Comparative Evaluation of Four Different Anti-CCP Assays for the Diagnosis of Rheumatoid Arthritis: A Diagnostic Performance Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- The first group of 100 patients (85 women and 15 men, mean age 51.3 ± 14.2 years, range 19–88) with a diagnosis of RA according to the 2010 ACR/EULAR classification criteria [2].
- The second group of 99 patients (80 women and 19 men, mean age 40.8 ± 18.5 years, range 4–80), for whom anti-CCP testing was requested by a physician, was ultimately diagnosed as not having RA after further workups (the control group).
2.2. Anti-CCP Assays
2.3. Statistical Analysis
3. Results
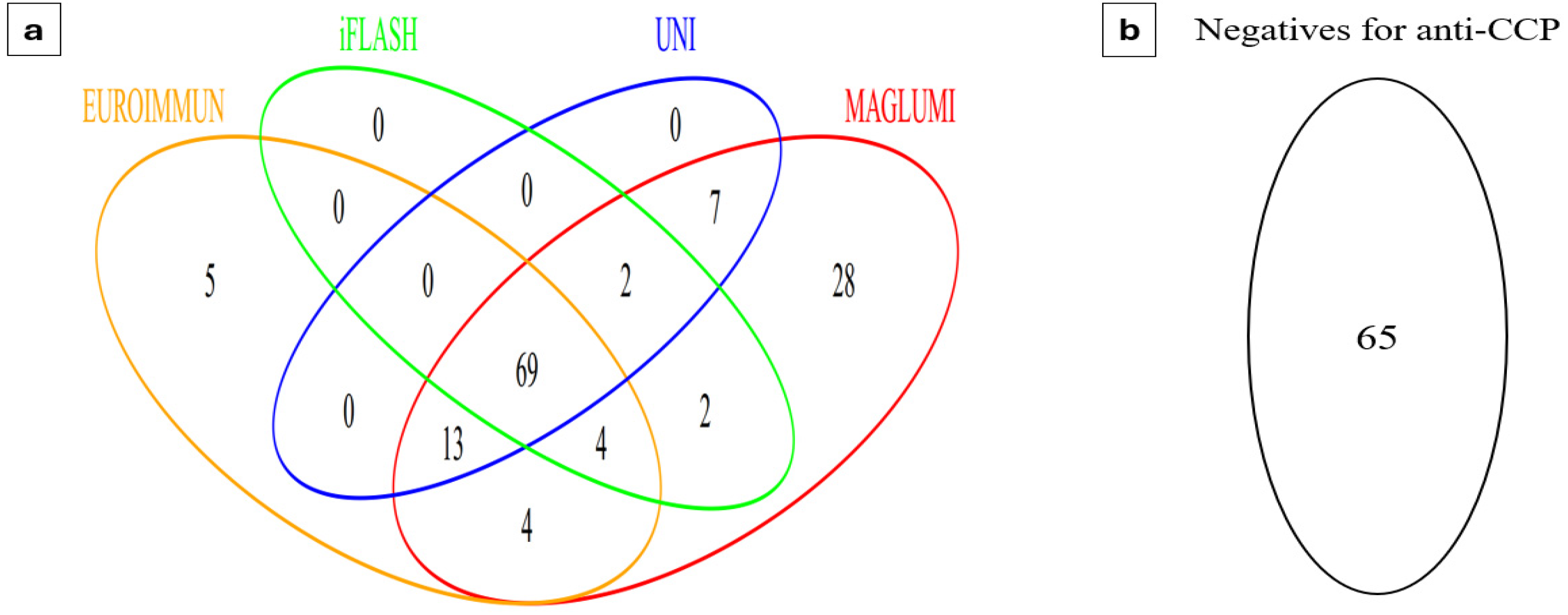
3.1. Prevalence of Anti-CCP Antibodies According to Each Assay
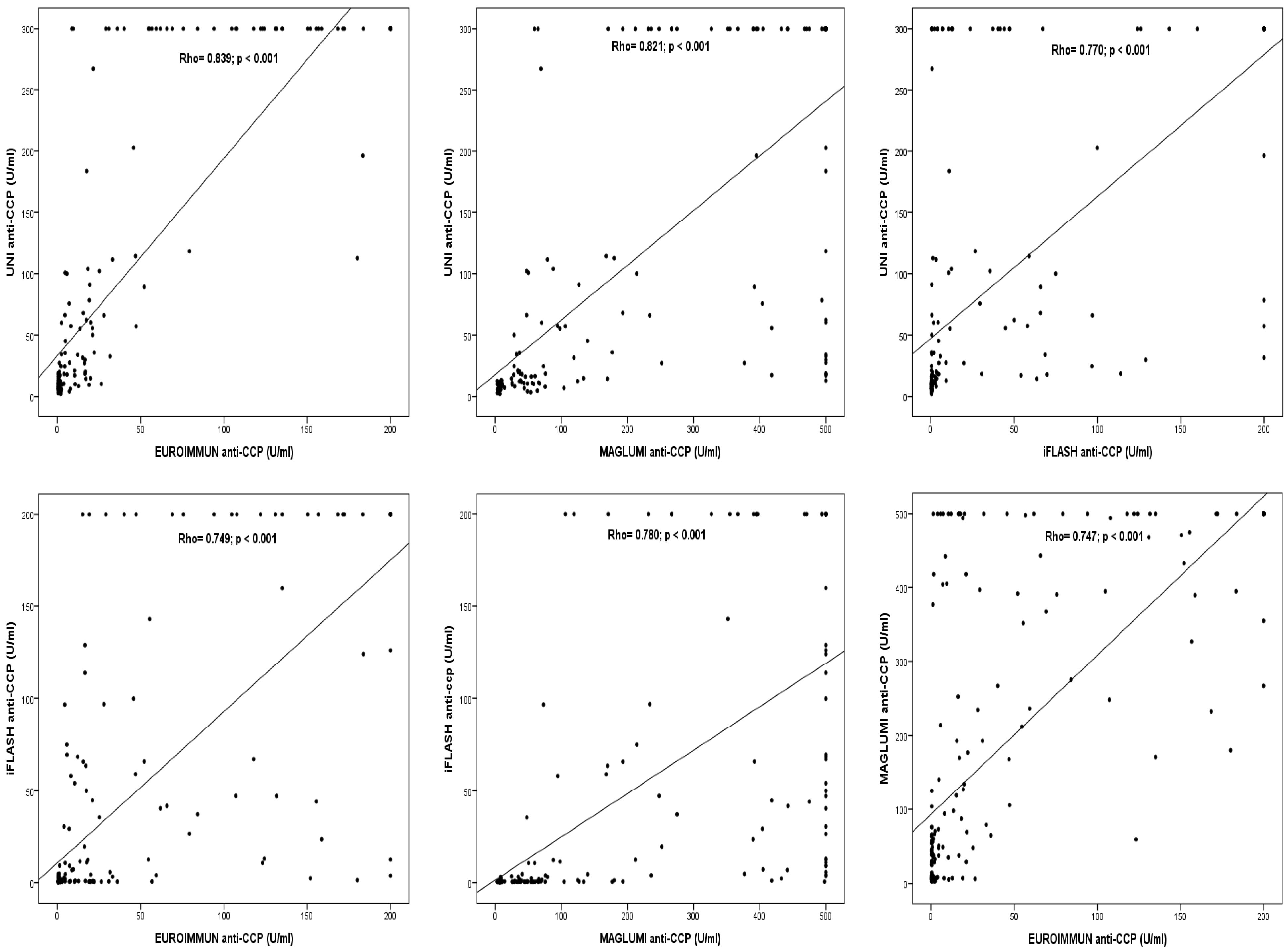
3.2. Qualitative and Quantitative Agreement Between the Results of the Four Immunoassays
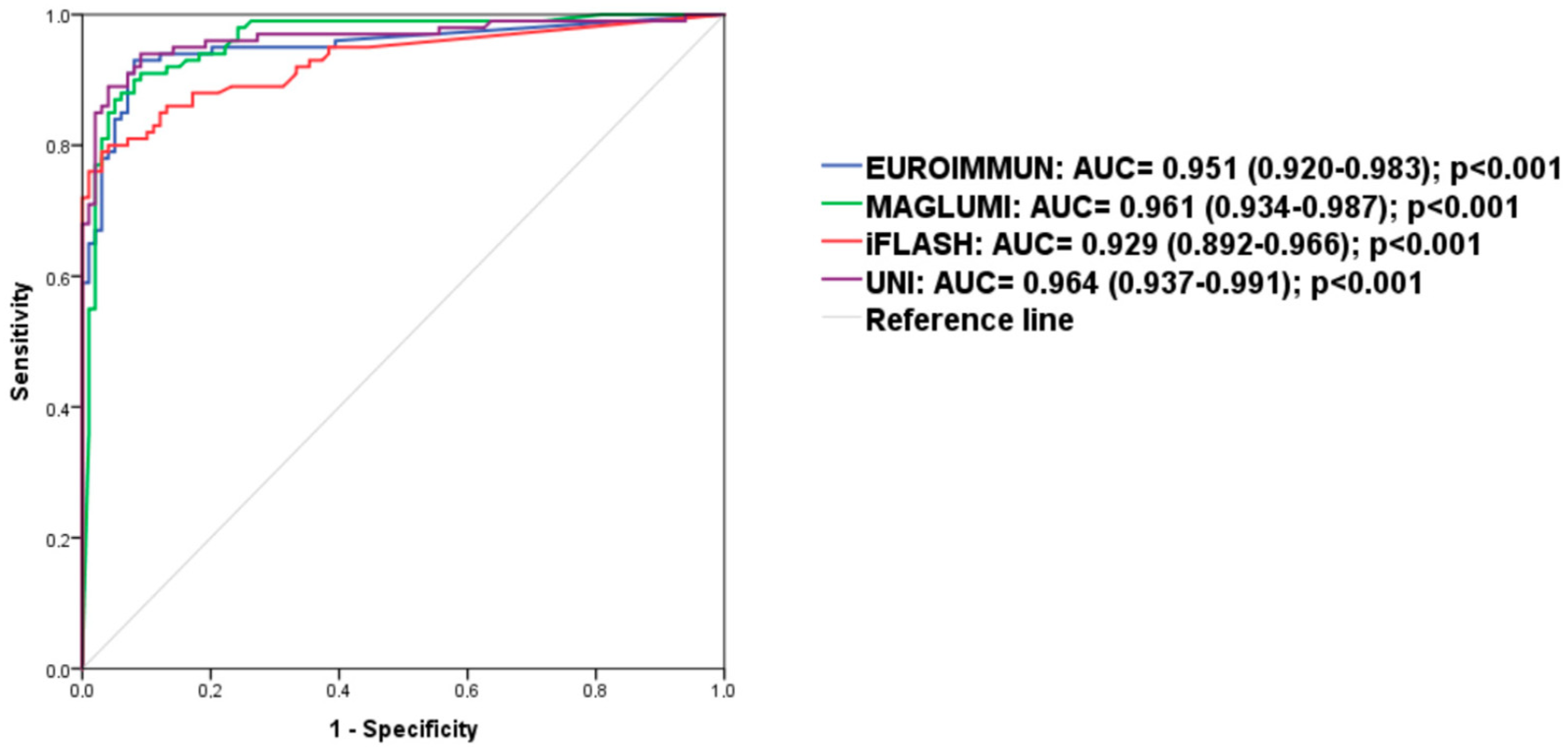
3.3. Performance of the Four Immunoassays for Diagnosis of Patients with RA in Our Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Nishimura, K.; Sugiyama, D.; Kogata, Y.; Tsuji, G.; Nakazawa, T.; Kawano, S.; Saigo, K.; Morinobu, A.; Koshiba, M.; Kuntz, K.M.; et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann. Intern. Med. 2007, 146, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J.; Wu, Z.; Mi, L.; Li, N.; Wang, Y.; Peng, X.; Xu, K.; Wu, F.; Zhang, L. Anti-citrullinated Protein Antibody Generation, Pathogenesis, Clinical Application, and Prospects. Front. Med. 2022, 8, 802934. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.F.; Redwan, E.M. Advances in the diagnosis of autoimmune diseases based on citrullinated peptides/proteins. Expert. Rev. Mol. Diagn. 2021, 21, 685–702. [Google Scholar] [CrossRef]
