Immune Cells as Mediators of Lipidome Influence on Osteoporosis: Evidence from a Mediation Analysis
Abstract
1. Introduction
2. Methods
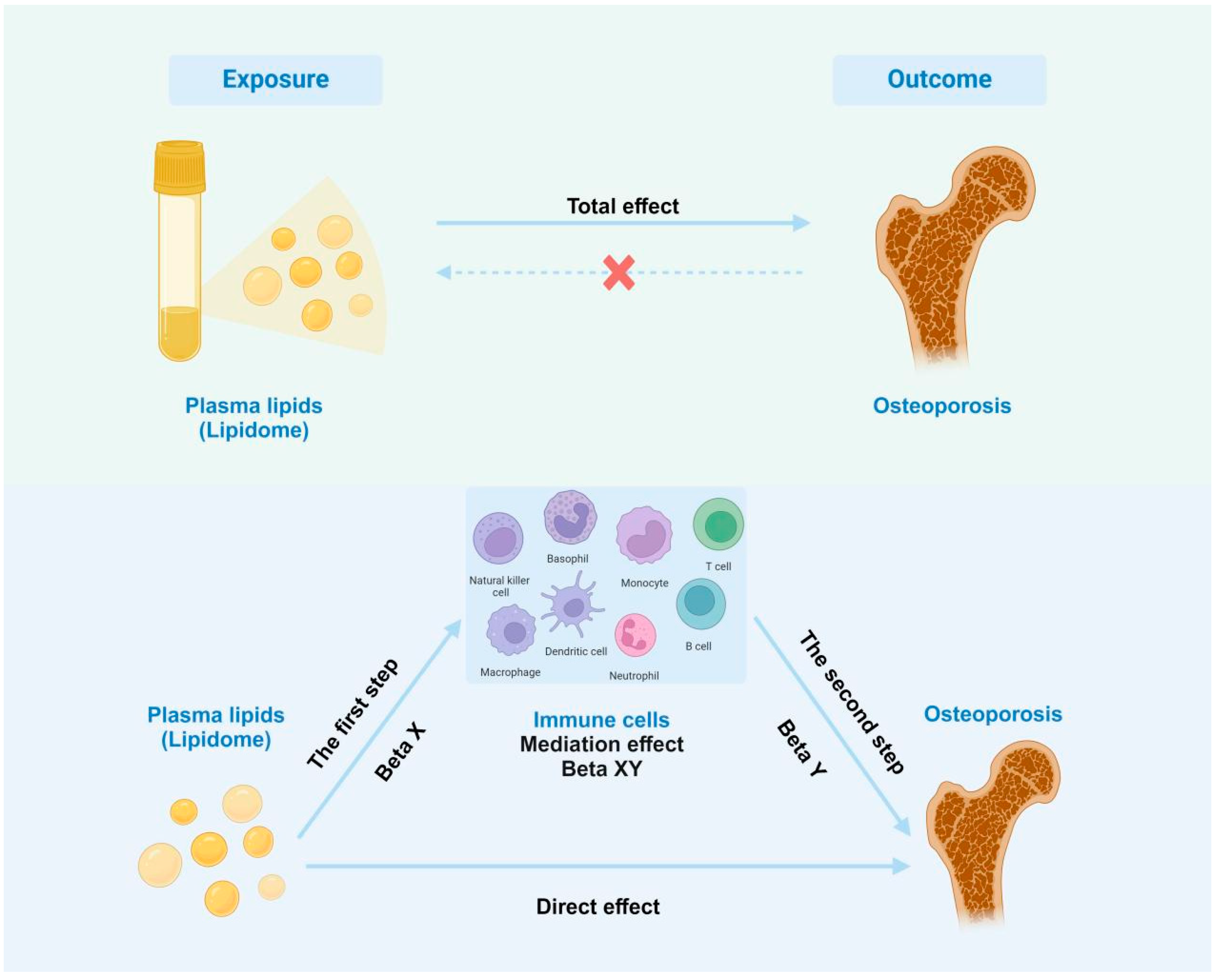
2.1. Study Design
2.2. GWAS Data for the Lipidome
2.3. GWAS Data for Osteoporosis (OP)
2.4. GWAS Data for Immune Traits
2.5. Instrumental Variable Selection
2.6. Statistical Analysis
3. Results
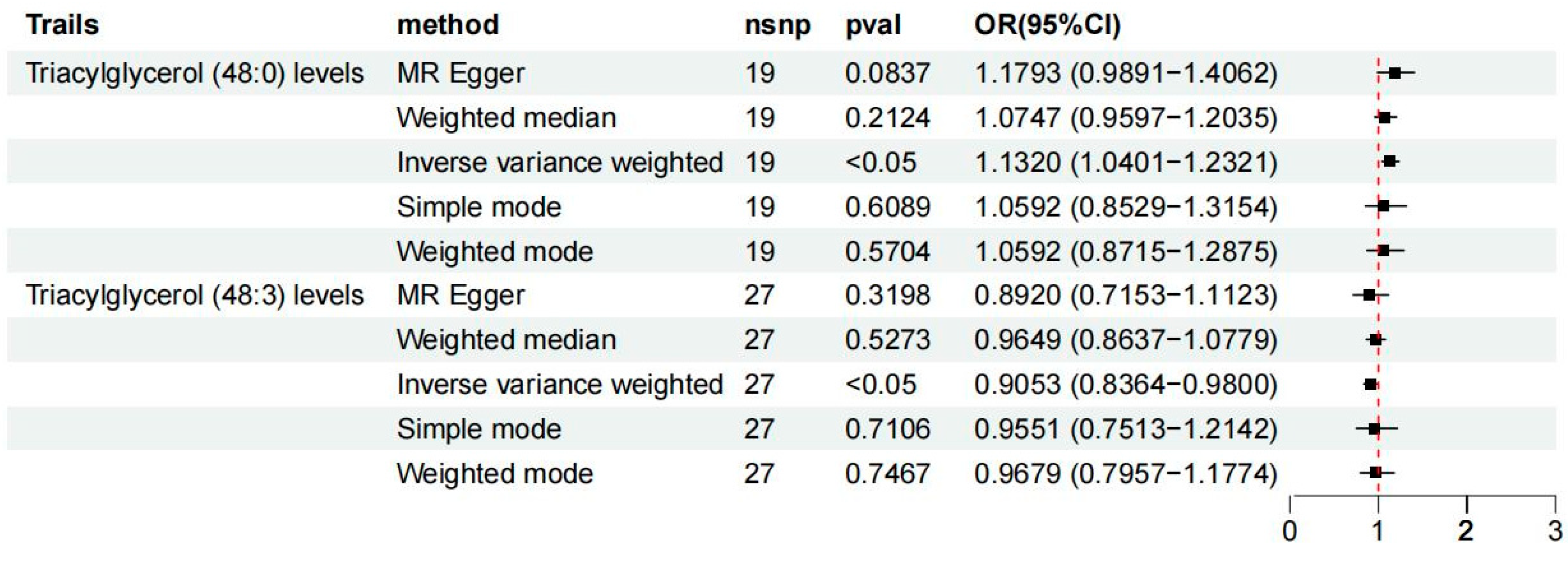
3.1. Exploration of the Causal Effect of Lipidome on Osteoporosis
3.2. Exploration of the Causal Effects of Immune Cell Characteristics on Osteoporosis
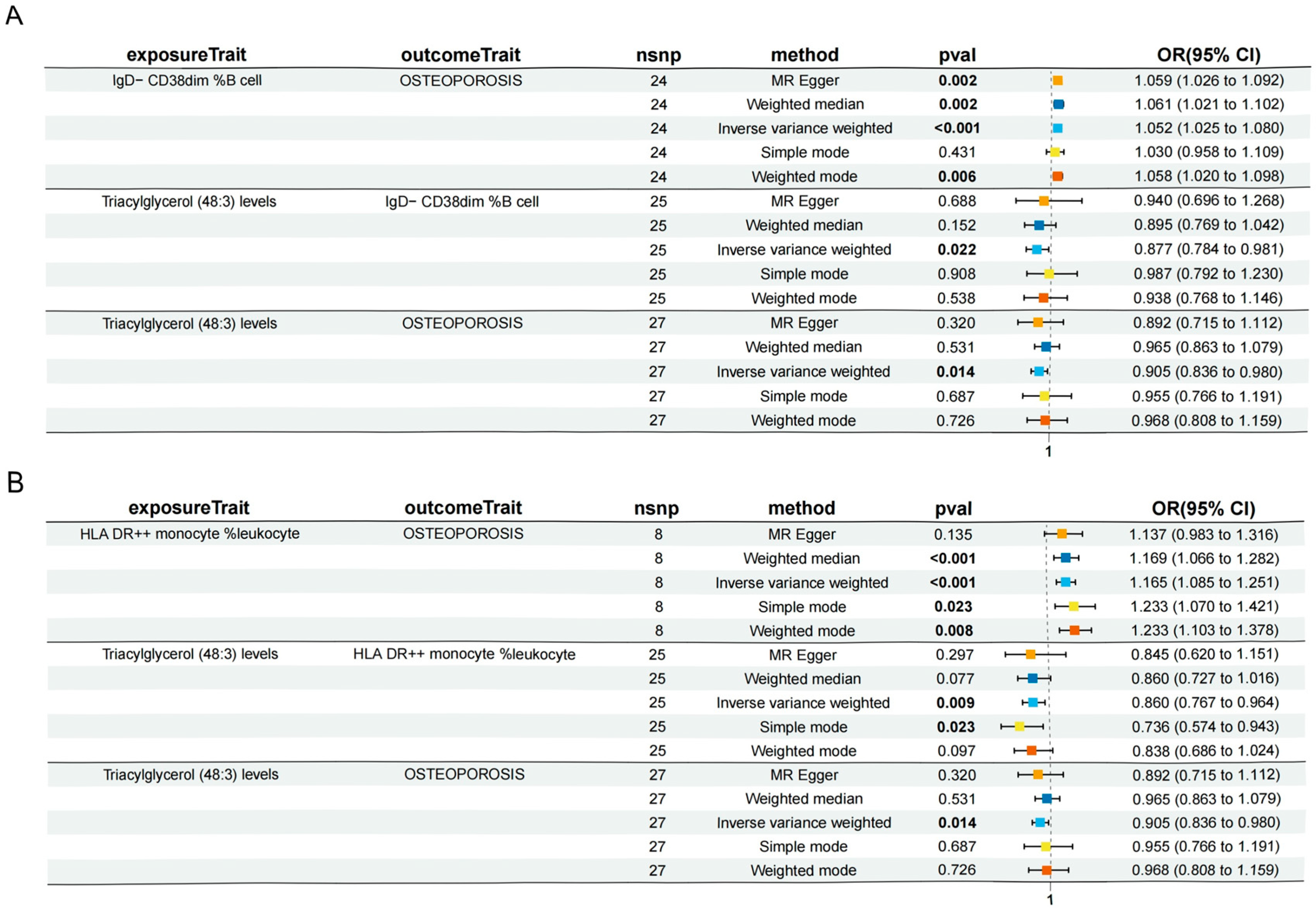
3.3. Exploring the Relationship Between Lipidomes and Osteoporosis-Associated Immune Cells
3.4. Immune Cell Characteristics Partly Mediate the Association Between the Lipidome and Osteoporosis
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.-Y. Healthy bone tissue homeostasis. Exp. Mol. Med. 2020, 52, 1165. [Google Scholar] [CrossRef] [PubMed]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern Med. 2017, 167, ITC17–ITC32. [Google Scholar] [CrossRef]
- Parhami, F.; Garfinkel, A.; Demer, L.L. Role of lipids in osteoporosis. Arter. Thromb. Vasc. Biol. 2000, 20, 2346–2348. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Zhang, M.; Qi, L.; Tang, Y. Blood lipid levels in patients with osteopenia and osteoporosis:a systematic review and meta-analysis. J. Bone Miner. Metab. 2021, 39, 510–520. [Google Scholar] [CrossRef]
- Zhan, H.; Liu, X.; Piao, S.; Rong, X.; Guo, J. Association between triglyceride-glucose index and bone mineral density in US adults: A cross sectional study. J. Orthop. Surg. Res. 2023, 18, 810. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Zou, Z.; Li, Y.; Kang, F.; Li, J.; Dong, S. The role of lipid metabolism in osteoporosis: Clinical implication and cellular mechanism. Genes. Dis. 2023, 11, 101122. [Google Scholar] [CrossRef]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 47–57. [Google Scholar] [CrossRef]
