The Application of Circulating Tumour DNA (ctDNA) in the Diagnosis, Prognosis, and Treatment Monitoring of Gynaecological and Breast Cancers (Review)
Abstract
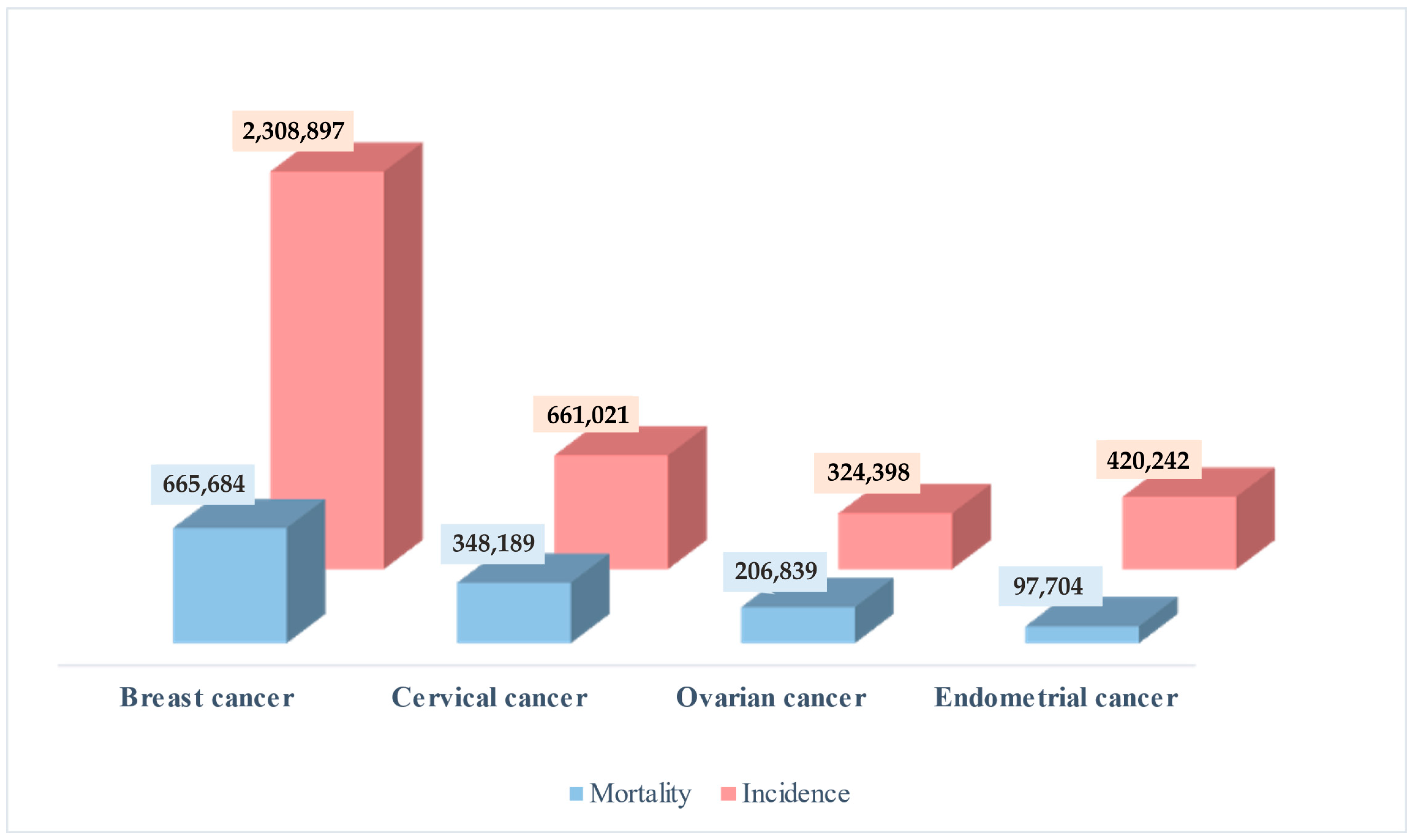
1. Introduction
1.1. Liquid Biopsy
1.2. ctDNA
2. Use of ctDNA as a Biomarker in Gynaecological Cancers and Cancers of the Breast
2.1. Ovarian Cancer (OC)
ctDNA and Ovarian Cancer
2.2. Endometrial Cancer (EC)
ctDNA and Endometrial Cancer
2.3. Cervical Cancer (CC)
ctDNA and Cervical Cancer
2.4. Breast Cancer (BC)
ctDNA and Breast Cancer
| Type of Cancer | Role of ctDNA | Ref. |
|---|---|---|
| Ovarian cancer | Prediction of the presence of microscopic residual disease and recurrence in a patient with OC | [11] |
| Undetectable ctDNA 6 months after initial treatment is related to better PFS and OS | [27] | |
| Association of the presence of ctDNA mutations after surgery with worse PFS and OS | [62] | |
| The presence of ctDNA in the last sample during first-line treatment is associated with rapid progression and shortened OS in HGSC patients | [63] | |
| Monitoring of the efficacy of PARP1 inhibitors and detection of secondary reversion of BRCA1/2 mutations | [64] | |
| Association of the detection of hMLH1 methylation in plasma DNA at relapse with worse OS | [65] | |
| Diagnosis, monitoring response to treatment, and prognosis in EOC | [67] | |
| Endometrial cancer | A potential biomarker for early detection and monitoring of EC relapse | [69] |
| Real-time monitoring of treatment response and earlier identification of relapse in EC and OC | [70] | |
| Evaluation of tumour recurrence in EC (SN 100%, SP 83.3); monitoring of high-risk EC recurrence during postoperative follow-up | [28] | |
| Association between preoperative ctDNA detection and stage and presence of cancer with aggressive tumour characteristics | [71] | |
| Association with more aggressive disease | [72] | |
| Cervical cancer | Presence of HPV ctDNA related to high FIGO stage; association of complete absence of HPV ctDNA before the end of treatment with longer PFS | [74] |
| Detectable HPV ctDNA at the end of chemoradiotherapy is associated with worse PFS | [75] | |
| Presence of HPV ctDNA before chemotherapy is associated with tumour stage; presence of HPV ctDNA after completion of therapy and during the follow-up period is associated with shorter DFS and OS | [76] | |
| HPV ctDNA as a prognostic biomarker for locally advanced CC | [77] | |
| Monitoring of response to therapy; identification of early relapses | [78] | |
| Breast cancer | BM may be a potential new source for liquid biopsy for PPBC detection | [29] |
| Detection of minimal residual disease, early detection of recurrence, monitoring, and treatment planning of advanced disease | [86] | |
| Ability to predict relapse | [88] | |
| Evaluation of the effectiveness of NCT and relapse | [89] | |
| Diagnosis and differential diagnosis of early-stage BC patients | [91] | |
| Potential usefulness of urine DNA in early-stage breast cancer; prediction of disease recurrence | [93] | |
| ESR1 mutation in baseline ctDNA is associated with worse PFS and OS in patients treated with exemestane compared with fulvestrant | [100] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhu, J.W.; Charkhchi, P.; Akbari, M.R. Potential clinical utility of liquid biopsies in ovarian cancer. Mol. Cancer 2022, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.M. Liquid Biopsy, ctDNA Diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.M.; Yekula, A.; Khanna, P.; Hsia, T.; Gamblin, A.S.; Ekanayake, E.; Escobedo, A.K.; You, D.G.; Castro, C.M.; Im, H.; et al. The Liquid Biopsy Consortium: Challenges and opportunities for early cancer detection and monitoring. Cell Rep. Med. 2023, 4, 101198. [Google Scholar] [CrossRef]