- Rahali, F.Z.; Tarmidi, M.; Hazime, R.; Admou, B. Clinical significance of anti-cyclic citrullinated peptide (anti-CCP) antibodies in rheumatoid arthritis: Literature review. SN Compr. Clin. Med. 2023, 5, 272. [Google Scholar] [CrossRef]
- Lutteri, L.; Malaise, M.; Chapelle, J.P. Comparison of second- and third-generation anti-cyclic citrullinated peptide antibodies assays for detecting rheumatoid arthritis. Clin. Chim. Acta Int. J. Clin. Chem. 2007, 386, 76–81. [Google Scholar] [CrossRef]
- Correia, M.L.; Carvalho, S.; Fortuna, J.; Pereira, M.H. Comparison of three anti-CCP antibody tests and rheumatoid factor in RA and control patients. Clin. Rev. Allergy Immunol. 2008, 34, 21–25. [Google Scholar] [CrossRef]
- Shidara, K.; Inoue, E.; Tanaka, E.; Hoshi, D.; Seto, Y.; Nakajima, A.; Momohara, S.; Taniguchi, A.; Yamanaka, H. Comparison of the second and third generation anti-cyclic citrullinated peptide antibody assays in the diagnosis of Japanese patients with rheumatoid arthritis. Rheumatol. Int. 2011, 31, 617–622. [Google Scholar] [CrossRef]
- Ji, M.; Hur, M.; Moon, H.W.; Park, M.; Yun, Y.M.; Lee, S.H. Comparison of second- and third-generation immunoassays for detection of anti-cyclic citrullinated peptide antibodies. Scand. J. Clin. Lab. Investig. 2018, 78, 477–482. [Google Scholar] [CrossRef]
- Cho, J.; Pyo, J.Y.; Fadriquela, A.; Uh, Y.; Lee, J.H. Comparison of the analytical and clinical performances of four anti-cyclic citrullinated peptide antibody assays for diagnosing rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Debaugnies, F.; Servais, G.; Badot, V.; Noubouossie, D.; Willems, D.; Corazza, F. Anti-cyclic citrullinated peptide antibodies: A comparison of different assays for the diagnosis of rheumatoid arthritis. Scand. J. Rheumatol. 