- Tan, A.; Shu, J.; Huang, H.; Shao, H.; Yang, J. The correlation between the serum LDL-C/Apo B ratio and lumbar bone mineral density in young adults. BMC Musculoskelet. Disord. 2023, 24, 213. [Google Scholar] [CrossRef]
- Ambrogini, E.; Que, X.; Wang, S.; Yamaguchi, F.; Weinstein, R.S.; Tsimikas, S.; Manolagas, S.C.; Witztum, J.L.; Jilka, R.L. Oxidation-specific epitopes restrain bone formation. Nat. Commun. 2018, 9, 2193. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, N.; Khosla, S.; Atkinson, E.J.; McCready, L.K.; Riggs, B.L. Effect of blockade of TNF-α and interleukin-1 action on bone resorption in early postmenopausal women. J. Bone Miner. Res. 2007, 22, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Ticchioni, M.; Testa, J.; Roux, C.H.; Ferrari, P.; Breittmayer, J.P.; Albert-Sabonnadière, C.; Durant, J.; De Perreti, F.; Bernard, A.; et al. Immune changes in post-menopausal osteoporosis: The Immunos study. Osteoporos. Int. 2010, 21, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Holmes, M.V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods 2019, 10, 486–496. [Google Scholar] [CrossRef]
- Ottensmann, L.; Tabassum, R.; Ruotsalainen, S.E.; Gerl, M.J.; Klose, C.; Widén, E.; Gen, F.; Simons, K.; Ripatti, S.; Pirinen, M. Genome-wide association analysis of plasma lipidome identifies 495 genetic associations. Nat. Commun. 2023, 14, 6934. [Google Scholar] [CrossRef]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between gut microbiota and preeclampsia-eclampsia: A two-sample Mendelian randomization study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef]
- During, A.; Penel, G.; Hardouin, P. Understanding the local actions of lipids in bone physiology. Prog. Lipid Res. 2015, 59, 126–146. [Google Scholar] [CrossRef]
- Elbaz, A.; Wu, X.; Rivas, D.; Gimble, J.M.; Duque, G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J. Cell. Mol. Med. 2010, 14, 982–991. [Google Scholar] [CrossRef]
