The Biochemical–Imaging Connection: Urinary Noradrenaline and Fluorodeoxyglucose-Positron Emission Tomography in Unresectable or Metastatic Pheochromocytomas and Paragangliomas
Abstract
1. Introduction
2. Materials and Methods
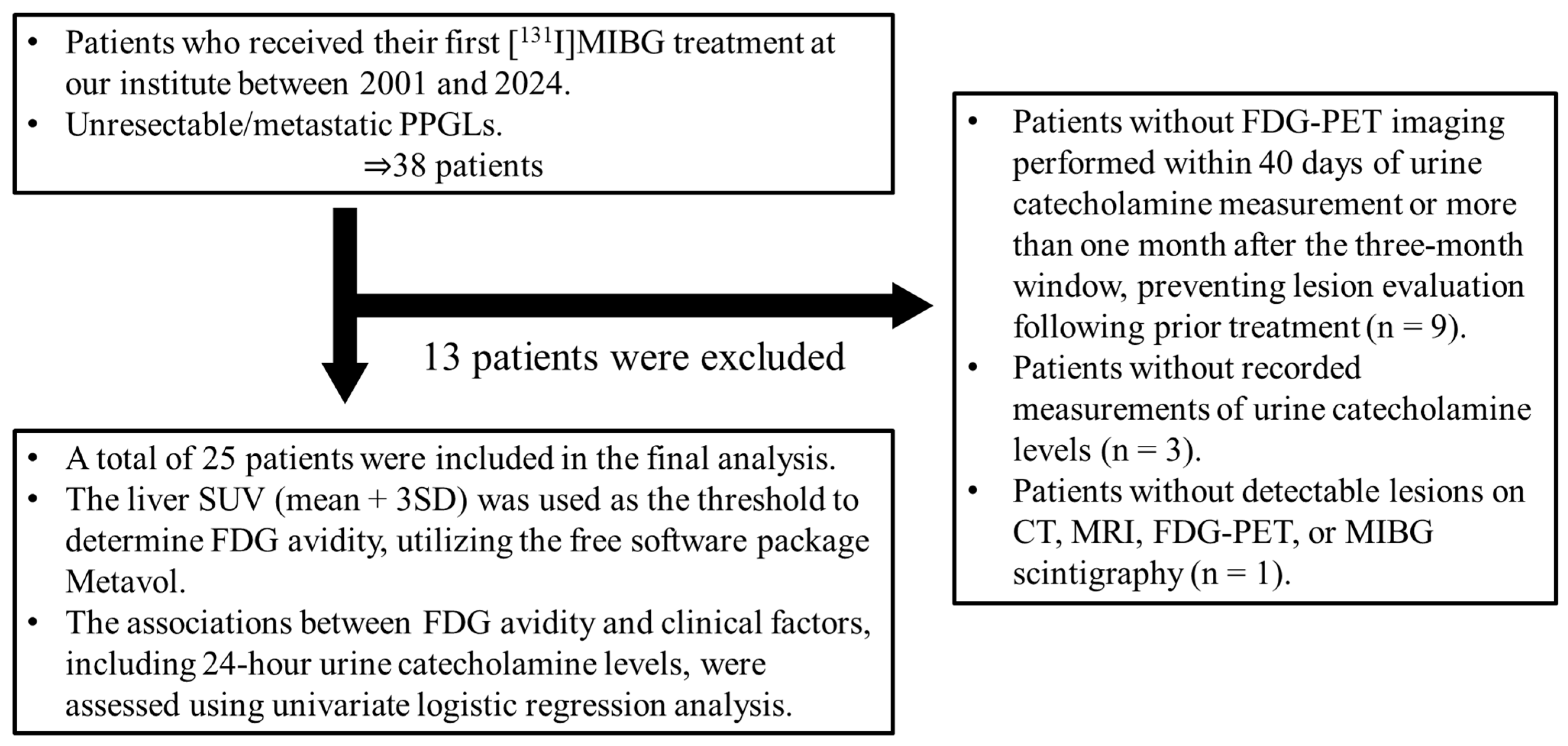
2.1. Patients
2.2. FDG-PET Acquisition
2.3. Image Analysis
2.4. Biochemical Parameters
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
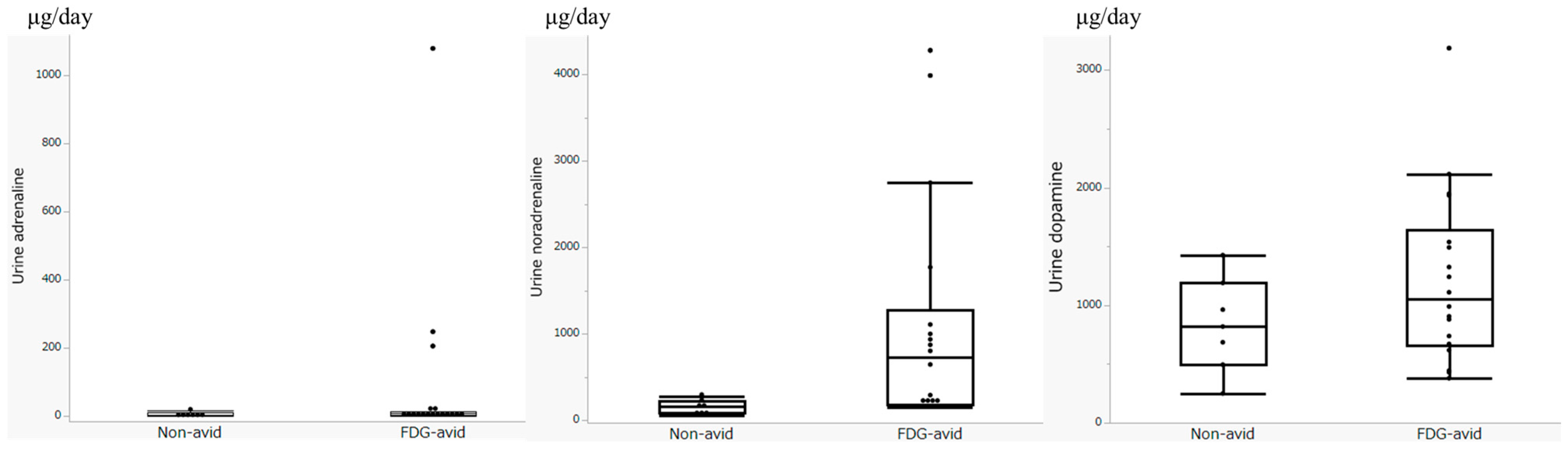
3.2. Biochemical Parameters
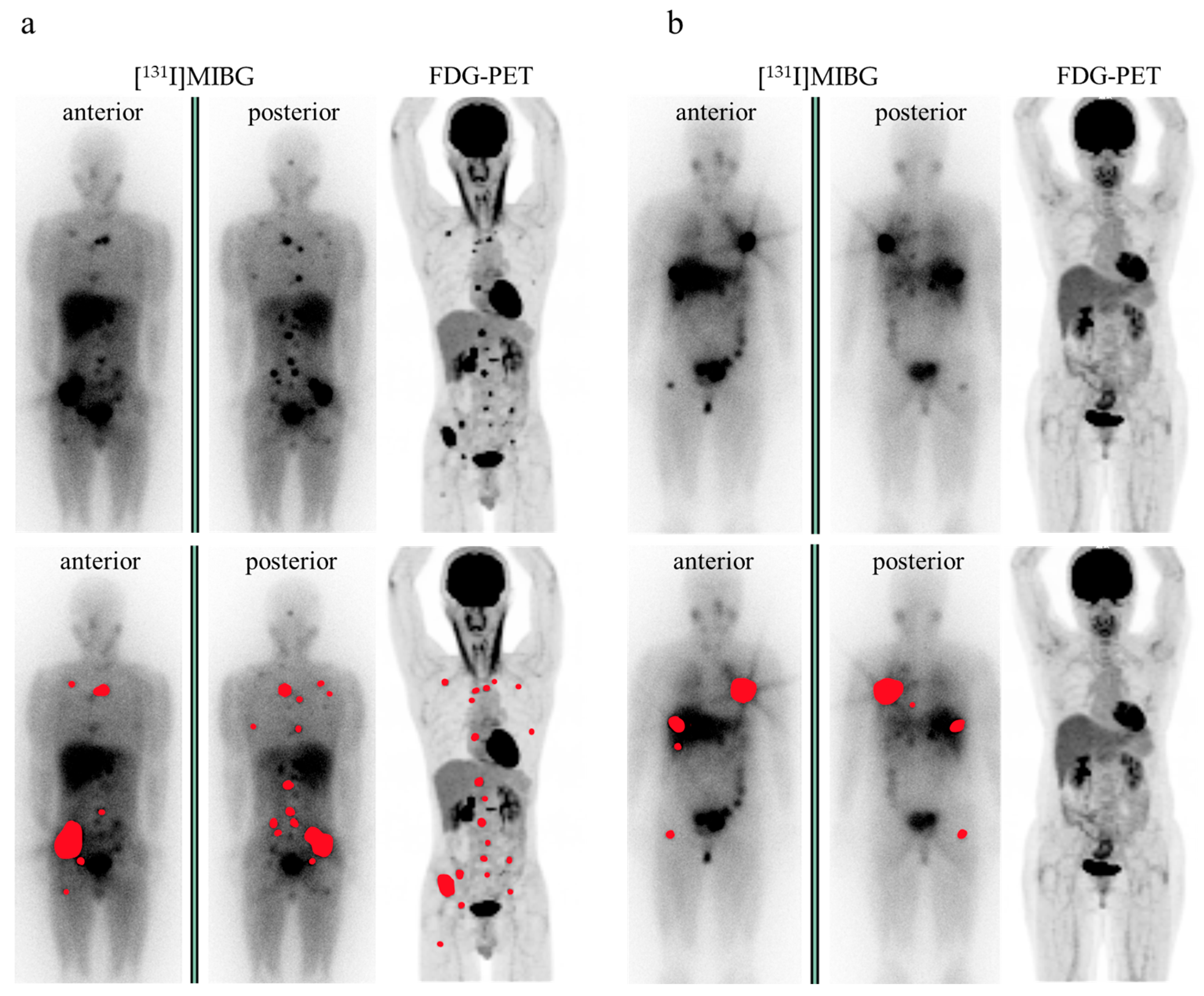
3.3. FDG-PET Imaging Parameters
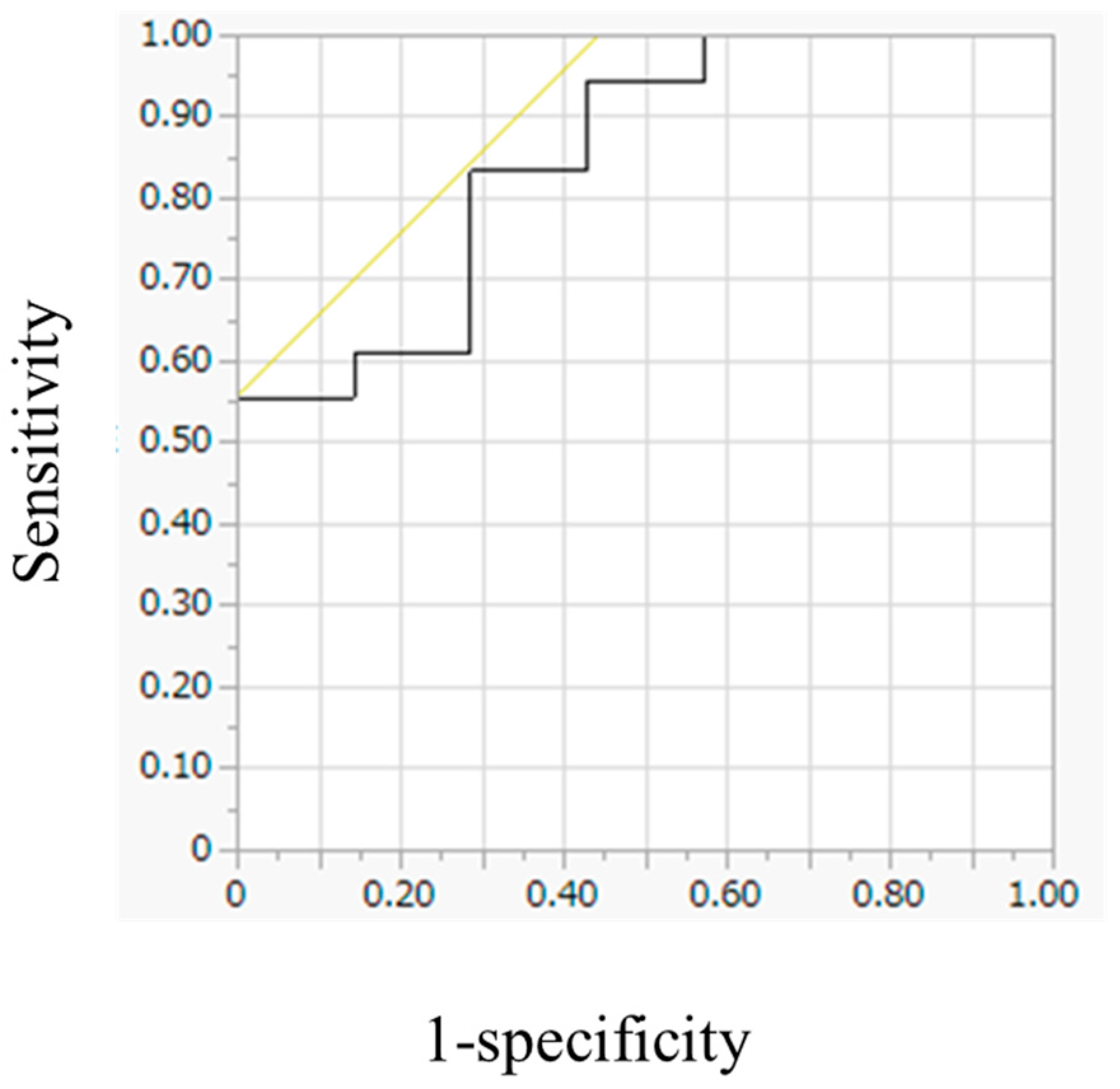
3.4. Evaluation of FDG Avidity and Associated Clinical Data, Including Urinary Catecholamine Levels
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CT | computed tomography |
| DOPA | dihydroxyphenylalanine |
| FDG | [18F]fluorodeoxyglucose |
| FH | fumarate hydratase |
| HIF-1 | hypoxia-inducible factor-1 |
| IDH | isocitrate dehydrogenase |
| IQR | interquartile range |
| MIBG | metaiodobenzylguanidine |
| MRI | magnetic resonance imaging |
| MTV | total metabolic tumor volume |
| OSEM | ordered subset expectation maximization |
| PET | positron emission tomography |
| PHD | prolyl hydroxylase domain-containing protein |
| PPGLs | pheochromocytomas and paragangliomas |
| PRRT | peptide receptor radionuclide therapy |
| ROC | receiver operating characteristic |
| SD | standard deviation |
| SDH | succinate dehydrogenase |
| SRE | skeletal-related event |
| SSTR | somatostatin receptor |
| SUV | standardized uptake value |
| SUVmax | maximum standardized uptake value |
| TCA | tricarboxylic acid |
| TLG | total tumor lesion glycolysis |
| VHL | von Hippel–Lindau |
References
- Mete, O.; Asa, S.L.; Gill, A.J.; Kimura, N.; de Krijger, R.R.; Tischler, A. Overview of the 2022 WHO classification of paragangliomas and pheochromocytomas. Endocr. Pathol. 2022, 33, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Sung, T.Y.; Kim, W.G.; Lee, J.J.; Ryu, J.S.; Kim, T.Y.; Kim, W.B.; Hong, S.J.; Song, D.E.; Shong, Y.K. Clinical course and prognostic factors in patients with malignant pheochromocytoma and paraganglioma: A single institution experience. J. Surg. Oncol. 2015, 112, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Ezzat Abdel-Aziz, T.; Prete, F.; Conway, G.; Gaze, M.; Bomanji, J.; Bouloux, P.; Khoo, B.; Caplin, M.; Mushtaq, I.; Smart, J.; et al. Phaeochromocytomas and paragangliomas: A difference in disease behaviour and clinical outcomes. J. Surg. Oncol. 2015, 112, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Nölting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized management of pheochromocytoma and paraganglioma. Endocr. Rev. 2022, 43, 199–239. [Google Scholar] [CrossRef]