2013, 42, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Malher, M.; Bentow, C.; Albesa, R.; Cesana, L.; Martinez-Prat, L.; Roux-Lombard, P.; Nissen, M.; Lamacchia, C.; Gabay, C. P024 Comparison of CCP2 and CCP3 assays in a large cohort of established rheumatoid arthritis and controls. Ann. Rheum. Dis. 2018, 77 (Suppl. 1), A24. [Google Scholar] [CrossRef]
- Bizzaro, N.; Tampoia, M. Diagnostic Accuracy of Immunoassays for the Detection of Antibodies to Citrullinated Proteins. Clin. Rev. Allergy Immunol. 2008, 34, 16–20. [Google Scholar] [CrossRef]
- Tanaka, R.; Takemura, M.; Sato, M.; Yamada, Y.; Nakagawa, T.; Horibe, T.; Hoshi, M.; Otaki, H.; Ito, H.; Seishima, M.; et al. Comparison of chemiluminescence enzyme immunoassay (CLEIA) with ELISA for the determination of anti-cyclic citrullinated peptide antibodies. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.; Lakos, G.; Swart, A.; Gürtler, I.; Favalli, E.G.; Schioppo, T.; Mahler, M. Clinical evaluation of a novel chemiluminescent immunoassay for the detection of anti-citrullinated peptide antibodies. Clin. Chim. Acta Int. J. Clin. Chem. 2014, 437, 161–167. [Google Scholar] [CrossRef]
- Vos, I.; Van Mol, C.; Trouw, L.A.; Mahler, M.; Bakker, J.A.; Van Offel, J.; De Clerck, L.; Huizinga, T.W. Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): Diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays. Clin. Rheumatol. 2017, 36, 1487–1492. [Google Scholar] [CrossRef]
