google Scholar] [CrossRef]
- Studer Bruengger, A.A.; Kaufmann, B.A.; Buser, M.; Hoffmann, M.; Bader, F.; Bernheim, A.M. Diastolic stress echocardiography in the young: A study in nonathletic and endurance-trained healthy subjects. J. Am. Soc. Echocardiogr. 2014, 27, 1053–1059. [Google Scholar] [CrossRef]
- Ha, J.W.; Oh, J.K.; Pellikka, P.A.; Ommen, S.R.; Stussy, V.L.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Diastolic stress echocardiography: A novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J. Am. Soc. Echocardiogr. 2005, 18, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Miranda, W.R.; Yeung, D.F.; Kane, G.C.; Oh, J.K. Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1490–1499. [Google Scholar] [CrossRef]
- Bok, Y.; Kim, J.Y.; Park, J.H. Prognostic Role of Right Ventricular-Pulmonary Artery Coupling Assessed by TAPSE/PASP Ratio in Patients with Acute Heart Failure. J. Cardiovasc. Imaging 2023, 31, 200–206. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure with Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging 2017, 10 Pt B, 1211–1221. [Google Scholar] [CrossRef]
- Sun, B.J.; Park, J.H.; Lee, M.; Choi, J.O.; Lee, J.H.; Shin, M.S.; Kim, M.J.; Jung, H.O.; Park, J.R.; Sohn, I.S.; et al. Normal Reference Values for Left Atrial Strain and Its Determinants from a Large Korean Multicenter Registry. J. Cardiovasc. Imaging 2020, 28, 186–198. [Google Scholar] [CrossRef]
- Miyoshi, T.; Addetia, K.; Citro, R.; Daimon, M.; Desale, S.; Fajardo, P.G.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; et al. Left Ventricular Diastolic Function in Healthy Adult Individuals: Results of the World Alliance Societies of Echocardiography Normal Values Study. J. Am. Soc. Echocardiogr. 2020, 33, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Amano, M.; Obokata, M.; Izumo, M.; Utsunomiya, H. Practice guidance for stress echocardiography. J. Echocardiogr. 2024, 22, 1–15. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Schiros, C.G.; Aban, I.; Denney, T.S.; Gupta, H. Diagnostic Accuracy of Tissue Doppler Index E/e’ for Evaluating Left Ventricular Filling Pressure and Diastolic Dysfunction/Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002530. [Google Scholar] [CrossRef]
- Kane, G.C.; Sachdev, A.; Villarraga, H.R.; Ammash, N.M.; Oh, J.K.; McGoon, M.D.; Pellikka, P.A.; McCully, R.B. Impact of age on pulmonary artery systolic pressures at rest and with exercise. Echo Res. Pract. 2016, 3, 53–61. [Google Scholar] [CrossRef]
- Lewis, G.D.; Bossone, E.; Naeije, R.; Grünig, E.; Saggar, R.; Lancellotti, P.; Ghio, S.; Varga, J.; Rajagopalan, S.; Oudiz, R.; et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013, 128, 1470–1479. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.; Redfield, M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Meyer, P.; Marwick, T.H.; Lam, C.S.P.; Kaye, D.M. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 198–205. [Google Scholar] [CrossRef]