- Lenders, J.W.M.; Kerstens, M.N.; Amar, L.; Prejbisz, A.; Robledo, M.; Taieb, D.; Pacak, K.; Crona, J.; Zelinka, T.; Mannelli, M.; et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1443–1456. [Google Scholar] [CrossRef]
- Hescot, S.; Curras-Freixes, M.; Deutschbein, T.; van Berkel, A.; Vezzosi, D.; Amar, L.; de la Fouchardière, C.; Valdes, N.; Riccardi, F.; Do Cao, C.; et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors retrospective study. J. Clin. Endocrinol. Metab. 2019, 104, 2367–2374. [Google Scholar] [CrossRef]
- Dahia, P.L.M.; Clifton-Bligh, R.; Gimenez-Roqueplo, A.P.; Robledo, M.; Jimenez, C. Hereditary endocrine tumours: Current state-of-the-art and research opportunities: Metastatic pheochromocytomas and paragangliomas: Proceedings of the MEN2019 workshop. Endocr. Relat. Cancer 2020, 27, T41–T52. [Google Scholar] [CrossRef]
- Bechmann, N.; Moskopp, M.L.; Ullrich, M.; Calsina, B.; Wallace, P.W.; Richter, S.; Friedemann, M.; Langton, K.; Fliedner, S.M.J.; Timmers, H.J.L.M.; et al. HIF2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr. Relat. Cancer 2020, 27, 625–640. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Deutschbein, T.; Constantinescu, G.; Langton, K.; Pamporaki, C.; Calsina, B.; Monteagudo, M.; Peitzsch, M.; Fliedner, S.; Timmers, H.J.L.M.; et al. Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma. Clin. Chem. Lab. Med. 2020, 59, 353–363. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Huynh, T.T.; Pacak, K.; Brouwers, F.M.; Walther, M.M.; Linehan, W.M.; Munson, P.J.; Mannelli, M.; Goldstein, D.S.; Elkahloun, A.G. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: Activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr. Relat. Cancer 2004, 11, 897–911. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Rhodes, L.; Blanco, I.; Chung, W.K.; Eng, C.; Maher, E.R.; Richard, S.; Giles, R.H. Von Hippel-Lindau disease: Genetics and role of genetic counseling in a multiple neoplasia syndrome. J. Clin. Oncol. 2016, 34, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Lenders, J.W.; Linehan, W.M.; Walther, M.M.; Goldstein, D.S.; Keiser, H.R. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N. Engl. J. Med. 1999, 340, 1872–1879. [Google Scholar] [CrossRef]
- Rednam, S.P.; Erez, A.; Druker, H.; Janeway, K.A.; Kamihara, J.; Kohlmann, W.K.; Nathanson, K.L.; States, L.J.; Tomlinson, G.E.; Villani, A.; et al. Von Hippel-Lindau and hereditary pheochromocytoma/paraganglioma syndromes: Clinical features, genetics, and surveillance recommendations in childhood. Clin. Cancer Res. 2017, 23, e68–e75. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Lenders, J.W.; Timmers, H.; Mannelli, M.; Grebe, S.K.; Hofbauer, L.C.; Bornstein, S.R.; Tiebel, O.; Adams, K.; Bratslavsky, G.; et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin. Chem. 2011, 57, 411–420. [Google Scholar] [CrossRef]
