Diagnostic Performance of Magnetic Resonance Sequences in Staging Lymph Node Involvement and Extranodal Extension in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
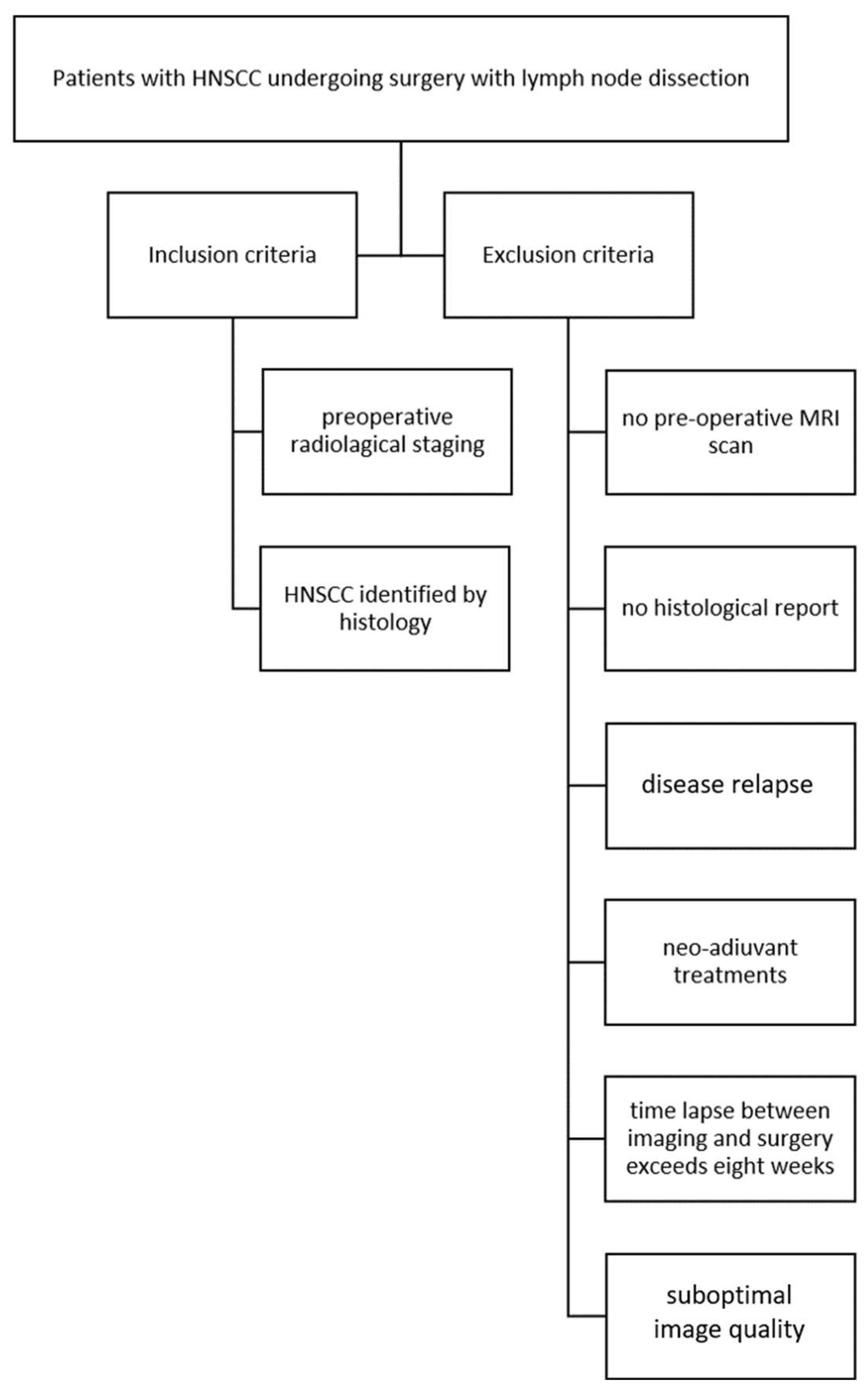
2.1. The Study Population
2.2. MRI Scans
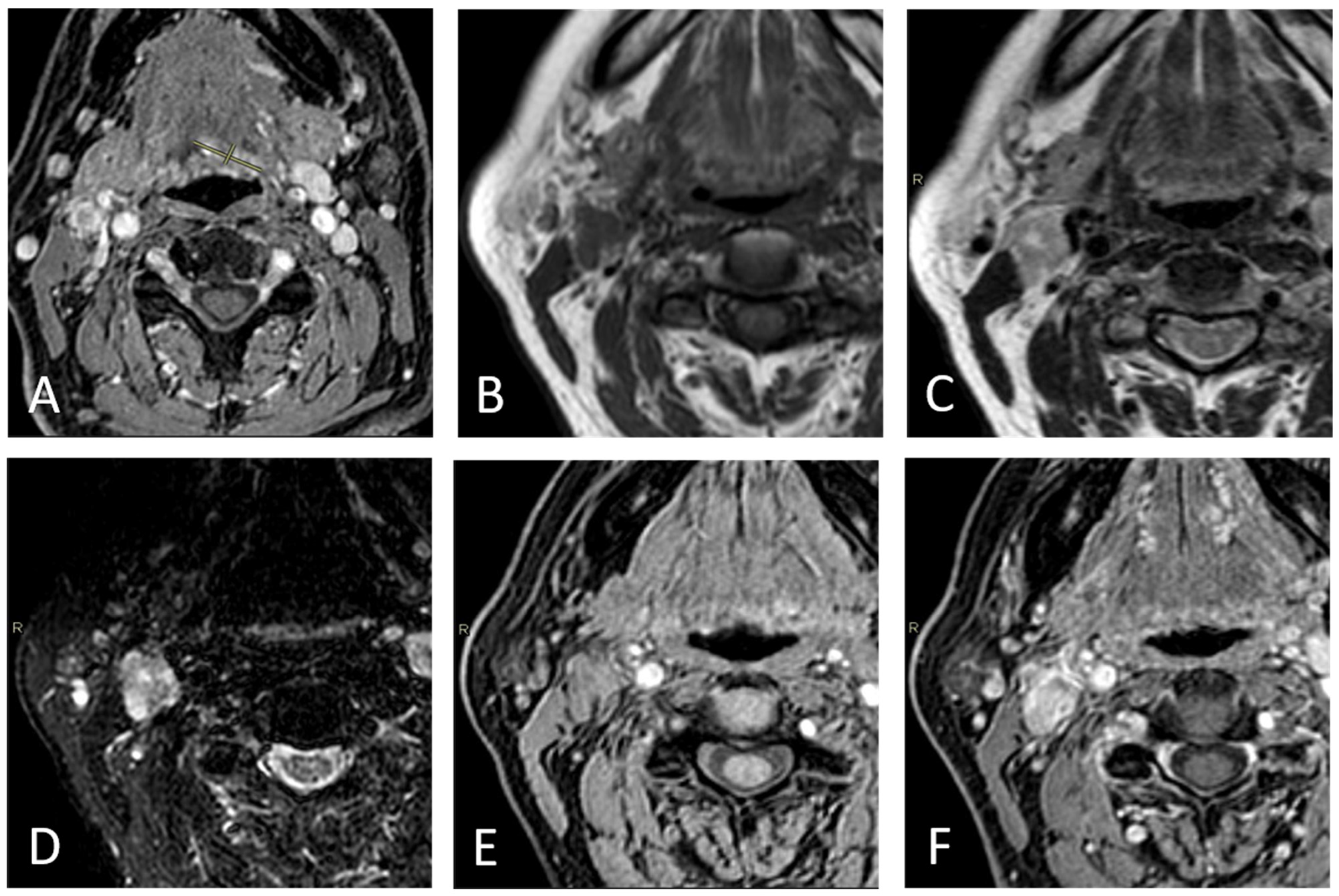
2.3. Imaging Assessment
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HNSCC | head and neck squamous cell carcinoma |
| STIR | short tau inversion recovery |
| THRIVE | T1WI High-Resolution Volume Excitation |
| TSE | turbo spin echo |
| IR | inversion recovery |
| GRE | gradient echo |
| TR | repetition time |
| PPV | positive predictive value |
| NPV | negative predictive value |
| CE | contrast-enhanced |
| WI | weighted imaging |
| ENE+ | extranodal extension |
| N+ | nodal involvement |
| SAD | short-axis diameter |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Ahuja, A.; Sable, N.; Stambuk, H.E. Imaging in oral cancers: A comprehensive review. Oral Oncol. 2020, 104, 104658, Erratum in Oral. Oncol. 2020, 111, 104956. [Google Scholar] [CrossRef] [PubMed]
- Trotta, B.M.; Pease, C.S.; Rasamny, J.J.; Raghavan, P.; Mukherjee, S. Oral cavity and oropharyngeal squamous cell cancer: Key imaging findings for staging and treatment planning. Radiographics 2011, 31, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasperts, N.; Slotman, B.J.; Leemans, C.R.; de Bree, R.; Doornaert, P.; Langendijk, J.A. Results of postoperative reirradiation for recurrent or second primary head and neck carcinoma. Cancer 2006, 106, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Sagowski, C.; Kehrl, W.; Metternich, F.U. The prognostic impact of metastatic pattern of lymph nodes in patients with oral and oropharyngeal squamous cell carcinomas. Eur. Arch. Otorhinolaryngol. 2004, 261, 270–275. [Google Scholar]
- Myers, J.N.; Greenberg, J.S.; Mo, V.; Roberts, D. Extracapsular spread: A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer 2001, 92, 3030–3036. [Google Scholar] [CrossRef]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; Van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef]
- Horvath, L.; Kraft, M. Evaluation of ultrasound and fine-needle aspiration in the assessment of head and neck lesions. Eur. Arch. Otorhinolaryngol. 2019, 276, 2903–2911. [Google Scholar] [CrossRef]