- Domínguez-Vigil, I.G.; Moreno-Martínez, A.K.; Wang, J.Y.; Roehrl, M.H.A.; Barrera-Saldaña, H.A. The dawn of the liquid biopsy in the fight against cancer. Oncotarget 2017, 9, 2912–2922. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Freitas, A.J.A.; Causin, R.L.; Varuzza, M.B.; Calfa, S.; Hidalgo Filho, C.M.T.; Komoto, T.T.; Souza, C.P.; Marques, M.M.C. Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 9952. [Google Scholar] [CrossRef]
- Asante, D.B.; Calapre, L.; Ziman, M.; Meniawy, T.M.; Gray, E.S. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett. 2020, 468, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, X.; Pan, Q.; Zhao, B. Liquid biopsy techniques and pancreatic cancer: Diagnosis, monitoring, and evaluation. Mol. Cancer 2023, 22, 167. [Google Scholar] [CrossRef]
- Zhu, J.W.; Wong, F.; Szymiczek, A.; Ene, G.E.V.; Zhang, S.; May, T.; Narod, S.A.; Kotsopoulos, J.; Akbari, M.R. Evaluating the Utility of ctDNA in Detecting Residual Cancer and Predicting Recurrence in Patients with Serous Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14388. [Google Scholar] [CrossRef] [PubMed]
- Mari, R.; Mamessier, E.; Lambaudie, E.; Provansal, M.; Birnbaum, D.; Bertucci, F.; Sabatier, R. Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease. Cancers 2019, 11, 774. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Pomerantz, T.; Brooks, R. Circulating Tumor DNA (ctDNA) and Its Role in Gynecologic Malignancies. Curr. Treat. Options Oncol. 2024, 25, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Su, L.; Qian, C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Gonzalez, N.S.; Pimentel, I.; Suñol, A.; Zamora, E.; Ortiz, C.; Espinosa-Bravo, M.; Peg, V.; Vivancos, A.; Saura, C.; et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat. Rev. 2022, 104, 102362. [Google Scholar] [CrossRef] [PubMed]
- Panet, F.; Papakonstantinou, A.; Borrell, M.; Vivancos, J.; Vivancos, A.; Oliveira, M. Use of ctDNA in early breast cancer: Analytical validity and clinical potential. NPJ Breast Cancer 2024, 10, 50. [Google Scholar] [CrossRef]
- Duffy, M.J. Circulating tumor DNA (ctDNA) as a biomarker for lung cancer: Early detection, monitoring and therapy prediction. Tumour Biol. 2024, 46, S283–S295. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef]
- Fonseca, N.M.; Maurice-Dror, C.; Herberts, C.; Tu, W.; Fan, W.; Murtha, A.J.; Kollmannsberger, C.; Kwan, E.M.; Parekh, K.; Schönlau, E.; et al. Prediction of plasma ctDNA fraction and prognostic implications of liquid biopsy in advanced prostate cancer. Nat. Commun. 2024, 15, 1828. [Google Scholar] [CrossRef]
- Helal, C.; Pobel, C.; Bayle, A.; Vasseur, D.; Nicotra, C.; Blanc-Durand, F.; Naoun, N.; Bernard-Tessier, A.; Patrikidou, A.; Colomba, E.; et al. Clinical utility of plasma ctDNA sequencing in metastatic urothelial cancer. Eur. J. Cancer 2023, 195, 113368. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J.A.; Alig, S.K.; Esfahani, M.S.; Lauer, E.M.; Mitschke, J.; Kurtz, D.M.; Kühn, J.; Bleul, S.; Olsen, M.; Liu, C.L.; et al. Circulating Tumor DNA Profiling for Detection, Risk Stratification, and Classification of Brain Lymphomas. J. Clin. Oncol. 2023, 41, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Antonouli, S.; Di Nisio, V.; Daponte, N.; Daponte, A.I.; Daponte, A. Cervical Cancer Genetic Profile through Circulating Tumor DNA: What Can We Learn from Blood? Biomolecules 2024, 14, 825. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Wahab, M.R.A.; Palaniyandi, T.; Ravi, M.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Rajendran, B.K. Biomarkers and biosensors for early cancer diagnosis, monitoring and prognosis. Pathol. Res. Pract. 2023, 250, 154812. [Google Scholar] [CrossRef]