- Feldman, J.M.; Blalock, J.A.; Zern, R.T.; Shelburne, J.D.; Gaede, J.T.; Farrell, R.E.; Wells, S.A. Deficiency of dopamine-β-hydroxylase. A new mechanism for normotensive pheochromocytomas. Am. J. Clin. Pathol. 1979, 72, 175–185. [Google Scholar] [CrossRef]
- Takenaka, J.; Watanabe, S.; Abe, T.; Takeuchi, S.; Hirata, K.; Kimura, R.; Ishii, H.; Wakabayashi, N.; Majigsuren, M.; Kudo, K. Urinary dopamine levels can predict the avidity of post-therapy [131I]MIBG scintigraphy in unresectable or metastatic pheochromocytomas and paragangliomas: A preliminary clinical study. Pharmaceuticals 2025, 18, 165. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Taïeb, D.; Hicks, R.J.; Hindié, E.; Guillet, B.A.; Avram, A.; Ghedini, P.; Timmers, H.J.; Scott, A.T.; Elojeimy, S.; Rubello, D.; et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2112–2137. [Google Scholar] [CrossRef]
- Han, S.; Suh, C.H.; Woo, S.; Kim, Y.J.; Lee, J.J. Performance of 68Ga-DOTA-conjugated somatostatin receptor–targeting peptide PET in detection of pheochromocytoma and paraganglioma: A systematic review and metaanalysis. J. Nucl. Med. 2019, 60, 369–376. [Google Scholar] [CrossRef]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Severi, S.; Bongiovanni, A.; Ferrara, M.; Nicolini, S.; Di Mauro, F.; Sansovini, M.; Lolli, I.; Tardelli, E.; Cittanti, C.; Di Iorio, V.; et al. Peptide receptor radionuclide therapy in patients with metastatic progressive pheochromocytoma and paraganglioma: Long-term toxicity, efficacy and prognostic biomarker data of phase II clinical trials. ESMO Open 2021, 6, 100171. [Google Scholar] [CrossRef]
- Janssen, I.; Chen, C.C.; Zhuang, Z.; Millo, C.M.; Wolf, K.I.; Ling, A.; Lin, F.I.; Adams, K.T.; Herscovitch, P.; Feelders, R.A.; et al. Functional imaging signature of patients presenting with polycythemia/paraganglioma syndromes. J. Nucl. Med. 2017, 58, 1236–1242. [Google Scholar] [CrossRef]
- Fischer, A.; Kloos, S.; Maccio, U.; Friemel, J.; Remde, H.; Fassnacht, M.; Pamporaki, C.; Eisenhofer, G.; Timmers, H.J.L.M.; Robledo, M.; et al. Metastatic pheochromocytoma and paraganglioma: Somatostatin receptor 2 expression, genetics, and therapeutic responses. J. Clin. Endocrinol. Metab. 2023, 108, 2676–2685. [Google Scholar] [CrossRef]
- Giammarile, F.; Chiti, A.; Lassmann, M.; Brans, B.; Flux, G. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1039–1047. [Google Scholar] [CrossRef]
