In Vitro Evaluation of Accuracy of CBCT-Derived Volumes in Maxillary Defects: Effects of kVp, Device, and Software
Abstract
1. Introduction
- Different CBCT devices would not affect the accuracy of maxillary defect volume measurements.
- Different kilovolt peak (kVp) settings of CBCT devices would not influence the accuracy of maxillary defect volume measurements.
- There would be no difference in defect volume measurements segmented using different software programs.
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- (1)
- Different CBCT devices did not significantly affect the accuracy of volume measurements, confirming the consistency and reliability of these devices under standard conditions.
- (2)
- kVp settings had a significant effect on measurement accuracy, with higher kVp levels yielding results closer to the GS.
- (3)
- There were no significant differences between volume measurements obtained using the two software programs utilized suggesting their reliability in defect volume estimation.
- (4)
- As defect volume increased, the measurements became more closely aligned with the GS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beumer, J.; Curtis, T.A.; Firtell, D.N. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations; Mosby Co.: St. Louis, MO, USA, 1979; pp. 188–243. [Google Scholar]
- Amirlak, B.; Tang, C.J.; Becker, D.; Palomo, J.M.; Gosain, A.K. Volumetric analysis of simulated alveolar cleft defects and bone grafts using cone beam computed tomography. Plast. Reconstr. Surg. 2013, 131, 854–859. [Google Scholar] [CrossRef]
- Linderup, B.W.; Küseler, A.; Jensen, J.; Cattaneo, P.M. A novel semiautomatic technique for volumetric assessment of the alveolar bone defect using cone beam computed tomography. Cleft Palate Craniofac. J. 2015, 52, e47–e55. [Google Scholar] [CrossRef] [PubMed]
- de Rezende Barbosa, G.L.; Wood, J.S.; Pimenta, L.A.; Almeida, S.M.D.; Tyndall, D.A. Comparison of different methods to assess alveolar cleft defects in cone beam CT images. Dentomaxillofac. Radiol. 2016, 45, 20150332. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.G.; Schreurs, R.; Bittermann, G.K.P.; Borstlap, W.A.; Koole, R.; Meijer, G.J.; Maal, T.J.J. A novel semi-automatic segmentation protocol for volumetric assessment of alveolar cleft grafting procedures. J. Craniomaxillofac. Surg. 2017, 45, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Jotikasthira, P.; Chaiworawitkul, M.; Khwanngern, K.; Powcharoen, W. Correlation Between Presurgical Alveolar Cleft Volume Measured by Simulation Software Using CBCT and Actual Bone Volume Used for Grafting. Cleft. Palate. Craniofac J. 2025, 62, 692–700. [Google Scholar] [CrossRef]
- Stoop, C.C.; Janssen, N.G.; Ten Harkel, T.C.; Rosenberg, A.J.W.P. A Novel and Practical Protocol for Three-Dimensional Assessment of Alveolar Cleft Grafting Procedures. Cleft Palate Craniofac J. 2023, 60, 601–607. [Google Scholar] [CrossRef]
- Scarfe, W.C.; Farman, A.G.; Sukovic, P. Clinical applications of cone beam computed tomography in dental practice. J. Can. Dent. Assoc. 2006, 72, 75–80. [Google Scholar]
- Angelopoulos, C.; Scarfe, W.C.; Farman, A.G. A comparison of maxillofacial CBCT and medical CT. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2012, 20, 1–17. [Google Scholar] [CrossRef]
- Kamburoğlu, K.; Murat, S.; Kolsuz, E.; Kurt, H.; Yüksel, S.; Paksoy, C. Comparative assessment of subjective image quality of cross-sectional cone-beam computed tomography scans. J. Oral. Sci. 2011, 53, 501–508. [Google Scholar] [CrossRef]
- Spin-Neto, R.; Gotfredsen, E.; Wenzel, A. Impact of voxel size variation on CBCT-based diagnostic outcome in dentistry: A systematic review. J. Digit. Imaging 2013, 26, 813–820. [Google Scholar] [CrossRef]
- Koç, A.; Kaya, S. Is it possible to estimate volume of bone defects formed on dry sheep mandibles more practically by secondarily reconstructing section thickness of cone beam computed tomography images? Dentomaxillofac. Radiol. 2021, 50, 20200400. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.H.; Nguyen, K.T.; Kaipatur, N.R.; Alexiou, M.; La, T.G.; Lagravère Vich, M.O.; Major, P.W.; Punithakumar, K.; Lou, E.H.; Le, L.H. Ultrasonic mapping of midpalatal suture—An ex-vivo study. J. Dent. 2024, 145, 105024. [Google Scholar] [CrossRef]