- Pereira, E.; Camacho-Vanegas, O.; Anand, S.; Sebra, R.; Catalina Camacho, S.; Garnar-Wortzel, L.; Nair, N.; Moshier, E.; Wooten, M.; Uzilov, A.; et al. Limarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers. PLoS ONE 2015, 10, e0145754. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Jia, N.; Jiao, H.; Chen, J.; Chen, Y.; Zhang, Y.; Zhu, M.; Zhu, C.; Shen, L.; Long, W. Circulating tumor DNA as a prognostic marker in high-risk endometrial cancer. J. Transl. Med. 2021, 19, 51. [Google Scholar] [CrossRef]
- Saura, C.; Ortiz, C.; Matito, J.; Arenas, E.J.; Suñol, A.; Martín, Á.; Córdoba, O.; Martínez-Sabadell, A.; García-Ruiz, I.; Miranda, I.; et al. Early-Stage Breast Cancer Detection in Breast Milk. Cancer Discov. 2023, 13, 2180–2191. [Google Scholar] [CrossRef]
- Gu, Y.; Wan, C.; Qiu, J.; Cui, Y.; Jiang, T.; Zhuang, Z. Circulating HPV cDNA in the blood as a reliable biomarker for cervical cancer: A meta-analysis. PLoS ONE 2020, 15, e0224001. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Xiao, M.; Zhao, J.; Du, S.; Wang, Z.; Hu, S.; Yang, L.; Cai, J. PBRM1 presents a potential ctDNA marker to monitor response to neoadjuvant chemotherapy in cervical cancer. iScience 2024, 27, 109160. [Google Scholar] [CrossRef] [PubMed]
- Sowamber, R.; Lukey, A.; Huntsman, D.; Hanley, G. Ovarian Cancer: From Precursor Lesion Identification to Population-Based Prevention Programs. Curr. Oncol. 2023, 30, 10179–10194. [Google Scholar] [CrossRef]
- De Leo, A.; Santini, D.; Ceccarelli, C.; Santandrea, G.; Palicelli, A.; Acquaviva, G.; Chiarucci, F.; Rosini, F.; Ravegnini, G.; Pession, A.; et al. What Is New on Ovarian Carcinoma: Integrated Morphologic and Molecular Analysis Following the New 2020 World Health Organization Classification of Female Genital Tumors. Diagnostics 2021, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Hanley, G.E.; Pearce, C.L.; Talhouk, A.; Kwon, J.S.; Finlayson, S.J.; McAlpine, J.N.; Huntsman, D.G.; Miller, D. Outcomes From Opportunistic Salpingectomy for Ovarian Cancer Prevention. JAMA Netw. Open 2022, 5, e2147343. [Google Scholar] [CrossRef]
- Shih, I.-M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. Pathogenesis of ovarian cancer: Lessons from morphology and molecular biology and their clinical implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160. [Google Scholar] [CrossRef]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Bandera, E.V.; Lee, V.S.; Rodriguez-Rodriguez, L.; Powell, C.B.; Kushi, L.H. Racial/Ethnic Disparities in Ovarian Cancer Treatment and Survival. Clin. Cancer Res. 2016, 22, 5909–5914. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Lee, A.W.; Ness, R.B.; Roman, L.D.; Terry, K.L.; Schildkraut, J.M.; Chang-Claude, J.; Doherty, J.A.; Menon, U.; Cramer, D.W.; Gayther, S.A.; et al. Association Between Menopausal Estrogen-Only Therapy and Ovarian Carcinoma Risk. Obstet. Gynecol. 2016, 127, 828–836. [Google Scholar] [CrossRef]