- Hiromasa, T.; Wakabayashi, H.; Kayano, D.; Inaki, A.; Watanabe, S.; Mori, H.; Akatani, N.; Yamase, T.; Kunita, Y.; Saito, S.; et al. Prognostic factors for refractory pheochromocytoma and paraganglioma after 131I-metaiodobenzylguanidine therapy. Ann. Nucl. Med. 2022, 36, 61–69. [Google Scholar] [CrossRef]
- Kayano, D.; Taki, J.; Fukuoka, M.; Wakabayashi, H.; Inaki, A.; Nakamura, A.; Kinuya, S. Low-dose 123I-metaiodobenzylguanidine diagnostic scan is inferior to 131I-metaiodobenzylguanidine posttreatment scan in detection of malignant pheochromocytoma and paraganglioma. Nucl. Med. Commun. 2011, 32, 941–946. [Google Scholar] [CrossRef]
- Inaki, A.; Shiga, T.; Tsushima, Y.; Jinguji, M.; Wakabayashi, H.; Kayano, D.; Akatani, N.; Yamase, T.; Kunita, Y.; Watanabe, S.; et al. An open-label, single-arm, multi-center, phase II clinical trial of single-dose [131I]meta-iodobenzylguanidine therapy for patients with refractory pheochromocytoma and paraganglioma. Ann. Nucl. Med. 2022, 36, 267–278. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Abe, T.; Okamoto, S.; Uchiyama, Y.; Manabe, O.; Ito, Y.M.; Tamura, N.; Ito, N.; Yoshioka, N.; Washino, K.; et al. Effects of repeated 131I-meta-iodobenzylguanidine radiotherapy on tumor size and tumor metabolic activity in patients with metastatic neuroendocrine tumors. J. Nucl. Med. 2021, 62, 685–694. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Watanabe, S.; Abe, T.; Takenaka, J.; Hirata, K.; Kimura, R.; Sakamoto, K.; Shinohara, N.; Kudo, K. Safety and efficacy of multiple-dose versus single-dose MIBG therapy in patients with refractory pheochromocytoma and paraganglioma: A single-center retrospective analysis. Ann. Nucl. Med. 2024, 38, 553–562. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Taki, J.; Inaki, A.; Nakamura, A.; Kayano, D.; Fukuoka, M.; Matsuo, S.; Nakajima, K.; Kinuya, S. Prognostic values of initial responses to low-dose 131I-MIBG therapy in patients with malignant pheochromocytoma and paraganglioma. Ann. Nucl. Med. 2013, 27, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Taïeb, D.; Carrasquillo, J.A.; Pryma, D.A.; Patel, M.; Millo, C.; de Herder, W.W.; Del Rivero, J.; Crona, J.; Shulkin, B.L.; et al. High-specific-activity-131I-MIBG versus 177Lu-DOTATATE targeted radionuclide therapy for metastatic pheochromocytoma and paraganglioma. Clin. Cancer Res. 2021, 27, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Timmers, H.J.; Chen, C.C.; Carrasquillo, J.A.; Whatley, M.; Ling, A.; Eisenhofer, G.; King, K.S.; Rao, J.U.; Wesley, R.A.; Adams, K.T.; et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J. Natl. Cancer Inst. 2012, 104, 700–708. [Google Scholar] [CrossRef]