- Chai, R.L.; Rath, T.J.; Johnson, J.T.; Ferris, R.L.; Kubicek, G.J.; Duvvuri, U.; Branstetter, B.F., 4th. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.S.; Ailianou, A.; Dulguerov, N.; Becker, C.D.; Ratib, O.; Becker, M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights Imaging 2014, 5, 585–602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zwittag, P.; Asel, C.; Gabriel, M.; Rubicz, N.; Bauer, B.; Poier-Fabian, N. MRI and PET/CT in the assessment of lymph node metastases in head and neck cancer. Sci. Rep. 2023, 13, 19347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elsholtz, F.H.J.; Asbach, P.; Haas, M.; Becker, M.; Beets-Tan, R.G.H.; Thoeny, H.C.; Padhani, A.R.; Hamm, B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): A concept for standardized assessment of lymph nodes in cancer. Eur. Radiol. 2021, 31, 6116–6124, Erratum in Eur. Radiol. 2021, 31, 7217. https://doi.org/10.1007/s00330-021-07795-z. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jan, W.D.; Frank, A.P. Radiological assessment of extranodal extension in patients with head and neck squamous cell carcinoma. J. Cancer Metastasis Treat. 2021, 7, 56. [Google Scholar]
- Liao, C.T.; Lee, L.Y.; Huang, S.F.; Chen, I.H.; Kang, C.J.; Lin, C.Y.; Fan, K.-H.; Wang, H.-M.; Ng, S.-H.; Yen, T.-C. Outcome analysis of patients with oral cavity cancer and extracapsular spread in neck lymph nodes. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 930–937. [Google Scholar] [CrossRef]
- Su, Z.; Duan, Z.; Pan, W.; Wu, C.; Jia, Y.; Han, B.; Li, C. Predicting extracapsular spread of head and neck cancers using different imaging techniques: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Roh, J.L.; Lee, J.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Extranodal extension and thickness of metastatic lymph node as a significant prognostic marker of recurrence and survival in head and neck squamous cell carcinoma. J. Craniomaxillofac. Surg. 2015, 43, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Guenette, J.P.; Suh, C.H.; Hanna, G.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Choi, Y.J. The diagnostic performance of CT and MRI for detecting extranodal extension in patients with head and neck squamous cell carcinoma: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2021, 31, 2048–2061. [Google Scholar] [CrossRef]
- Url, C.; Schartinger, V.H.; Riechelmann, H.; Gluckert, R.; Majer, H.; Trumpp, M.; Widmann, G. Radiological detection of extracapsular spread in head and neck squamous cell carcinoma (HNSCC) cervical metastases. Eur. J. Radiol. 2013, 82, 1783–1787. [Google Scholar] [CrossRef]