- Hempling, R.E.; Wong, C.; Piver, M.S.; Natarajan, N.; Mettlin, C.J. Hormone replacement therapy as a risk factor for epithelial ovarian cancer: Results of a case-control study. Obstet. Gynecol. 1997, 89, 1012–1016. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Lubinski, J.; Neuhausen, S.L.; Lynch, H.T.; Rosen, B.; Ainsworth, P.; Moller, P.; Ghadirian, P.; Isaacs, C.; Karlan, B.; et al. Hormone replacement therapy and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Gynecol. Oncol. 2006, 100, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gaitskell, K.; Green, J.; Pirie, K.; Barnes, I.; Hermon, C.; Reeves, G.K.; Beral, V.; Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int. J. Cancer 2018, 142, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Shanmughapriya, S.; Senthilkumar, G.; Arun, S.; Das, B.C.; Natarajaseenivasan, K. Risk Factors for Epithelial Ovarian Carcinoma in India: A Case Control Study in Low-Incidence Population. Int. J. Cancer Res. 2016, 12, 61–68. [Google Scholar] [CrossRef]
- Faber, M.T.; Kjær, S.K.; Dehlendorff, C.; Chang-Claude, J.; Andersen, K.K.; Høgdall, E.; Webb, P.M.; Jordan, S.J.; Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer Study Group; et al. Cigarette smoking and risk of ovarian cancer: A pooled analysis of 21 case-control studies. Cancer Causes Control 2013, 24, 989–1004. [Google Scholar] [CrossRef]
- Ali, A.T.; Al-Ani, O.; Al-Ani, F. Epidemiology and risk factors for ovarian cancer. Przegląd Menopauzalny 2023, 22, 93–104. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Atallah, G.A.; Abd Aziz, N.H.; Teik, C.K.; Shafiee, M.N.; Kampan, N.C. New Predictive Biomarkers for Ovarian Cancer. Diagnostics 2021, 11, 465. [Google Scholar] [CrossRef]
- Scaletta, G.; Plotti, F.; Luvero, D.; Capriglione, S.; Montera, R.; Miranda, A.; Lopez, S.; Terranova, C.; De Cicco Nardone, C.; Angioli, R. The role of novel biomarker HE4 in the diagnosis, prognosis and follow-up of ovarian cancer: A systematic review. Expert Rev. Anticancer Ther. 2017, 17, 827–839. [Google Scholar] [CrossRef]
- Ghafoor, A.; Thomas, A.; Hassan, R. Targeting mesothelin in ovarian cancer. Oncotarget 2018, 9, 36050–36051. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, H.; Liu, H.Q.; Hao, M. Correlation between ovarian neoplasm and serum levels of osteopontin: A meta-analysis. Tumour Biol. 2014, 35, 11799–11808. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, S.; Li, L.; Liu, X.; Liu, X.; Dai, S.; Zhang, P.; Lu, H.; Lin, Z.; Yu, Y.; et al. Evaluation of HE4 and TTR for diagnosis of ovarian cancer: Comparison with CA-125. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 227–230. [Google Scholar] [CrossRef]
- Kumari, S. Serum Biomarker Based Algorithms in Diagnosis of Ovarian Cancer: A Review. Indian J. Clin. Biochem. 2018, 33, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Shimada, C.; Xu, R.; Al-Alem, L.; Stasenko, M.; Spriggs, D.R.; Rueda, B.R. Galectins and Ovarian Cancer. Cancers 2020, 12, 1421. [Google Scholar] [CrossRef]
- Kukla, A.; Piotrowska, K.; Misiek, M.; Chudecka-Glaz, A.M. Role of adipokines in ovarian cancer epidemiology and prognosis. Ginekol. Pol. 2022, 93, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, J.R.; Wilczyński, M.; Paradowska, E. Cancer Stem Cells in Ovarian Cancer—A Source of Tumor Success and a Challenging Target for Novel Therapies. Int. J. Mol. Sci. 2022, 23, 2496. [Google Scholar] [CrossRef]
- Duan, Y.; Cui, C.; Qiu, C.; Sun, G.; Wang, X.; Wang, P.; Ye, H.; Dai, L.; Shi, J. Serum Autoantibodies against LRDD, STC1, and FOXA1 as Biomarkers in the Detection of Ovarian Cancer. Dis. Markers 2022, 2022, 6657820. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. miRNA profile in ovarian cancer. Exp. Mol. Pathol. 2020, 113, 104381. [Google Scholar] [CrossRef]