- Takenaka, J.; Watanabe, S.; Abe, T.; Hirata, K.; Uchiyama, Y.; Kimura, R.; Shinohara, N.; Kudo, K. Prognostic value of [18F]FDG-PET prior to [131I]MIBG treatment for pheochromocytoma and paraganglioma (PPGL). Ann. Nucl. Med. 2023, 37, 10–17. [Google Scholar] [CrossRef]
- Takenaka, J.; Watanabe, S.; Abe, T.; Tsuchikawa, T.; Takeuchi, S.; Hirata, K.; Kimura, R.; Wakabayashi, N.; Shinohara, N.; Kudo, K. Predictive factors of early 18F-fluorodeoxyglucose-positron emission tomography response to [131I] metaiodobenzylguanidine treatment for unresectable or metastatic pheochromocytomas and paragangliomas. Neuroendocrinology 2024, 114, 816–826. [Google Scholar] [CrossRef]
- Timmers, H.J.L.M.; Taïeb, D.; Pacak, K.; Lenders, J.W.M. Imaging of pheochromocytomas and paragangliomas. Endocr. Rev. 2024, 45, 414–434. [Google Scholar] [CrossRef]
- Urso, L.; Panareo, S.; Castello, A.; Ambrosio, M.R.; Zatelli, M.C.; Caracciolo, M.; Tonini, E.; Valpiani, G.; Boschi, A.; Uccelli, L.; et al. Glucose metabolism modification induced by radioligand therapy with [177Lu]Lu/[90Y]Y-DOTATOC in advanced neuroendocrine neoplasms: A prospective pilot study within FENET-2016 trial. Pharmaceutics 2022, 14, 2009. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Hirata, K.; Kobayashi, K.; Wong, K.P.; Manabe, O.; Surmak, A.; Tamaki, N.; Huang, S.C. A semi-automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET-CT. PLoS ONE 2014, 9, e105682. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef]
- Crona, J.; Lamarca, A.; Ghosal, S.; Welin, S.; Skogseid, B.; Pacak, K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: A systematic review and individual patient meta-analysis. Endocr. Relat. Cancer 2019, 26, 539–550. [Google Scholar] [CrossRef]
- Taïeb, D.; Sebag, F.; Barlier, A.; Tessonnier, L.; Palazzo, F.F.; Morange, I.; Niccoli-Sire, P.; Fakhry, N.; De Micco, C.; Cammilleri, S.; et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: A new molecular imaging signature. J. Nucl. Med. 2009, 50, 711–717. [Google Scholar] [CrossRef]
- Yoshioka, K.; Nakano, Y.; Horichi, M.; Aono, D.; Takeshita, Y.; Takamura, T. Metastatic pheochromocytoma/paraganglioma overproducing multiple catecholamines. JCEM Case Rep. 2025, 3, luae241. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Nakazawa, A.; Higuchi, T.; Oriuchi, N.; Arisaka, Y.; Endo, K. Clinical Significance of 2-[18F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography for the Assessment of 131I-Metaiodobenzylguanidine Therapy in Malignant Phaeochromocytoma. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1869–1875. [Google Scholar] [CrossRef]