- Ma, L.; Wang, W.; Li, L.; Chen, Y.; Chen, B.; Shao, M.; Cheng, Y.; Zhou, R. Comparison of different assays for the detection of anticyclic citrullinated peptide antibodies in patients with rheumatoid arthritis. Front. Immunol. 2022, 13, 940713. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- International Union of Immunological Societies (IUIS)/Antibody Standardization Committee (ACS). Reference Reagent for Human Reference Serum for Citrullinated Peptide/Protein Antibodies (ACPA). Available online: https://asc.dental.ufl.edu/reference-sera/ (accessed on 26 August 2024).
- Rönnelid, J.; Turesson, C.; Kastbom, A. Autoantibodies in Rheumatoid Arthritis—Laboratory and Clinical Perspectives. Front. Immunol. 2021, 12, 685312. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Fontana, D.E.; Bizzaro, N. Chemiluminescent immunoassay technology: What does it change in autoantibody detection? Auto-Immun. Highlights 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, P. Diagnostic accuracy measures. Cerebrovasc. Dis. 2013, 36, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding diagnostic tests 1: Sensitivity, specificity and predictive values. Acta Paediatr. 2007, 96, 338–341. [Google Scholar] [CrossRef]
- Schellekens, G.A.; de Jong, B.A.; van den Hoogen, F.H.; van de Putte, L.B.; van Venrooij, W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998, 101, 273–281. [Google Scholar] [CrossRef]
- Ru, Z.; Zhang, H.; Huang, X.; Lou, J.; Liao, J.; Chen, Z.; Yang, X. A new pattern of citrullinated peptides improves the sensitivity for diagnosing rheumatoid arthritis. Clin. Biochem. 2022, 105–106, 87–93. [Google Scholar] [CrossRef]
- Catrina, A.; Krishnamurthy, A.; Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 2021, 7, e001228. [Google Scholar] [CrossRef]
| EUROIMMUN | MAGLUMI X3 | iFlash 1800 | UNI | |