- Golara, A.; Kozłowski, M.; Cymbaluk-Płoska, A. The Role of Circulating Tumor DNA in Ovarian Cancer. Cancers 2024, 16, 3117. [Google Scholar] [CrossRef]
- Yurkovetsky, Z.; Skates, S.; Lomakin, A.; Nolen, B.; Pulsipher, T.; Modugno, F.; Marks, J.; Godwin, A.; Gorelik, E.; Jacobs, I.; et al. Development of a multimarker assay for early detection of ovarian cancer. J. Clin. Oncol. 2010, 28, 2159–2166. [Google Scholar] [CrossRef]
- McIntosh, M.W.; Drescher, C.; Karlan, B.; Scholler, N.; Urban, N.; Hellstrom, K.E.; Hellstrom, I. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol. Oncol. 2004, 95, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chen, S.J.; Chen, H.C.; Tan, K.T.; Hsiao, W.; Jung, S.M.; Yang, L.Y.; Huang, K.G.; Chou, H.H.; Huang, H.J.; et al. Mutations in circulating tumor DNA detected in the postoperative period predict poor survival in patients with ovarian cancer. Biomed. J. 2023, 46, 100563. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.M.; Savolainen, K.; Virtanen, T.; Ryyppö, L.; Selin, H.; Martikainen, P.; Staff, S.; Kivinummi, K.; Sipola, J.; Vuorinen, J.; et al. Sensitive circulating tumor DNA–based residual disease detection in epithelial ovarian cancer. Life Sci. Alliance 2024, 7, e202402658. [Google Scholar] [CrossRef] [PubMed]
- Ratajska, M.; Koczkowska, M.; Żuk, M.; Gorczyński, A.; Kuźniacka, A.; Stukan, M.; Biernat, W.; Limon, J.; Wasąg, B. Detection of BRCA1/2 mutations in circulating tumor DNA from patients with ovarian cancer. Oncotarget 2017, 8, 101325–101332. [Google Scholar] [CrossRef]
- Gifford, G.; Paul, J.; Vasey, P.A.; Kaye, S.B.; Brown, R. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin. Cancer Res. 2004, 10, 4420–4426. [Google Scholar] [CrossRef]
- Hou, J.Y.; Chapman, J.S.; Kalashnikova, E.; Pierson, W.; Smith-McCune, K.; Pineda, G.; Vattakalam, R.M.; Ross, A.; Mills, M.; Suarez, C.J.; et al. Circulating tumor DNA monitoring for early recurrence detection in epithelial ovarian cancer. Gynecol. Oncol. 2022, 167, 334–341. [Google Scholar] [CrossRef]
- Thusgaard, C.F.; Korsholm, M.; Koldby, K.M.; Kruse, T.A.; Thomassen, M.; Jochumsen, K.M. Epithelial ovarian cancer and the use of circulating tumor DNA: A systematic review. Gynecol. Oncol. 2021, 161, 884–895. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef]
- Moss, E.L.; Gorsia, D.N.; Collins, A.; Sandhu, P.; Foreman, N.; Gore, A.; Wood, J.; Kent, C.; Silcock, L.; Guttery, D.S. Utility of Circulating Tumor DNA for Detection and Monitoring of Endometrial Cancer Recurrence and Progression. Cancers 2020, 12, 2231. [Google Scholar] [CrossRef]
- Jamieson, A.; McConechy, M.K.; Lum, A.; Senz, J.; Dowhy, T.; Huntsman, D.G.; McAlpine, J.N. Selective utilization of circulating tumor DNA testing enables disease monitoring in endometrial and ovarian carcinomas. J. Gynecol. Oncol. 2025, 36, e5. [Google Scholar] [CrossRef]
- Grassi, T.; Harris, F.R.; Smadbeck, J.B.; Murphy, S.J.; Block, M.S.; Multinu, F.; Schaefer Klein, J.L.; Zhang, P.; Karagouga, G.; Liu, M.C.; et al. Personalized tumor-specific DNA junctions to detect circulating tumor in patients with endometrial cancer. PLoS ONE 2021, 16, e0252390. [Google Scholar] [CrossRef] [PubMed]