- King, A.D.; Tse, G.M.; Yuen, E.H.; To, E.W.; Vlantis, A.C.; Zee, B.; Chan, A.B.; van Hasselt, A.C.; Ahuja, A.T. Comparison of CT and MR imaging for detection of extranodal neoplastic spread in metastatic neck nodes. Eur. J. Radiol. 2004, 52, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, H.J.; Beck, A.; Werk, M.; Felix, R. Extra capsular spread of cervical lymph node metastases: Diagnostic value of magnetic resonance imaging. Rofo 2002, 174, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zoumalan, R.A.; Kleinberger, A.J.; Morris, L.G.; Ranade, A.; Yee, H.; DeLacure, M.D.; Myssiorek, D. Lymph node central necrosis on computed tomography as predictor of extracapsular spread in metastatic head and neck squamous cell carcinoma: Pilot study. J. Laryngol. Otol. 2010, 124, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Santer, M.; Kloppenburg, M.; Gottfried, T.M.; Runge, A.; Schmutzhard, J.; Vorbach, S.M.; Mangesius, J.; Riedl, D.; Mangesius, S.; Widmann, G.; et al. Current Applications of Artificial Intelligence to Classify Cervical Lymph Nodes in Patients with Head and Neck Squamous Cell Carcinoma-A Systematic Review. Cancers 2022, 14, 5397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maggialetti, N.; Villanova, I.; Greco, S.; Sardaro, A.; Lagrasta, M.T.; Dipalma, C.; Maglitto, F.; Bicci, E.; Lucarelli, N.M.; Copelli, C.; et al. Nodal assessment and extranodal extension in head and neck squamous cell cancer: Insights from computed tomography and magnetic resonance imaging. Radiol. Med. 2024, 130, 202–213. [Google Scholar] [CrossRef]
- Kawai, Y.; Sumi, M.; Nakamura, T. Turbo short tau inversion recovery imaging for metastatic node screening in patients with head and neck cancer. AJNR Am. J. Neuroradiol. 2006, 27, 1283–1287. [Google Scholar] [PubMed] [PubMed Central]
- Touska, P.; Connor, S.E.J. Recent advances in MRI of the head and neck, skull base and cranial nerves:new and evolving sequences, analyses and clinical application. Br. J. Radiol. 2019, 92, 20190513. [Google Scholar] [CrossRef]




| Parameter | T1WI Axial | T1WI Coronal | T2WI Axial | T2WI Sagittal | STIR Axial | THRIVE | CE THRIVE |
|---|---|---|---|---|---|---|---|
| Sequence type | TSE | TSE | TSE | TSE | IR | GRE 3D | GRE 3D |
| TR/TE (ms) | 650/20 | 650/20 | 4000/80 | 4000/80 | 2100/70 | 8/4 | 8/4 |
| Fat suppression | No | No | No | No | Yes | Yes | Yes |
| Thickness (mm) | 4 | 4 | 4 | 4 | 4 | 1.5 | 1.5 |