|---|---|---|---|---|
| Manufacturer | EUROIMMUN | Snibe | YHLO | NeoMedica |
| Principal of assay | ELISA | CLIA | CLIA | EIA |
| Citrullinated antigen | Second-generation synthetic CCPs | Second-generation synthetic CCPs | Second-generation synthetic CCPs | Second-generation synthetic CCPs |
| Range detection (U/mL) | 0.3–200 | 0.783–500 | 0.5–200 | 0.5–300 |
| Conjugate | Peroxidase-labelled anti-human IgG | ABEI labeled anti-human IgG | Acridinium-ester-labeled anti-human IgG | HRP-labelled anti-human IgG |
| Interpretation of the results (U/mL) | ≤5 negative >5 positive | <17 negative ≥17 positive | >5 negative ≥5 positive | >18 negative 18–22 borderline >22 positive |
| Diagnostic Group | Number (%) of Positive Results | |||
|---|---|---|---|---|
| EUROIMMUN | MAGLUMI | iFlash | UNI | |
| RA (N = 100) | 88 (88%) | 99 (99%) | 76 (76%) | 87 (87%) |
| Non-RA (N = 99) | 7 (07.07%) | 30 (30.30%) | 1 (01.01%) | 4 (04.04%) |
| EUROIMMUN | MAGLUMI | iFlash | |
|---|---|---|---|
UNI
|
95% CI [0.690–0.866] |
95% CI [0.530–0.726] |
95% CI [0.640–0.828] |
iFlash
|
95% CI [0.644–0.828] |
95% CI [0.410–0.610] | |
MAGLUMI
|
95% CI [0.456–0.672] |
| EUROIMMUN | MAGLUMI | iFlash | UNI | |
|---|---|---|---|---|
Manufacturer’s cut-off (U/mL)
| 5 88 (83.4–92.5) 92.9 (89.3–96.5) 12.39 0.13 | 17 99 (97.6–99.9) 69.7 (63.3–76.1) 3.27 0.01 | 5 76 (70.1–81.9) 99 (97.6–99.9) 76 0.24 | 22 87 (82.3–91.7) 96 (93.3–98.7) 21.75 0.14 |
3× Manufacturer’s cut-off (U/mL)
| 15 76 (70.1–81.9) 97 (94.6–99.4) 25.3 0.25 | 51 91 (87–95) 86.9 (82.2–91.6) 6.9 0.10 | 15 64 (57.3–70.7) 100 / 0.36 | 66 68 (61.5–74.5) 100 / 0.32 |
Optimal cut-off (U/mL)