- Casas-Arozamena, C.; Díaz, E.; Moiola, C.P.; Alonso-Alconada, L.; Ferreirós, A.; Abalo, A.; Gil, C.L.; Oltra, S.S.; de Santiago, J.; Cabrera, S.; et al. Genomic Profiling of Uterine Aspirates and cfDNA as an Integrative Liquid Biopsy Strategy in Endometrial Cancer. J. Clin. Med. 2020, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tong, Y.; Wu, J.; Xu, X. Clinical applications and utility of ctDNA in cervical cancer and its precursor lesions: From screening to predictive biomarker. Cancer Cell Int. 2023, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, E.; Latouche, A.; Bonneau, C.; Calméjane, M.A.; Beaufort, C.; Ruigrok-Ritstier, K.; Bataillon, G.; Larbi Chérif, L.; Dupain, C.; Lecerf, C.; et al. Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer. Clin. Cancer Res. 2021, 27, 5869–5877. [Google Scholar] [CrossRef]
- Han, K.; Zou, J.; Zhao, Z.; Baskurt, Z.; Zheng, Y.; Barnes, E.; Croke, J.; Ferguson, S.E.; Fyles, A.; Gien, L.; et al. Clinical Validation of Human Papilloma Virus Circulating Tumor DNA for Early Detection of Residual Disease After Chemoradiation in Cervical Cancer. J. Clin. Oncol. 2024, 42, 431–440. [Google Scholar] [CrossRef]
- Cabel, L.; Bonneau, C.; Bernard-Tessier, A.; Héquet, D.; Tran-Perennou, C.; Bataillon, G.; Rouzier, R.; Féron, J.G.; Fourchotte, V.; Le Brun, J.F.; et al. HPV ctDNA detection of high-risk HPV types during chemoradiotherapy for locally advanced cervical cancer. ESMO Open 2021, 6, 100154. [Google Scholar] [CrossRef]
- Sivars, L.; Hellman, K.; Crona Guterstam, Y.; Holzhauser, S.; Nordenskjöld, M.; Falconer, H.; Palsdottir, K.; Tham, E. Circulating cell-free tumor human papillomavirus DNA is a promising biomarker in cervical cancer. Gynecol. Oncol. 2022, 167, 107–114. [Google Scholar] [CrossRef]
- Bellone, S.; McNamara, B.; Mutlu, L.; Demirkiran, C.; Hartwich, T.M.P.; Harold, J.; Yang-Hartwich, Y.; Siegel, E.R.; Santin, A.D. Monitoring Treatment Response, Early Recurrence, and Survival in Uterine Serous Carcinoma and Carcinosarcoma Patients Using Personalized Circulating Tumor DNA Biomarkers. Int. J. Mol. Sci. 2023, 24, 8873. [Google Scholar] [CrossRef]
- Tzanikou, E.; Lianidou, E. The potential of ctDNA analysis in breast cancer. Crit. Rev. Clin. Lab. Sci. 2020, 57, 54–72. [Google Scholar] [CrossRef]
- Pesapane, F.; Suter, M.B.; Rotili, A.; Penco, S.; Nigro, O.; Cremonesi, M.; Bellomi, M.; Jereczek-Fossa, B.A.; Pinotti, G.; Cassano, E. Will traditional biopsy be substituted by radiomics and liquid biopsy for breast cancer diagnosis and characterisation? Med. Oncol. 2020, 37, 29. [Google Scholar] [CrossRef]
- Beňačka, R.; Szabóová, D.; Guľašová, Z.; Hertelyová, Z.; Radoňák, J. Classic and New Markers in Diagnostics and Classification of Breast Cancer. Cancers 2022, 14, 5444. [Google Scholar] [CrossRef]
- Seale, K.N.; Tkaczuk, K.H.R. Circulating Biomarkers in Breast Cancer. Clin. Breast Cancer 2022, 22, e319–e331. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, P.M.M.; Jucoski, T.S.; Vieira, E.; Carvalho, T.M.; Malheiros, D.; Ribeiro, E.M.S.F. Liquid biopsy for breast cancer using extracellular vesicles and cell-free microRNAs as biomarkers. Transl. Res. 2020, 223, 40–60. [Google Scholar] [CrossRef]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid biopsy in breast cancer: A comprehensive review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Y.; Ma, Z.; Ju, J.; Wei, T.; Gao, S.; Kang, Y.; Yang, Z.; Wang, X.; Yue, J.; Yuan, P. Liquid-based biomarkers in breast cancer: Looking beyond the blood. J. Transl. Med. 2023, 21, 809. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Bernat-Peguera, A.; Felip, E.; Margelí, M. Role of ctDNA in Breast Cancer. Cancers 2022, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Su, F.; Joshi, M.; Masuda, N.; Ishikawa, T.; Aruga, T.; Zarate, J.P.; Babbar, N.; Balbin, O.A.; Yap, Y.S. Potential value of ctDNA monitoring in metastatic HR + /HER2 - breast cancer: Longitudinal ctDNA analysis in the phase Ib MONALEESASIA trial. BMC Med. 2023, 21, 306. [Google Scholar] [CrossRef]