| Acquisition time | 3′25″ | 3′25″ | 3′30″ | 3′30″ | 3′40″ | 3′00″ | 3′00″ |
| Patient Characteristics | N = 42 Patients |
|---|---|
| Sex | |
| Male; Female | 24 (57%); 18 (42%) |
| Age (mean; SD) | 66; 14 |
| Primary tumor site | |
| Oral cavity | 6 (14%) |
| Nasopharynx | 9 (21%) |
| Hypopharynx | 25 (60%) |
| Oropharynx | 0 (0%) |
| Larynx | 2 (5%) |
| Sensitivity | Specificity | PPV | NPV | Accuracy | ||
|---|---|---|---|---|---|---|
| T1WI | N+ | 65% | 80% | 49% | 89% | 77% |
| ENE+ | 38% | 92% | 25% | 95% | 88% | |
| T2WI | N+ | 58% | 79% | 44% | 87% | 75% |
| ENE+ | 50% | 92% | 31% | 96% | 89% | |
| STIR | N+ | 65% | 79% | 47% | 89% | 76% |
| ENE+ | 75% | 92% | 40% | 98% | 91% | |
| THRIVE | N+ | 58% | 84% | 50% | 88% | 78% |
| ENE+ | 63% | 95% | 45% | 97% | 92% | |
| CE THRIVE | N+ | 62% | 84% | 48% | 88% | 78% |
| ENE+ | 63% | 94% | 42% | 97% | 92% |
| Sensitivity | Specificity | PPV | NPV | Accuracy | ||
|---|---|---|---|---|---|---|
| T1WI | Loss of fatty hilum | 62% | 59% | 30% | 84% | 59% |
| Necrosis | 8% | 97% | 40% | 79% | 77% | |
| Round shape | 50% | 93% | 68% | 87% | 84% | |
| Capsular irregularity | 42% | 95% | 69% | 85% | 83% | |
| Short-axis diameter | 54% | 61% | 28% | 82% | 59% | |
| T2WI | Loss of fatty hilum | 54% | 57% | 26% | 81% | 56% |
| Necrosis | 15% | 98% | 67% | 80% | 80% | |
| Round shape | 38% | 93% | 63% | 84% | 81% | |
| Capsular irregularity | 35% | 95% | 64% | 84% | 81% | |
| Short-axis diameter | 54% | 61% | 28% | 82% | 59% | |
| STIR | Loss of fatty hilum | 58% | 64% | 31% | 84% | 63% |
| Necrosis | 27% | 95% | 58% | 82% | 80% | |
| Round shape | 42% | 93% | 65% | 85% | 82% | |
| Capsular irregularity | 42% | 95% | 69% | 85% | 83% | |
| Short-axis diameter | 54% | 61% | 28% | 82% | 59% | |
| THRIVE | Loss of fatty hilum | 46% | 67% | 29% | 82% | 63% |
| Necrosis | 12% | 97% | 50% | 79% | 78% | |
| Round shape | 38% | 93% | 63% | 84% | 81% | |
| Capsular irregularity | 35% | 95% | 64% | 84% | 81% | |
| Short-axis diameter | 54% | 61% | 28% | 82% | 59% | |
| CE THRIVE | Loss of fatty hilum | 50% | 58% | 25% | 80% | 56% |
| Necrosis | 31% | 95% | 62% | 83% | 81% | |
| Round shape | 46% | 93% | 67% | 86% | 83% | |
| Capsular irregularity | 38% | 93% | 63% | 84% | 81% | |
| Short-axis diameter | 54% | 61% | 28% | 82% | 59% |
| Imaging Characteristics of ENE+ | ||||||
|---|---|---|---|---|---|---|
| Capsular Irregularity | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| T1WI | Fat stranding | 13% | 100% | 100% | 94% | 94% |