- Jung, M.H.; Ihm, S.H.; Lee, D.H.; Han, S.; Jung, H.O.; Youn, H.J.; Ryu, K.H. Sex-specific associations of obesity with exercise capacity and diastolic function in Koreans. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 254–262. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, M.H. Sex-related differences among young adults with heart failure: Does sex matter? Int. J. Cardiol. 2022, 364, 91–92. [Google Scholar] [CrossRef]
- Beale, A.L.; Nanayakkara, S.; Segan, L.; Mariani, J.A.; Maeder, M.T.; van Empel, V.; Vizi, D.; Evans, S.; Lam, C.S.; Kaye, D.M. Sex Differences in Heart Failure with Preserved Ejection Fraction Pathophysiology: A Detailed Invasive Hemodynamic and Echocardiographic Analysis. JACC Heart Fail. 2019, 7, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Ihm, S.H.; Park, S.M.; Jung, H.O.; Hong, K.S.; Baek, S.H.; Youn, H.J. Effects of sarcopenia, body mass indices, and sarcopenic obesity on diastolic function and exercise capacity in Koreans. Metabolism 2019, 97, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.P.; Kirby, M.; Singh, G.V.; Tan, W.C.; Bourbeau, J.; Eves, N.D.; CanCOLD Collaborative Research Group. Sex-related differences in pulmonary vascular volume distribution. Pulm. Circ. 2024, 14, e12436. [Google Scholar] [CrossRef] [PubMed]
- Radakrishnan, A.; Agrawal, S.; Singh, N.; Barbieri, A.; Shaw, L.J.; Gulati, M.; Lala, A. Underpinnings of Heart Failure with Preserved Ejection Fraction in Women—From Prevention to Improving Function. A Co-publication with the American Journal of Preventive Cardiology and the Journal of Cardiac Failure. J. Card. Fail. 2025; in press. [Google Scholar] [CrossRef]
- Beale, A.L.; Cosentino, C.; Segan, L.; Mariani, J.A.; Vizi, D.; Evans, S.; Nanayakkara, S.; Kaye, D.M. The effect of parity on exercise physiology in women with heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 213–222. [Google Scholar] [CrossRef]
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef]

| Variable | All Patients (n = 28) | Female (n = 14) | Male (n = 14) | p |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, y | 60.9 ± 8.4 | 59.7 ± 8.0 | 62.0 ± 8.9 | 0.480 |
| Systolic blood pressure, mmHg | 119.3 ± 7.7 | 118.1 ± 7.4 | 120.4 ± 8.0 | 0.426 |
| Diastolic blood pressure, mmHg | 75.0 ± 8.5 | 71.7 ± 7.2 | 78.4 ± 8.6 | 0.036 |
| Body mass index, kg/m2 | 24.6 ± 4.0 | 22.6 ± 4.3 | 26.6 ± 2.3 | 0.007 |
| Hypertension, n (%) | 17 (60.7) | 7 (50.0) | 10 (71.4) | 0.440 |
| Diabetes mellitus, n (%) | 7 (25.0) | 1 (7.1) | 6 (42.9) | 0.077 |
| Echocardiographic variables | ||||
| LV ejection fraction, % | 62.9 ± 2.3 | 63.3 ± 1.8 | 62.4 ± 2.7 | 0.325 |
| IVSd, mm | 9.9 ± 1.3 | 9.1 ± 0.9 | 10.8 ± 1.1 | <0.001 |
| LVMI, g/m2 | 87.1 ± 16.7 | 83.5 ± 18.6 | 90.8 ± 14.1 | 0.247 |
| Septal E/e’ ratio | 10.2 ± 1.6 | 10.5 ± 1.6 | 9.9 ± 1.7 | 0.383 |
| Average E/e’ ratio | 8.3 ± 1.6 | 8.4 ± 1.6 | 8.2 ± 1.7 | 0.743 |
| SPAP, mmHg | 23.0 ± 3.9 | 23.3 ± 3.8 | 22.8 ± 4.1 | 0.721 |