- Coombes, R.C.; Page, K.; Salari, R.; Hastings, R.K.; Armstrong, A.; Ahmed, S.; Ali, S.; Cleator, S.; Kenny, L.; Stebbing, J.; et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin. Cancer Res. 2019, 25, 4255–4263. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Wang, C.; Gong, Y.; Zhang, Y.; Yao, R.; Li, P.; Zhu, X.; Bai, J.; Guan, Y.; et al. Serial circulating tumor DNA identification associated with the efficacy and prognosis of neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2021, 188, 661–673. [Google Scholar] [CrossRef]
- Venetis, K.; Pepe, F.; Pescia, C.; Cursano, G.; Criscitiello, C.; Frascarelli, C.; Mane, E.; Russo, G.; Taurelli Salimbeni, B.; Troncone, G.; et al. ESR1 mutations in HR+/HER2-metastatic breast cancer: Enhancing the accuracy of ctDNA testing. Cancer Treat. Rev. 2023, 121, 102642. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Wei, W.; You, Z.; Ou, X.; Sun, M.; Yin, Y.; Tang, X.; Zhao, Z.; Hu, C.; et al. Parallel Analyses of Somatic Mutations in Plasma Circulating Tumor DNA (ctDNA) and Matched Tumor Tissues in Early-Stage Breast Cancer. Clin. Cancer Res. 2019, 25, 6546–6553. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Wang, Y.; Sun, Q.; Wang, L.; Xie, F.; Yan, J.; Huang, H.; Liu, H. Utility of urinary ctDNA to monitoring minimal residual disease in early breast cancer patients. Cancer Biomark. 2020, 28, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, W. Association of urinary and plasma DNA in early breast cancer patients and its links to disease relapse. Clin. Transl. Oncol. 2018, 20, 1053–1060. [Google Scholar] [CrossRef]
- Cullinane, C.; Fleming, C.; O’Leary, D.P.; Hassan, F.; Kelly, L.; O’Sullivan, M.J.; Corrigan, M.A.; Redmond, H.P. Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2026921. [Google Scholar] [CrossRef]
- Siravegna, G.; Geuna, E.; Mussolin, B.; Crisafulli, G.; Bartolini, A.; Galizia, D.; Casorzo, L.; Sarotto, I.; Scaltriti, M.; Sapino, A.; et al. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open 2017, 2, e000253. [Google Scholar] [CrossRef]
- Turner, N.; Huang-Bartlett, C.; Kalinsky, K.; Cristofanilli, M.; Bianchini, G.; Chia, S.; Iwata, H.; Janni, W.; Ma, C.X.; Mayer, E.L.; et al. Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment. Future Oncol. 2023, 19, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Najim, O.; Papadimitriou, K.; Broeckx, G.; Huizing, M.; Tjalma, W. Validation of liquid biopsy for ESR1-mutation analysis in hormone-sensitive breast cancer: A pooled meta-analysis. Front. Oncol. 2023, 13, 1221773. [Google Scholar] [CrossRef]
- Betz, M.; Massard, V.; Gilson, P.; Witz, A.; Dardare, J.; Harlé, A.; Merlin, J.L. ESR1 Gene Mutations and Liquid Biopsy in ER-Positive Breast Cancers: A Small Step Forward, a Giant Leap for Personalization of Endocrine Therapy? Cancers 2023, 15, 5169. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial-PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Kilburn, L.; Fribbens, C.; Beaney, M.; Garcia-Murillas, I.; Budzar, A.U.; Robertson, J.F.R.; Gradishar, W.; Piccart, M.; et al. ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor-Positive Breast Cancer: A Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin. Cancer Res. 2020, 26, 5172–5177. [Google Scholar] [CrossRef]