| Fat invasion | 0% | 95% | 0% | 93% | 89% | |
| Muscle/vessel invasion | 25% | 95% | 29% | 95% | 91% | |
| T2WI | Fat stranding | 13% | 100% | 100% | 94% | 94% |
| Fat invasion | 13% | 94% | 13% | 94% | 88% | |
| Muscle/vessel invasion | 25% | 97% | 40% | 95% | 92% | |
| STIR | Fat stranding | 13% | 100% | 100% | 94% | 94% |
| Fat invasion | 38% | 95% | 33% | 95% | 91% | |
| Muscle/vessel invasion | 25% | 97% | 40% | 95% | 92% | |
| THRIVE | Fat stranding | 13% | 99% | 50% | 94% | 93% |
| Fat invasion | 38% | 96% | 43% | 95% | 92% | |
| Muscle/vessel invasion | 25% | 98% | 50% | 95% | 93% | |
| CE THRIVE | Fat stranding | 25% | 98% | 50% | 95% | 93% |
| Fat invasion | 13% | 94% | 13% | 94% | 88% | |
| Muscle/vessel invasion | 25% | 98% | 50% | 95% | 93% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, G.; Maggialetti, N.; Laugello, F.; Garofalo, A.; Villanova, I.; Greco, S.; Morelli, C.; Pignataro, P.; Lucarelli, N.M.; Stabile Ianora, A.A. Diagnostic Performance of Magnetic Resonance Sequences in Staging Lymph Node Involvement and Extranodal Extension in Head and Neck Squamous Cell Carcinoma. Diagnostics 2025, 15, 1251. https://doi.org/10.3390/diagnostics15101251
Lorusso G, Maggialetti N, Laugello F, Garofalo A, Villanova I, Greco S, Morelli C, Pignataro P, Lucarelli NM, Stabile Ianora AA. Diagnostic Performance of Magnetic Resonance Sequences in Staging Lymph Node Involvement and Extranodal Extension in Head and Neck Squamous Cell Carcinoma. Diagnostics. 2025; 15(10):1251. https://doi.org/10.3390/diagnostics15101251
Chicago/Turabian StyleLorusso, Giovanni, Nicola Maggialetti, Francesca Laugello, Annalisa Garofalo, Ilaria Villanova, Sara Greco, Chiara Morelli, Pasquale Pignataro, Nicola Maria Lucarelli, and Amato Antonio Stabile Ianora. 2025. "Diagnostic Performance of Magnetic Resonance Sequences in Staging Lymph Node Involvement and Extranodal Extension in Head and Neck Squamous Cell Carcinoma" Diagnostics 15, no. 10: 1251. https://doi.org/10.3390/diagnostics15101251
APA StyleLorusso, G., Maggialetti, N., Laugello, F., Garofalo, A., Villanova, I., Greco, S., Morelli, C., Pignataro, P., Lucarelli, N. M., & Stabile Ianora, A. A. (2025). Diagnostic Performance of Magnetic Resonance Sequences in Staging Lymph Node Involvement and Extranodal Extension in Head and Neck Squamous Cell Carcinoma. Diagnostics, 15(10), 1251. https://doi.org/10.3390/diagnostics15101251