- Gillet, C.; Spruyt, D.; Rigutto, S.; Valle, A.D.; Berlier, J.; Louis, C.; Debier, C.; Gaspard, N.; Malaisse, W.J.; Gangji, V.; et al. Oleate Abrogates Palmitate-Induced Lipotoxicity and Proinflammatory Response in Human Bone Marrow-Derived Mesenchymal Stem Cells and Osteoblastic Cells. Endocrinology 2015, 156, 4081–4093. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, B.; Kasonga, A.; Kruger, M.C.; Coetzee, M. Palmitoleic Acid Inhibits RANKL-Induced Osteoclastogenesis and Bone Resorption by Suppressing NF-κB and MAPK Signalling Pathways. Nutrients 2017, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.M.; Browner, W.S.; Blackwell, T.; Gore, R.; Cummings, S.R. Rate of bone loss is associated with mortality in older women: A Prospective Study. J. Bone Miner. Res. 2000, 15, 1974–1980. [Google Scholar] [CrossRef]
- Han, H.; Li, R.; Fu, D.; Zhou, H.; Zhan, Z.; Wu, Y.; Meng, B. Correlation between bone density, bone metabolism markers with lipid metabolism markers and body mass index. BMC Musculoskelet. Disord. 2024, 25, 162. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; A Venners, S.; A Terwedow, H.; Feng, Y.; Niu, T.; Li, Z.; Laird, N.; Brain, J.D.; Cummings, S.R.; Bouxsein, M.L.; et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am. J. Clin. Nutr. 2006, 83, 146–154. [Google Scholar] [CrossRef]
- Yin, W.; Li, Z.; Zhang, W. Modulation of Bone and Marrow Niche by Cholesterol. Nutrients 2019, 11, 1394. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Papachristou, D.J.; Blair, H.C. Bone and high-density lipoprotein: The beginning of a beautiful friendship. World J. Orthop. 2016, 7, 74–77. [Google Scholar] [CrossRef]
- Brodeur, M.R.; Brissette, L.; Falstrault, L.; Moreau, R. HDL3 reduces the association and modulates the metabolism of oxidized LDL by osteoblastic cells: A protection against cell death. J. Cell. Biochem. 2008, 105, 1374–1385. [Google Scholar] [CrossRef]