- Yavuz, M.S.; Buyukkurt, M.C.; Tozoglu, S.; Dagsuyu, I.M.; Kantarci, M. Evaluation of volumetry and density of mandibular symphysis bone grafts by three-dimensional computed tomography. Dent. Traumatol. 2009, 25, 475–479. [Google Scholar] [CrossRef]
- Kasaven, C.P.; McIntyre, G.T.; Mossey, P.A. Accuracy of both virtual and printed 3-dimensional models for volumetric measurement of alveolar clefts before grafting with alveolar bone compared with a validated algorithm: A preliminary investigation. Br. J. Oral. Maxillofac. Surg. 2017, 55, 31–36. [Google Scholar] [CrossRef]
- Liu, Y.; Olszewski, R.; Alexandroni, E.S.; Enciso, R.; Xu, T.; Mah, J.K. The validity of in vivo tooth volume determinations from cone beam computed tomography. Angle Orthod. 2010, 80, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Etemadi Sh, M.; Movahedian Attar, B.; Mehdizadeh, M.; Tajmiri, G. Evaluation of the CBCT imaging accuracy in the volumetric assessment of unilateral alveolar cleft. J. Stomatol. Oral. Maxillofac. Surg. 2021, 122, e1–e5. [Google Scholar] [CrossRef]
- El-Beblawy, Y.M.; Bakry, A.M.; Mohamed, M.E.A. Accuracy of formula-based volume and image segmentation-based volume in calculation of preoperative cystic jaw lesions’ volume. Oral. Radiol. 2024, 40, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Jiang, M.; Xu, X.; Li, J. A new method of volumetric assessment of alveolar bone grafting for cleft patients using cone beam computed tomography. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2017, 124, e171–e182. [Google Scholar] [CrossRef]
- Du, F.; Li, B.; Yin, N.; Cao, Y.; Wang, Y. Volumetric Analysis of Alveolar Bone Defect Using Three-Dimensional-Printed Models Versus Computer-Aided Engineering. J. Craniofac. Surg. 2017, 28, 383–386. [Google Scholar] [CrossRef]
- Ferrare, N.; Leite, A.F.; Caracas, H.C.; de Azevedo, R.B.; de Melo, N.S.; de Souza Figueiredo, P.T. Cone-beam computed tomography and microtomography for alveolar bone measurements. Surg. Radiol. Anat. 2013, 35, 495–502. [Google Scholar] [CrossRef]