- Laganà, M.; Habra, M.A.; Remde, H.; Almeida, M.Q.; Cosentini, D.; Pusceddu, S.; Grana, C.M.; Corssmit, E.P.M.; Bongiovanni, A.; De Filpo, G.; et al. Adverse skeletal related events in patients with bone-metastatic pheochromocytoma/paraganglioma. Eur. J. Cancer 2024, 208, 114122. [Google Scholar] [CrossRef]
- Ayala-Ramirez, M.; Palmer, J.L.; Hofmann, M.C.; de la Cruz, M.; Moon, B.S.; Waguespack, S.G.; Habra, M.A.; Jimenez, C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, 1492–1497. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Pacak, K.; Huynh, T.T.; Qin, N.; Bratslavsky, G.; Linehan, W.M.; Mannelli, M.; Friberg, P.; Grebe, S.K.; Timmers, H.J.; et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr. Relat. Cancer 2011, 18, 97–111. [Google Scholar] [CrossRef]
- van der Harst, E.; de Herder, W.W.; de Krijger, R.R.; Bruining, H.A.; Bonjer, H.J.; Lamberts, S.W.; van den Meiracker, A.H.; Stijnen, T.H.; Boomsma, F. The value of plasma markers for the clinical behaviour of phaeochromocytomas. Eur. J. Endocrinol. 2002, 147, 85–94. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Bhansali, A. ‘Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: A systematic review and meta-analysis’. Clin. Endocrinol. 2019, 91, 718–727. [Google Scholar] [CrossRef]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Schmitz, J.; Maric, I.; Pabst, K.; Umutlu, L.; Walz, M.; Herrmann, K.; Rischpler, C.; Weber, F.; Jentzen, W.; et al. Diagnostic performance of 124I-metaiodobenzylguanidine PET/CT in patients with pheochromocytoma. J. Nucl. Med. 2022, 63, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, T.; Cui, Y.; Zhuang, H.; Li, F.; Tong, A.; Jing, H. 18 F-MFBG PET/CT is an effective alternative of 68 Ga-DOTATATE PET/CT in the evaluation of metastatic pheochromocytoma and paraganglioma. Clin. Nucl. Med. 2023, 48, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Pacak, K. Precision medicine: An update on genotype/biochemical phenotype relationships in pheochromocytoma/paraganglioma patients. Endocr. Pract. 2017, 23, 690–704. [Google Scholar] [CrossRef]
- Mihai, R.; De Crea, C.; Guerin, C.; Torresan, F.; Agcaoglu, O.; Simescu, R.; Walz, M.K. Surgery for advanced adrenal malignant disease: Recommendations based on European Society of Endocrine Surgeons consensus meeting. Br. J. Surg. 2024, 111, znad266. [Google Scholar] [CrossRef]
| Characteristic | All | Pheochromocytoma | Paraganglioma |
|---|---|---|---|
| Number | 25 | 17 | 8 |
| Sex | |||
| Male | 11 (44.0%) | 6 (35.3%) | 5 (62.5%) |
| Female | 14 (56.0%) | 11 (64.7%) | 3 (37.5%) |