- Chen, H.; Shao, Z.; Gao, Y.; Yu, X.; Huang, S.; Zeng, P. Are blood lipids risk factors for fracture? Integrative evidence from instrumental variable causal inference and mediation analysis using genetic data. Bone 2020, 131, 115174. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Y.; Huo, J. Lipid Metabolism, Methylation Aberrant, and Osteoporosis: A Multi-omics Study Based on Mendelian Randomization. Calcif. Tissue Int. 2024, 114, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liu, L.; Lu, K.; Xu, M.-Z.; Ye, Y.-W.; Li, C.; Yin, Y. Association between triglycerides and lumbar bone mineral density in Chinese patients with osteoporotic fractures: A retrospective cross-sectional study. Sci. Rep. 2024, 14, 29473. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Chi, H. Lipid metabolism in dendritic cell biology. Immunol. Rev. 2023, 317, 137–151. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Döding, A.; Zimmermann, S.; Maghames, A.; Reimann, M.; Symmank, J.; Thürmer, M.; Gräler, M.H.; Wolf, M.; Jacobs, C.; Koeberle, A.; et al. Immunometabolic capacities of nutritional fatty acids in regulation of inflammatory bone cell interaction and systemic impact of periodontal infection. Front. Immunol. 2023, 14, 1213026. [Google Scholar] [CrossRef]
- Patel, C.H.; Leone, R.D.; Horton, M.R.; Powell, J.D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat. Rev. Drug Discov. 2019, 18, 669–688. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Walker, D.G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 1975, 190, 784–785. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-Gamma-Mediated Osteoimmunology. Front. Immunol. 2018, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Llorente, I.; García-Castañeda, N.; Valero, C.; González-Álvaro, I.; Castañeda, S. Osteoporosis in Rheumatoid Arthritis: Dangerous Liaisons. Front. Med. 2020, 7, 601618. [Google Scholar] [CrossRef]