- Costa, A.L.; Barbosa, B.V.; Perez-Gomes, J.P.; Calle, A.J.; Santamaria, M.P.; Lopes, S.C. Influence of voxel size on the accuracy of linear measurements of the condyle in images of cone beam computed tomography: A pilot study. J. Clin. Exp. Dent. 2018, 10, e876–e882. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, E.S.; Yamashita, F.C.; de Albuquerque, S.; Walewski, L.A.; Iwaki, L.C.V.; Takeshita, W.M.; Silva, M.C. Reliability and accuracy of linear measurements in cone-beam computed tomography using different software programs and voxel sizes. J. Conserv. Dent. 2018, 21, 607–612. [Google Scholar] [CrossRef]
- Fani, S.; Moudi, E.; Haghanifar, S.; Seyedmajidi, S.; Poursattar-Bejeh Mir, A. Evaluation of the linear and volumetric measuring changes in different positions in CBCT. Clin. Exp. Dent. Res. 2023, 9, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Xia, L.; Cai, C.; Yuan, L.; Ye, N.; Fang, B. Accuracy of in vitro mandibular volumetric measurements from CBCT of different voxel sizes with different segmentation threshold settings. BMC Oral. Health 2019, 19, 206. [Google Scholar] [CrossRef]
- da Silveira, P.F.; Vizzotto, M.B.; Liedke, G.S.; da Silveira, H.L.; Montagner, F.; da Silveira, H.E. Detection of vertical root fractures by conventional radiographic examination and cone beam computed tomography—an in vitro analysis. Dent. Traumatol. 2013, 29, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, H.M.; Dyda, S.; Pinsky, R.W.; Misch, K.A.; Sarment, D.P. Accuracy of three-dimensional measurements using cone-beam CT. Dentomaxillofac. Radiol. 2006, 35, 410–416. [Google Scholar] [CrossRef]
- Loubele, M.; Jacobs, R.; Maes, F.; Denis, K.; White, S.; Coudyzer, W.; Lambrichts, I.; van Steenberghe, D.; Suetens, P. Image quality vs radiation dose of four cone beam computed tomography scanners. Dentomaxillofac. Radiol. 2008, 37, 309–318. [Google Scholar] [CrossRef]
- Sahin, B.; Mazonakis, M.; Akan, H.; Kaplan, S.; Bek, Y. Dependence of computed tomography volume measurements upon section thickness: An application to human dry skulls. Clin. Anat. 2008, 21, 479–485. [Google Scholar] [CrossRef]
- Sezgin, O.S.; Kayıpmaz, S.; Sahin, B. The effect of slice thickness on the assessment of bone defect volumes by the Cavalieri principle using cone beam computed tomography. J. Digit. Imaging 2013, 26, 115–118. [Google Scholar] [CrossRef][Green Version]