| Age (y), median (range) | 53 (23–84) | 54 (35–84) | 48.5 (23–67) |
| Pre-treatment | |||
| Surgery | 24 (96.0%) | 15 (88.2%) | 8 (100.0%) |
| Chemotherapy | 8 (32.0%) | 6 (35.3%) | 2 (25.0%) |
| External radiation | 7 (28.0%) | 5 (29.4%) | 2 (25.0%) |
| MIBG | 1 (4.0%) | 0 (0.0%) | 1 (12.5%) |
| Radiofrequency ablation | 2 (8.0%) | 1 (5.9%) | 1 (12.5%) |
| Endovascular treatment | 1 (4.0%) | 1 (5.9%) | 0 (0.0%) |
| Residual primary tumor | 2 (8.0%) | 1 (5.9%) | 1 (12.5%) |
| Metastasis | |||
| Lymph node or soft tissue | 14 (56.0%) | 9 (52.9%) | 5 (62.5%) |
| Bone | 14 (56.0%) | 11 (64.7%) | 3 (37.5%) |
| Liver | 12 (48.0%) | 10 (58.8%) | 2 (25.0%) |
| Lung | 10 (40.0%) | 7 (41.2%) | 3 (37.5%) |
| Metastasis (no. of organs): | |||
| 1 | 9 (36.0%) | 5 (29.4%) | 4 (50.0%) |
| 2 | 10 (40.0%) | 7 (41.2%) | 3 (37.5%) |
| 3 | 4 (16.0%) | 3 (17.6%) | 1 (12.5%) |
| 4 | 2 (8.0%) | 2 (11.8%) | 0 (0.0%) |
| Excess catecholamine secretion | |||
| Adrenaline | 3 (12.0%) | 3 (17.6%) | 0 (0.0%) |
| Noradrenaline | 20 (80%) | 13 (76.5%) | 7 (87.5%) |
| Dopamine | 13 (52.0%) | 8 (47.1%) | 5 (62.5%) |
| Patients with FDG-avid lesions | 18 (72.0%) | 12 (70.6%) | 6 (75.0%) |
| Median | Range | IQR | Normal Range | ||
|---|---|---|---|---|---|
| Adrenaline | All | 6.9 | 1.8–1080 | 3.35–13.8 | 3.4–26.9 |
| FDG-avid | 7.7 | 2.3–1080 | 5.0–14.6 | ||
| Non-avid | 3.4 | 1.8–18.3 | 3.0–11.6 | ||
| Noradrenaline | All | 224.3 | 59.1–4282 | 172.9–945.95 | 48.6–168.4 |
| FDG-avid | 726.25 | 155–4020.1 | 186.7–1275.9 | ||
| Non-avid | 166.3 | 59.1–273.9 | 86.1–224.3 | ||
| Dopamine | All | 963.2 | 249.9–3187.5 | 644.95–1459.35 | 365.0–961.5 |
| FDG-avid | 1050.3 | 379.7–3187.5 | 658.2–1637.7 | ||
| Non-avid | 819.4 | 249.9–1426.3 | 495.3–1190.4 |
| Median | Range | IQR | |
|---|---|---|---|
| SUVmax | 10.65 | 4.64–64.45 | 7.425–42.51 |
| MTV | 83.54 | 1.21–395.31 | 19.32–118.82 |
| TLG | 396.65 | 4.50–4017.35 | 80.10–752.49 |
| Variable | With FDG-avid Lesions | Without FDG-avid Lesions | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Number | 18 | 7 | ||
| Sex | ||||
| Male | 9 | 2 | 1.620 (0.335–8.230) | 0.548 |
| Female | 9 | 5 | ||
| Age (y), median (range) | 61 (23–84) | 47 (35–53) | 1.047 (0.984–1.128) | 0.150 |
| Diagnosis | ||||
| Pheochromocytoma | 12 | 5 | 0.800 (0.095–5.071) | 0.818 |
| Paraganglioma | 6 | 2 | ||
| History of chemotherapy | 4 | 4 | 0.214 (0.030–1.341) | 0.100 |
| History of external radiation | 7 | 0 | >100 | 0.018 * |
| Metastatic lesion | ||||
| Lymph node or soft tissue | 12 | 2 | 5.000 (0.815–42.97) | 0.083 |
| Bone | 11 | 3 | 2.095 (0.357–13.58) | 0.410 |
| Liver | 8 | 4 | 0.600 (0.098–3.501) | 0.568 |