- Chen, K.; Cerutti, A. New insights into the enigma of immunoglobulin D. Immunol. Rev. 2010, 237, 160–179. [Google Scholar] [CrossRef]
- Piedra-Quintero, Z.L.; Wilson, Z.; Nava, P.; Guerau-De-Arellano, M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020, 11, 597959. [Google Scholar] [CrossRef]
- Nigam, L.; Vanikar, A.; Kanodia, K.; Patel, R.; Suthar, K. Small round tumour cells (CD38, CD 79a positive) in the adrenal gland. Urol. Case Rep. 2017, 16, 22–24. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Han, Y.; Duan, X.; Wang, J.; Yan, H.; Wang, S.; Xu, Y.; Zhu, Z.; Wang, L.; et al. Role of the major histocompatibility complex class II protein presentation pathway in bone immunity imbalance in postmenopausal osteoporosis. Front. Endocrinol. 2022, 13, 876067. [Google Scholar] [CrossRef]
- Benasciutti, E.; Mariani, E.; Oliva, L.; Scolari, M.; Perilli, E.; Barras, E.; Milan, E.; Orfanelli, U.; Fazzalari, N.L.; Campana, L.; et al. MHC class II transactivator is an in vivo regulator of osteoclast differentiation and bone homeostasis co-opted from adaptive immunity. J. Bone Miner. Res. 2014, 29, 290–303. [Google Scholar] [CrossRef]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Dou, C.; Ding, N.; Zhao, C.; Hou, T.; Kang, F.; Cao, Z.; Liu, C.; Bai, Y.; Dai, Q.; Ma, Q.; et al. Estrogen Deficiency–Mediated M2 Macrophage Osteoclastogenesis Contributes to M1/M2 Ratio Alteration in Ovariectomized Osteoporotic Mice. J. Bone Miner. Res. 2018, 33, 899–908. [Google Scholar] [CrossRef]

| Traits | Methods | Beta | Low | Up | p |
|---|---|---|---|---|---|
| IgD− CD38dim %B cell | Inverse-variance weighted | 0.051 | 0.025 | 0.077 | 0.000 |
| IgD+ CD38dim %lymphocyte | −0.030 | −0.051 | −0.009 | 0.004 | |
| CD11c+ CD62L− monocyte %monocyte | −0.087 | −0.148 | −0.027 | 0.005 | |
| HLA DR++ monocyte %leukocyte | 0.152 | 0.081 | 0.224 | 0.000 | |
| CD8br %leukocyte | 0.111 | 0.030 | 0.192 | 0.007 | |
| HLA DR+ CD4+ %lymphocyte | 0.140 | 0.058 | 0.222 | 0.001 | |