- Palankar, V.; Sattur, A.; Palankar, A.; Rajeswari, S.R.; Thakur, S.; Desai, A.K. Evaluation of Long-term Stability of Secondary Alveolar Bone Grafts in Cleft Palate Patients Using Multislice Computed Tomography and Three-Dimensional Printed Models: A Prospective Study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 2), S1496–S1500. [Google Scholar] [CrossRef]
- Phienwej, K.; Chaiworawitkul, M.; Jotikasthira, D.; Khwanngern, K.; Sriwilas, P. Comparison of preoperative measurement methods of alveolar cleft volume using cone beam computed tomography between computer simulation and water displacement methods. Cleft Palate Craniofac. J. 2023, 60, 115–121. [Google Scholar] [CrossRef]
- Ahlowalia, M.S.; Patel, S.; Anwar, H.M.; Cama, G.; Austin, R.S.; Wilson, R.; Mannocci, F. Accuracy of CBCT for volumetric measurement of simulated periapical lesions. Int. Endod. J. 2013, 46, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.A.; Gaia, B.F.; Cavalcanti, M.G. Comparison between multislice and cone-beam computerized tomography in the volumetric assessment of cleft palate. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011, 112, 249–257. [Google Scholar] [CrossRef]
- Kauke, M.; Safi, A.F.; Grandoch, A.; Nickenig, H.J.; Zoller, J.; Kreppel, M. Image segmentation-based volume approximation-volume as a factor in the clinical management of osteolytic jaw lesions. Dentomaxillofac. Radiol. 2019, 48, 20180113. [Google Scholar] [CrossRef]
- Dejaco, D.; Url, C.; Schartinger, V.H.; Haug, A.K.; Fischer, N.; Riedl, D.; Posch, A.; Riechelmann, H.; Widmann, G. Approximation of head and neck cancer volumes in contrast enhanced CT. Cancer Imaging 2015, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Weissheimer, A.; Menezes, L.M.; Sameshima, G.T.; Enciso, R.; Pham, J.; Grauer, D. Imaging software accuracy for 3-dimensional analysis of the upper airway. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Fyllingen, E.H.; Stensjøen, A.L.; Berntsen, E.M.; Solheim, O.; Reinertsen, I. Glioblastoma Segmentation: Comparison of Three Different Software Packages. PLoS ONE 2016, 11, e0164891. [Google Scholar] [CrossRef]
- Lee, D.K.; Yoon, U.; Kwak, K.; Lee, J.M. Automated Segmentation of Cerebellum Using Brain Mask and Partial Volume Estimation Map. Comput. Math. Methods Med. 2015, 2015, 167489. [Google Scholar] [CrossRef]