| LA volume index, mL/m2 | 25.2 ± 5.3 | 24.5 ± 4.8 | 25.9 ± 6.0 | 0.490 |
| LA strain | ||||
| LA reservoir, % | 28.1 ± 6.2 | 28.1 ± 5.5 | 28.2 ± 7.0 | 0.953 |
| LA conduit, % | −14.4 ± 4.5 | −15.4 ± 5.2 | −13.4 ± 3.7 | 0.250 |
| LA contraction, % | −13.8 ± 5.9 | −12.6 ± 5.2 | −14.9 ± 6.5 | 0.314 |
| Resting | 25 W | 50 W | 75 W | p | |
|---|---|---|---|---|---|
| Systolic BP, mmHg | 140.0 ± 14.9 | 161.2 ± 21.0 | 162.9 ± 40.8 | 179.1 ± 41.5 | <0.001 |
| Pulse pressure, mmHg | 56.6 ± 10.9 | 78.3 ± 20.1 | 81.4 ± 25.5 | 92.6 ± 27.8 | <0.001 |
| Heart rate, bpm | 70.6 ± 10.2 | 91.8 ± 9.5 | 105.1 ± 13.2 | 124.6 ± 19.8 | <0.001 |
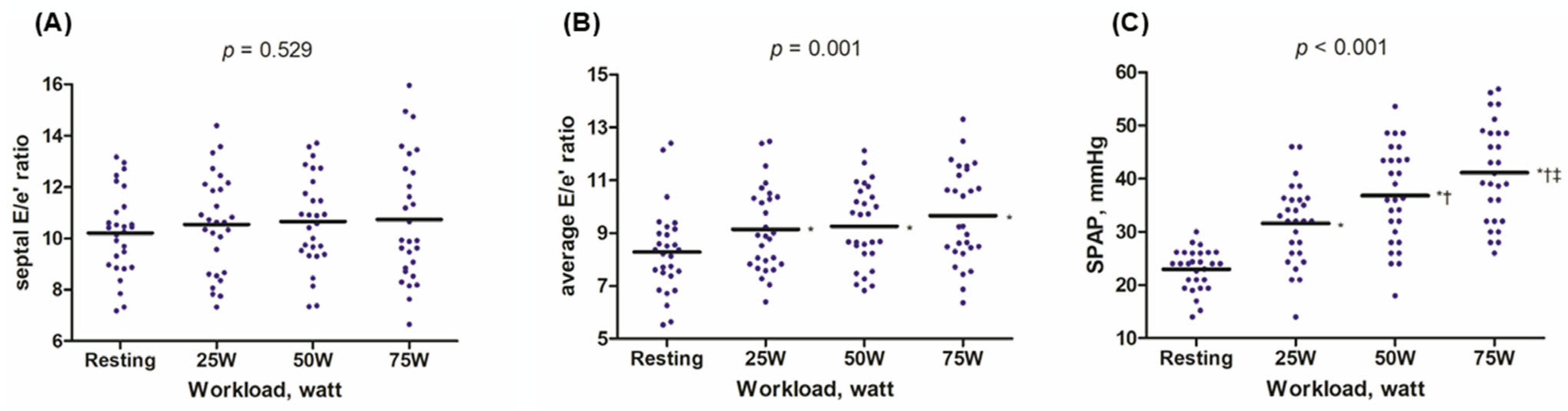
| Septal E/e’ ratio | 10.2 ± 1.6 | 10.5 ± 1.9 | 10.7 ± 1.8 | 10.7 ± 2.4 | 0.529 |
| Average E/e’ ratio | 8.3 ± 1.6 | 9.1 ± 1.6 | 9.3 ± 1.5 | 9.7 ± 1.8 | 0.001 |
| TR Vmax, m/s | 2.1 ± 0.2 | 2.5 ± 0.4 | 2.8 ± 0.4 | 3.0 ± 0.4 | <0.001 |
| SPAP, mmHg | 23.0 ± 3.9 | 31.6 ± 7.5 | 36.9 ± 9.1 | 41.2 ± 9.3 | <0.001 |
| TAPSE/SPAP * | 0.76 ± 0.18 | 0.62 ± 0.20 | 0.54 ± 0.18 | 0.48 ± 0.16 | <0.001 |
| Female (n = 14) | Male (n = 14) | p | |
|---|---|---|---|
| Resting profiles | |||
| Systolic blood pressure, mmHg | 140.4 ± 18.0 | 139.6 ± 11.7 | 0.882 |
| Pulse pressure, mmHg | 59.8 ± 10.0 | 53.4 ± 11.2 | 0.126 |
| Heart rate, bpm | 69.1 ± 10.4 | 72.1 ± 10.0 | 0.434 |
| Septal E/e’ ratio | 10.5 ± 1.6 | 9.9 ± 1.7 | 0.383 |
| Average E/e’ ratio | 8.4 ± 1.6 | 8.2 ± 1.7 | 0.743 |
| SPAP, mmHg | 23.3 ± 3.8 | 22.8 ± 4.1 | 0.721 |
| TAPSE/SPAP *, mm/Hg | 0.74 ± 0.12 | 0.82 ± 0.29 | 0.389 |
| 75 W exercise profiles | |||
| Systolic BP, mmHg | 192.6 ± 22.0 | 165.6 ± 51.9 | 0.085 |
| Pulse pressure, mmHg | 106.3 ± 21.5 | 78.9 ± 27.1 | 0.006 |
| Heart rate, bpm | 137.8 ± 13.7 | 111.4 ± 15.7 | <0.001 |
| Septal E/e’ ratio | 11.0 ± 2.5 | 10.5 ± 2.5 | 0.620 |
| Average E/e’ ratio | 9.8 ± 1.4 | 9.6 ± 2.2 | 0.762 |
| SPAP, mmHg | 45.5 ± 8.3 | 36.8 ± 8.3 | 0.011 |
| TAPSE/SPAP *, mm/Hg | 0.41 ± 0.10 | 0.59 ± 0.18 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.-H.; Lee, S.-Y.; Chung, W.-B.; Youn, J.-C.; Jung, H.O. Exercise Hemodynamics and Sex-Specific Data in Asymptomatic Adults: An Exploratory Pilot Study. Diagnostics 2025, 15, 1307. https://doi.org/10.3390/diagnostics15111307
Jung M-H, Lee S-Y, Chung W-B, Youn J-C, Jung HO. Exercise Hemodynamics and Sex-Specific Data in Asymptomatic Adults: An Exploratory Pilot Study. Diagnostics. 2025; 15(11):1307. https://doi.org/10.3390/diagnostics15111307
Chicago/Turabian StyleJung, Mi-Hyang, So-Young Lee, Woo-Baek Chung, Jong-Chan Youn, and Hae Ok Jung. 2025. "Exercise Hemodynamics and Sex-Specific Data in Asymptomatic Adults: An Exploratory Pilot Study" Diagnostics 15, no. 11: 1307. https://doi.org/10.3390/diagnostics15111307
APA StyleJung, M.-H., Lee, S.-Y., Chung, W.-B., Youn, J.-C., & Jung, H. O. (2025). Exercise Hemodynamics and Sex-Specific Data in Asymptomatic Adults: An Exploratory Pilot Study. Diagnostics, 15(11), 1307. https://doi.org/10.3390/diagnostics15111307