| HLA DR+ NK AC | −0.078 | −0.135 | −0.021 | 0.007 | |
| CD16− CD56+ on NK | −0.050 | −0.086 | −0.014 | 0.007 | |
| HLA DR on CD14+ CD16− monocyte | 0.098 | 0.063 | 0.133 | 0.000 | |
| HLA DR on CD14+ monocyte | 0.097 | 0.061 | 0.133 | 0.000 | |
| HLA DR on monocyte | 0.055 | 0.018 | 0.091 | 0.004 | |
| SSC-A on HLA DR+ T cell | 0.077 | 0.020 | 0.133 | 0.008 | |
| HLA DR on plasmacytoid DC | 0.048 | 0.017 | 0.080 | 0.003 | |
| HLA DR on DC | 0.069 | 0.025 | 0.114 | 0.002 | |
| HLA DR on CD33br HLA DR+ CD14dim | 0.058 | 0.021 | 0.094 | 0.002 |
| Lipidomes | Immune Cell | Outcome | Mediated Effect | Mediated Proportion |
|---|---|---|---|---|
| Triacylglycerol levels of 48:3 | IgD− CD38dim %B cell | Osteoporosis | −0.00669 (−0.0214, 0.00805) | 6.73% (21.6%, −8.09%) |
| Triacylglycerol levels of 48:3 | HLA DR++ monocyte %leukocyte | Osteoporosis | −0.023 (−0.0434, −0.00266) | 23.2% (43.7%, 2.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Zhou, W.; He, J.; Zhu, Y.; Zhang, Y.; Xiong, L. Immune Cells as Mediators of Lipidome Influence on Osteoporosis: Evidence from a Mediation Analysis. Diagnostics 2025, 15, 1287. https://doi.org/10.3390/diagnostics15101287
Xiao J, Zhou W, He J, Zhu Y, Zhang Y, Xiong L. Immune Cells as Mediators of Lipidome Influence on Osteoporosis: Evidence from a Mediation Analysis. Diagnostics. 2025; 15(10):1287. https://doi.org/10.3390/diagnostics15101287
Chicago/Turabian StyleXiao, Jiheng, Wei Zhou, Jiatai He, Yanbin Zhu, Yingze Zhang, and Liming Xiong. 2025. "Immune Cells as Mediators of Lipidome Influence on Osteoporosis: Evidence from a Mediation Analysis" Diagnostics 15, no. 10: 1287. https://doi.org/10.3390/diagnostics15101287
APA StyleXiao, J., Zhou, W., He, J., Zhu, Y., Zhang, Y., & Xiong, L. (2025). Immune Cells as Mediators of Lipidome Influence on Osteoporosis: Evidence from a Mediation Analysis. Diagnostics, 15(10), 1287. https://doi.org/10.3390/diagnostics15101287