- Rocchetti, V.; Cavarra, F.; Agnone, A.M.; Loro, L.; Manna, F.; Boffano, P. Digital workflow for the intraoral removable prosthesis of head and neck cancer patients. Dent. Cadmos 2023, 90, 8–12. [Google Scholar] [CrossRef]
- Brucoli, M.; Boffano, P.; Pezzana, A.; Corio, C.; Benech, A. The use of optical scanner for the fabrication of maxillary obturator prostheses. Oral. Maxillofac. Surg. 2020, 24, 157–161. [Google Scholar] [CrossRef]
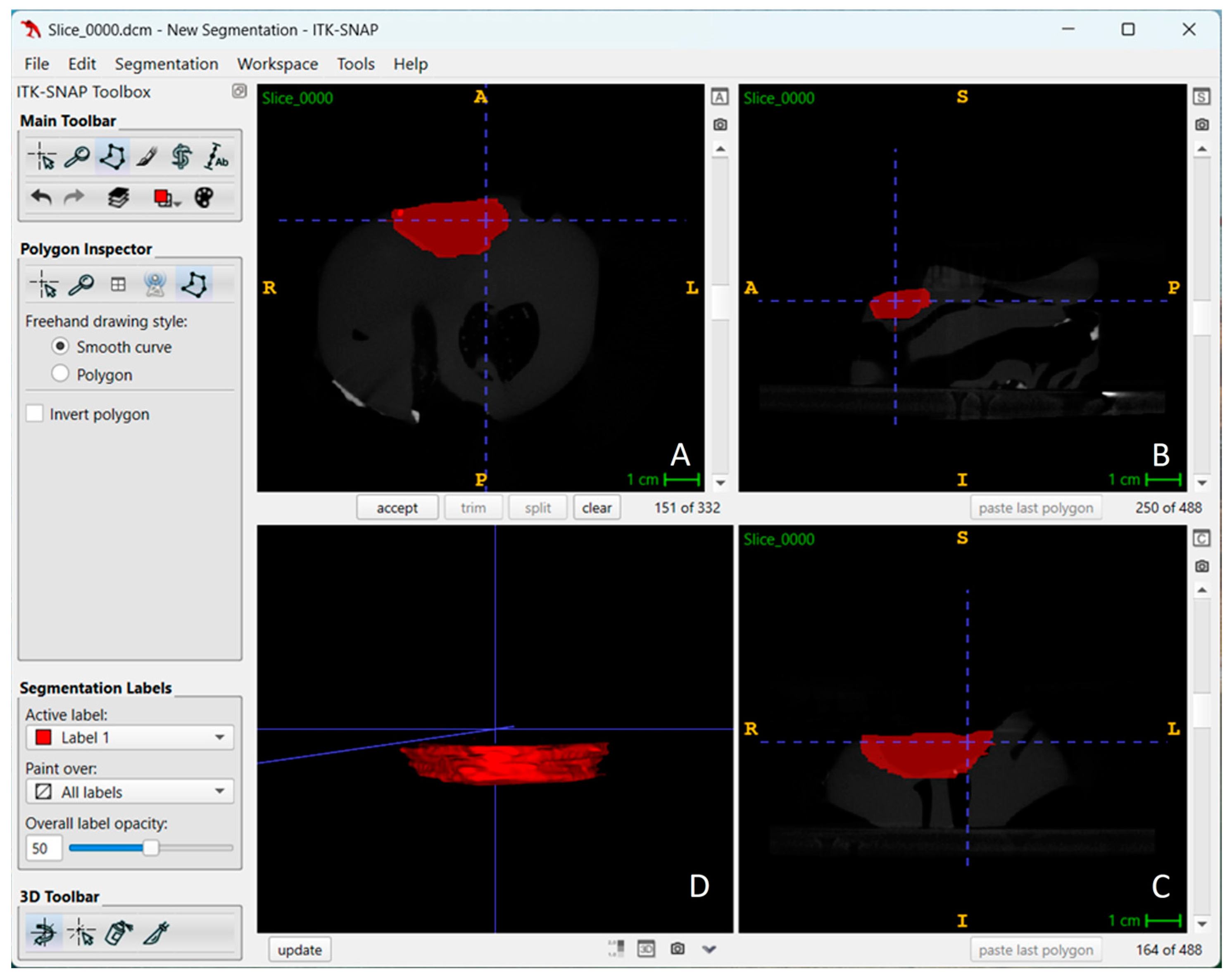
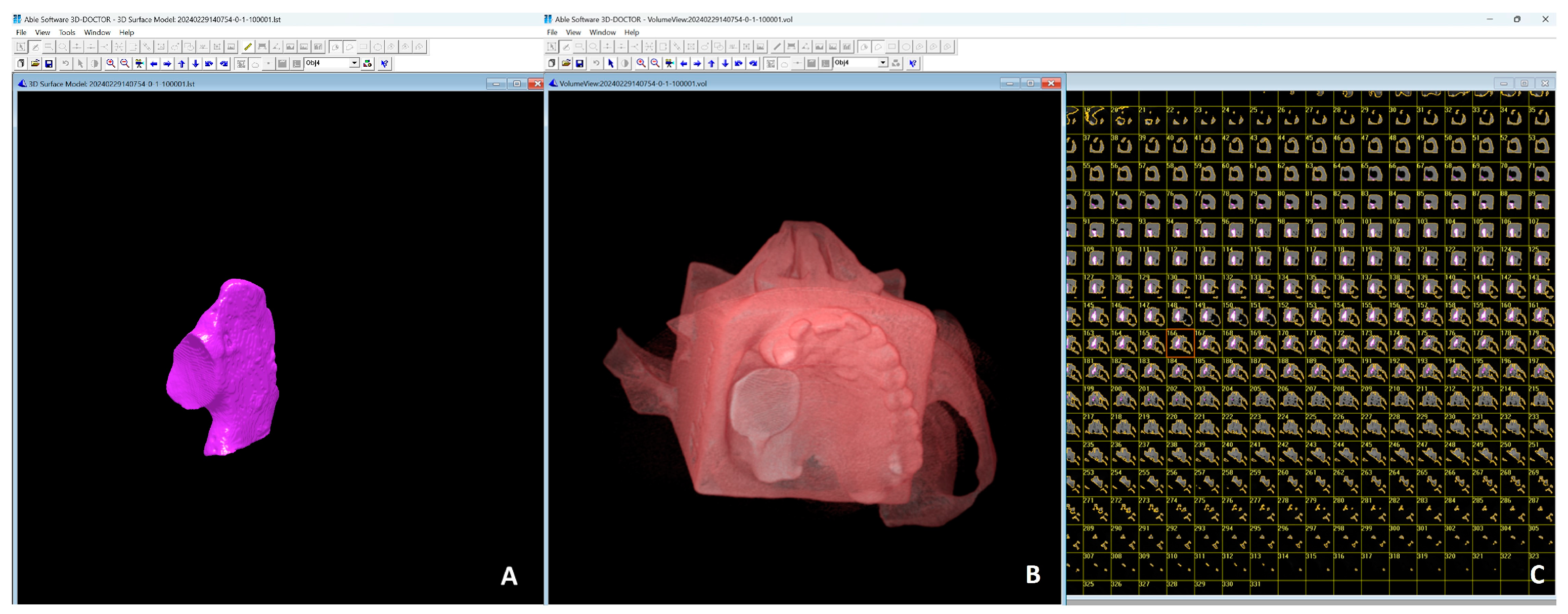

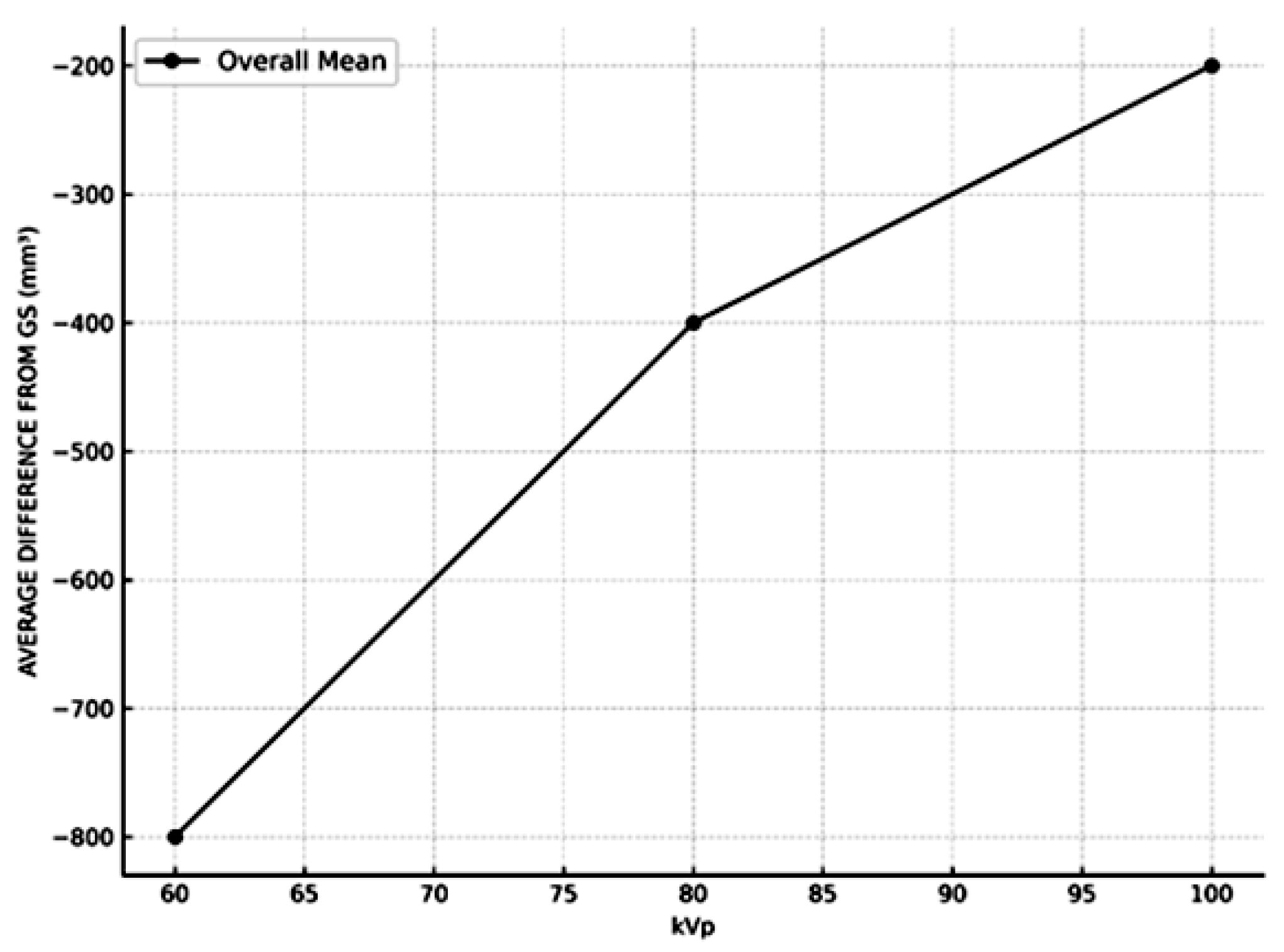
| CBCT | Software | kVp | Average Volume Measurement | Average Difference from Gs | Average Percentage Difference from Gs | |||
|---|---|---|---|---|---|---|---|---|
| Mean (mm3) | Standard Deviation | Mean (mm3) | Standard Deviation | Mean (%) | Standard Deviation | |||
| LARGEV | ITK SNAP | 1 | 9450.88 | 2250.12 | −901.25 | 200.67 | −8.47 | 3.25 |
| LARGEV | ITK SNAP | 2 | 10,673.88 | 2200.89 | −368 | 190.12 | −4.83 | 2.78 |
| LARGEV | ITK SNAP | 3 | 10,198.25 | 2230.65 | −153.88 | 170.54 | −3.85 | 2.56 |
| LARGEV | 3D DOC | 1 | 9452 | 2245.76 | −900.12 | 210.32 | −8.38 | 3.20 |
| LARGEV | 3D DOC | 2 | 10,673.88 | 2220.42 | −370.25 | 195.43 | −4.72 | 2.65 |
| LARGEV | 3D DOC | 3 | 10,200.88 | 2232.10 | −151.25 | 165.89 | −3.77 | 2.60 |
| PLANMECA | ITK SNAP | 1 | 9451.63 | 2245.12 | −900.50 | 195.87 | −8.45 | 3.18 |
| PLANMECA | ITK SNAP | 2 | 10,673.63 | 2210.32 | −370.25 | 185.42 | −4.75 | 2.70 |
| PLANMECA | ITK SNAP | 3 | 10,198 | 2222.12 | −154.13 | 175.34 | −3.80 | 2.55 |
| PLANMECA | 3D DOC | 1 | 9453.50 | 2230.25 | −898.62 | 200.34 | −8.42 | 3.22 |
| PLANMECA | 3D DOC | 2 | 10,674.63 | 2240.11 | −371.50 | 190.75 | −4.70 | 2.62 |
| PLANMECA | 3D DOC | 3 | 10,201.38 | 2235.32 | −150.75 | 180.21 | −3.78 | 2.58 |
| Source | Sum of Squares (SS) | df | Mean Square (MS) | F | p-Value |
|---|---|---|---|---|---|
| CBCT | 135,820.45 | 1 | 135,820.45 | 4.21 | 0.064 |
| SOFTWARE | 142,512.39 | 1 | 142,512.39 | 4.41 | 0.079 |
| kVp | 512,230.88 | 2 | 256,115.44 | 7.92 | <0.001 |
| CBCT × SOFTWARE | 32,145.78 | 1 | 32,145.78 | 1.00 | 0.321 |