| Lung | 8 | 2 | 2.000 (0.329–16.67) | 0.461 |
| Urine biochemistry | ||||
| Excess adrenaline secretion | 3 | 0 | >100 (0.448–NA) | 0.145 |
| Adrenaline (μg/day), median (range) | 7.7 (2.3–1080) | 3.4 (1.8–18.3) | 1.068 (0.999–1.347) | 0.110 |
| Excess noradrenaline secretion | 17 | 3 | 22.66 (2.399–541.7) | 0.005 * |
| Noradrenaline (μg/day), median (range) | 726.25 (155–4020.1) | 166.3 (59.1–273.9) | 1.013 (1.002–1.039) | 0.001 * |
| Excess dopamine secretion | 10 | 3 | 1.667 (0.286–10.66) | 0.568 |
| Dopamine (μg/day), median (range) | 1050.3 (379.7–3187.5) | 819.4 (249.9–1426.3) | 1.001 (1.000–1.004) | 0.145 |
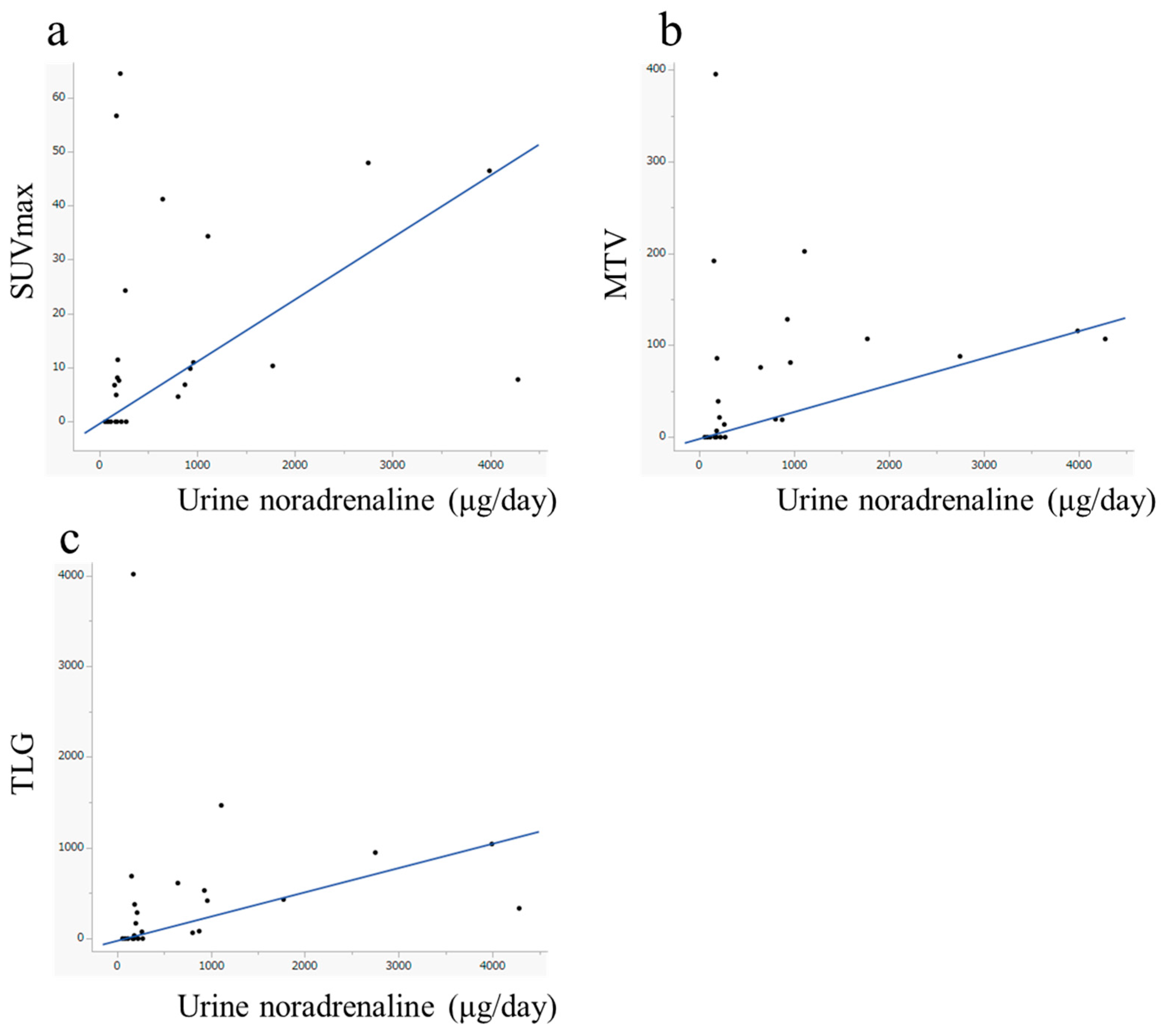
| Urine Biochemistry | ρ | p-Value | |
|---|---|---|---|
| SUVmax | Adrenaline | 0.246 | 0.236 |
| Noradrenaline | 0.527 | 0.007 * | |
| Dopamine | 0.369 | 0.069 | |
| MTV | Adrenaline | 0.274 | 0.185 |
| Noradrenaline | 0.541 | 0.004 * | |
| Dopamine | 0.299 | 0.147 | |
| TLG | Adrenaline | 0.296 | 0.151 |
| Noradrenaline | 0.557 | 0.004 * | |
| Dopamine | 0.336 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenaka, J.; Watanabe, S.; Abe, T.; Takeuchi, S.; Hirata, K.; Kimura, R.; Ishii, H.; Wakabayashi, N.; Majigsuren, M.; Kudo, K. The Biochemical–Imaging Connection: Urinary Noradrenaline and Fluorodeoxyglucose-Positron Emission Tomography in Unresectable or Metastatic Pheochromocytomas and Paragangliomas. Diagnostics 2025, 15, 1305. https://doi.org/10.3390/diagnostics15111305
Takenaka J, Watanabe S, Abe T, Takeuchi S, Hirata K, Kimura R, Ishii H, Wakabayashi N, Majigsuren M, Kudo K. The Biochemical–Imaging Connection: Urinary Noradrenaline and Fluorodeoxyglucose-Positron Emission Tomography in Unresectable or Metastatic Pheochromocytomas and Paragangliomas. Diagnostics. 2025; 15(11):1305. https://doi.org/10.3390/diagnostics15111305
Chicago/Turabian StyleTakenaka, Junki, Shiro Watanabe, Takashige Abe, Satoshi Takeuchi, Kenji Hirata, Rina Kimura, Hiroshi Ishii, Naoto Wakabayashi, Mungunkhuyag Majigsuren, and Kohsuke Kudo. 2025. "The Biochemical–Imaging Connection: Urinary Noradrenaline and Fluorodeoxyglucose-Positron Emission Tomography in Unresectable or Metastatic Pheochromocytomas and Paragangliomas" Diagnostics 15, no. 11: 1305. https://doi.org/10.3390/diagnostics15111305
APA StyleTakenaka, J., Watanabe, S., Abe, T., Takeuchi, S., Hirata, K., Kimura, R., Ishii, H., Wakabayashi, N., Majigsuren, M., & Kudo, K. (2025). The Biochemical–Imaging Connection: Urinary Noradrenaline and Fluorodeoxyglucose-Positron Emission Tomography in Unresectable or Metastatic Pheochromocytomas and Paragangliomas. Diagnostics, 15(11), 1305. https://doi.org/10.3390/diagnostics15111305