| 4 93 (89.5–96.5) 91.9 (88.1–95.7) 11.5 0.07 | 71.7 87 (82.3–91.7) 95 (92–98) 17.4 0.14 | 4 79 (73.3–84.7) 97 (94.6–99.4) 26.3 0.21 | 19.9 89 (84.7–93.4) 96 (93.3–98.7) 22.3 0.11 |
Cut-off at 95% specificity (U/mL)
| 8 84 (78.9–89.1) 16.8 0.17 | 71.7 87 (82.3–91.7) 17.4 0.14 | 3.4 80 (74.4–85.6) 16 0.21 | 19.2 89 (84.7–93.4) 17.8 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamara Mahammed, L.; Hadjout, T.; Bensaci, A.; Hamma, R.; Bousbia, G.; Dahmani, N.; Ismail, H.; Tamechmacht, N.; Djidjik, R. Comparative Evaluation of Four Different Anti-CCP Assays for the Diagnosis of Rheumatoid Arthritis: A Diagnostic Performance Analysis. Diagnostics 2025, 15, 1293. https://doi.org/10.3390/diagnostics15101293
Lamara Mahammed L, Hadjout T, Bensaci A, Hamma R, Bousbia G, Dahmani N, Ismail H, Tamechmacht N, Djidjik R. Comparative Evaluation of Four Different Anti-CCP Assays for the Diagnosis of Rheumatoid Arthritis: A Diagnostic Performance Analysis. Diagnostics. 2025; 15(10):1293. https://doi.org/10.3390/diagnostics15101293
Chicago/Turabian StyleLamara Mahammed, Lydia, Tamazouzt Hadjout, Asma Bensaci, Ryma Hamma, Ghalya Bousbia, Nawel Dahmani, Halima Ismail, Nadia Tamechmacht, and Reda Djidjik. 2025. "Comparative Evaluation of Four Different Anti-CCP Assays for the Diagnosis of Rheumatoid Arthritis: A Diagnostic Performance Analysis" Diagnostics 15, no. 10: 1293. https://doi.org/10.3390/diagnostics15101293
APA StyleLamara Mahammed, L., Hadjout, T., Bensaci, A., Hamma, R., Bousbia, G., Dahmani, N., Ismail, H., Tamechmacht, N., & Djidjik, R. (2025). Comparative Evaluation of Four Different Anti-CCP Assays for the Diagnosis of Rheumatoid Arthritis: A Diagnostic Performance Analysis. Diagnostics, 15(10), 1293. https://doi.org/10.3390/diagnostics15101293