- FBlitzer, G.C.; Zhao, S.G.; Bradley, K.A.; Hartenbach, E.M. The role of ctDNA in endometrial cancer: A tool for risk stratification and disease monitoring. Gynecol. Oncol. 2023, 178, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Wishart, G.; Templeman, A.; Hendry, F.; Miller, K.; Pailhes-Jimenez, A.S. Molecular Profiling of Circulating Tumour Cells and Circulating Tumour DNA: Complementary Insights from a Single Blood Sample Utilising the Parsortix® System. Curr. Issues Mol. Biol. 2024, 46, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calderón, D.; Pedraza, A.; Mancera Urrego, C.; Mejía-Mejía, A.; Montealegre-Páez, A.L.; Perdomo, S. Analysis of the Cost-Effectiveness of Liquid Biopsy to Determine Treatment Change in Patients with Her2-Positive Advanced Breast Cancer in Colombia. Clin. Outcomes Res. 2020, 12, 115–122. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | cfDNA | ctDNA | Refs. |
|---|---|---|---|
| Molecular structure | Single- and double-stranded DNA that circulates freely in the bloodstream, released from various cells in the body as a result of apoptosis and necrosis [14] | Fragments of single- or double-stranded DNA released into the bloodstream by tumour cells [14]; single- or double-stranded DNA found in plasma or serum [15] | [14,15] |
| Molecular size | About 150–200 bp [14] and 150–180 bp [10] | Shorter than cfDNA [15]; about 146 bp [14]; and 160 to 200 base pairs [11] | [10,11,14,15] |
| Serum level | Healthy subjects: 0–100 ng/mL; cancer patients: 0–5 ng/mL to more than 1000 ng/mL [13] | 0.01% of the total cfDNA detected in the patient’s plasma [14] | [13,14] |
| Half-life | 5–150 min [14] | Approximately 15 min to 2.5 h [10]; 23–52 min after surgical resection of the tumour [14]; and 16 min to 2.5 h [16] | [10,14,16] |
| Type of Cancer | Study Group | Method | SN/SP (%) | Ref. |
|---|---|---|---|---|
| Gynaecological cancers/ovarian cancer | 44 (including 22 with ovarian cancer) | ddPCR | 91/60 | [27] |
| High-risk endometrial cancer (assessment of postoperative tumour recurrence) | 9 | ddPCR | 100/83.3 | [28] |
| Breast cancer Breast milk | 19 (10 diagnosed during pregnancy and 9 diagnosed during breastfeeding) | NGS | 71.4/100 | [29] |
| Cervical cancer | 684 (fifteen studies; meta-analysis) | - | 27/94 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Englisz, A.; Smycz-Kubańska, M.; Królewska-Daszczyńska, P.; Błaut, M.; Duszyc, A.; Mielczarek-Palacz, A. The Application of Circulating Tumour DNA (ctDNA) in the Diagnosis, Prognosis, and Treatment Monitoring of Gynaecological and Breast Cancers (Review). Diagnostics 2025, 15, 1289. https://doi.org/10.3390/diagnostics15101289
Englisz A, Smycz-Kubańska M, Królewska-Daszczyńska P, Błaut M, Duszyc A, Mielczarek-Palacz A. The Application of Circulating Tumour DNA (ctDNA) in the Diagnosis, Prognosis, and Treatment Monitoring of Gynaecological and Breast Cancers (Review). Diagnostics. 2025; 15(10):1289. https://doi.org/10.3390/diagnostics15101289
Chicago/Turabian StyleEnglisz, Aleksandra, Marta Smycz-Kubańska, Patrycja Królewska-Daszczyńska, Magdalena Błaut, Agnieszka Duszyc, and Aleksandra Mielczarek-Palacz. 2025. "The Application of Circulating Tumour DNA (ctDNA) in the Diagnosis, Prognosis, and Treatment Monitoring of Gynaecological and Breast Cancers (Review)" Diagnostics 15, no. 10: 1289. https://doi.org/10.3390/diagnostics15101289
APA StyleEnglisz, A., Smycz-Kubańska, M., Królewska-Daszczyńska, P., Błaut, M., Duszyc, A., & Mielczarek-Palacz, A. (2025). The Application of Circulating Tumour DNA (ctDNA) in the Diagnosis, Prognosis, and Treatment Monitoring of Gynaecological and Breast Cancers (Review). Diagnostics, 15(10), 1289. https://doi.org/10.3390/diagnostics15101289