| CBCT × kVp | 42,320.67 | 2 | 21,160.33 | 0.65 | 0.523 |
| SOFTWARE × kVp | 45,612.23 | 2 | 22,806.12 | 0.71 | 0.491 |
| CBCT × SOFTWARE × kVp | 28,750.12 | 2 | 14,375.06 | 0.45 | 0.642 |
| Error | 2,120,034.56 | 63 | 33,650.23 | ||
| Total | 2,928,726.28 | 71 |
| Source | Sum of Squares (SS) | df | Mean Square (MS) | F | p-Value |
|---|---|---|---|---|---|
| CBCT | 124,580.25 | 1 | 124,580.25 | 0.62 | 0.435 |
| SOFTWARE | 135,420.12 | 1 | 135,420.12 | 0.67 | 0.414 |
| kVp | 215,420.43 | 2 | 107,710.22 | 10.45 | <0.001 |
| CBCT × SOFTWARE | 38,210.45 | 1 | 38,210.45 | 1.87 | 0.176 |
| CBCT × kVp | 54,325.34 | 2 | 27,162.67 | 1.32 | 0.271 |
| SOFTWARE × kVp | 45,210.67 | 2 | 22,605.33 | 1.10 | 0.335 |
| CBCT × SOFTWARE × kVp | 32,120.56 | 2 | 16,060.28 | 0.78 | 0.462 |
| Error | 1,412,034.89 | 63 | 22,413.25 | ||
| Total | 1,812,345.69 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murat, S.; Kamburoğlu, K.; Vazquez, D.; Nart, L.J.; Azcona, V.; Benitez, L.E.; Awawdeh, M.; Aboelmaaty, W. In Vitro Evaluation of Accuracy of CBCT-Derived Volumes in Maxillary Defects: Effects of kVp, Device, and Software. Diagnostics 2025, 15, 1247. https://doi.org/10.3390/diagnostics15101247
Murat S, Kamburoğlu K, Vazquez D, Nart LJ, Azcona V, Benitez LE, Awawdeh M, Aboelmaaty W. In Vitro Evaluation of Accuracy of CBCT-Derived Volumes in Maxillary Defects: Effects of kVp, Device, and Software. Diagnostics. 2025; 15(10):1247. https://doi.org/10.3390/diagnostics15101247
Chicago/Turabian StyleMurat, Sema, Kıvanç Kamburoğlu, Diego Vazquez, Leonardo Jorge Nart, Victoria Azcona, Lorena Elizabeth Benitez, Mohammed Awawdeh, and Wael Aboelmaaty. 2025. "In Vitro Evaluation of Accuracy of CBCT-Derived Volumes in Maxillary Defects: Effects of kVp, Device, and Software" Diagnostics 15, no. 10: 1247. https://doi.org/10.3390/diagnostics15101247
APA StyleMurat, S., Kamburoğlu, K., Vazquez, D., Nart, L. J., Azcona, V., Benitez, L. E., Awawdeh, M., & Aboelmaaty, W. (2025). In Vitro Evaluation of Accuracy of CBCT-Derived Volumes in Maxillary Defects: Effects of kVp, Device, and Software. Diagnostics, 15(10), 1247. https://doi.org/10.3390/diagnostics15101247







