The Incidence of Adverse Events in Adults Undergoing Procedural Sedation with Propofol Administered by Non-Anesthetists: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Study Protocol
2.4. Data Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Study Characteristics
3.3. Quality and Risk of Bias Assessment
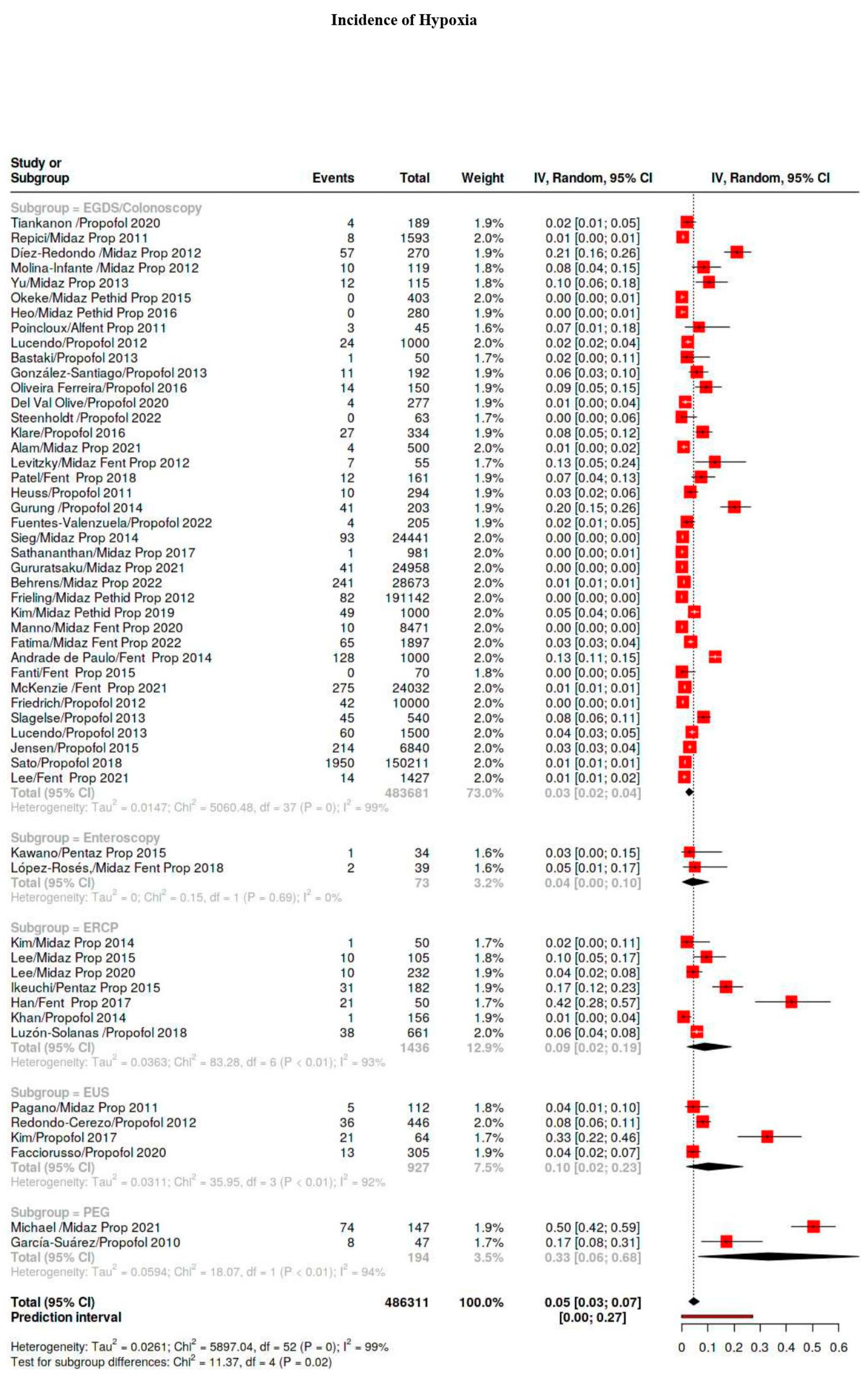
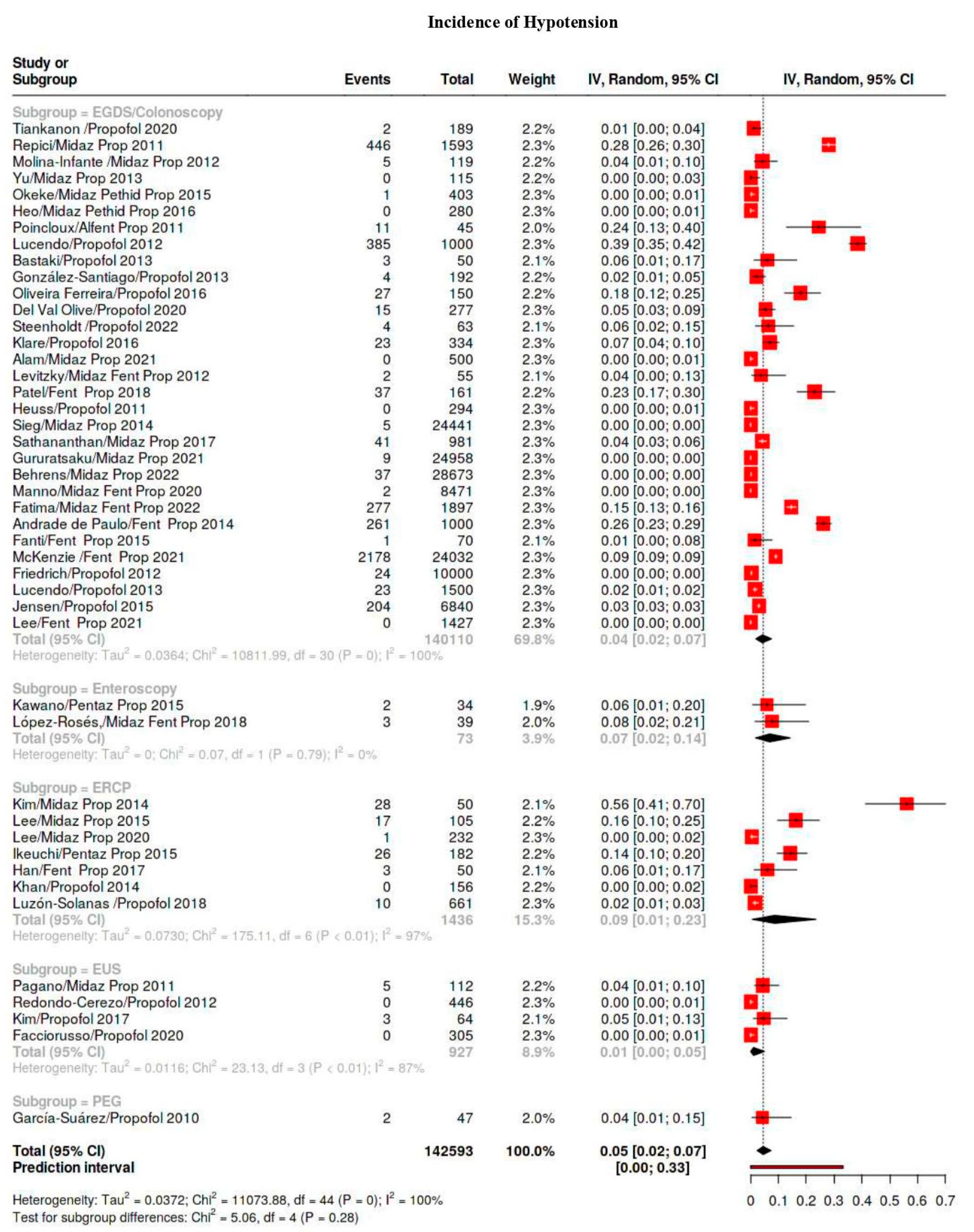
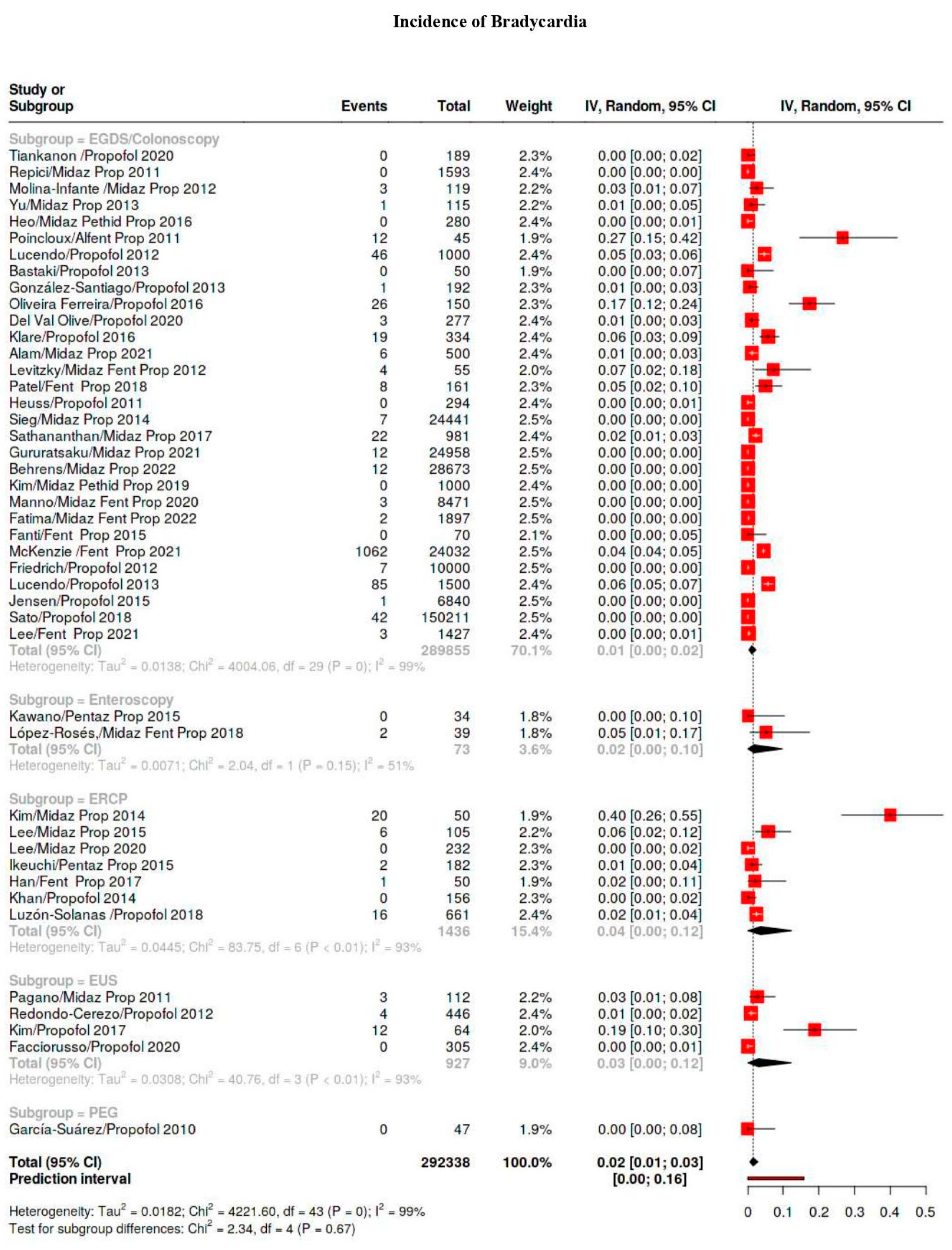
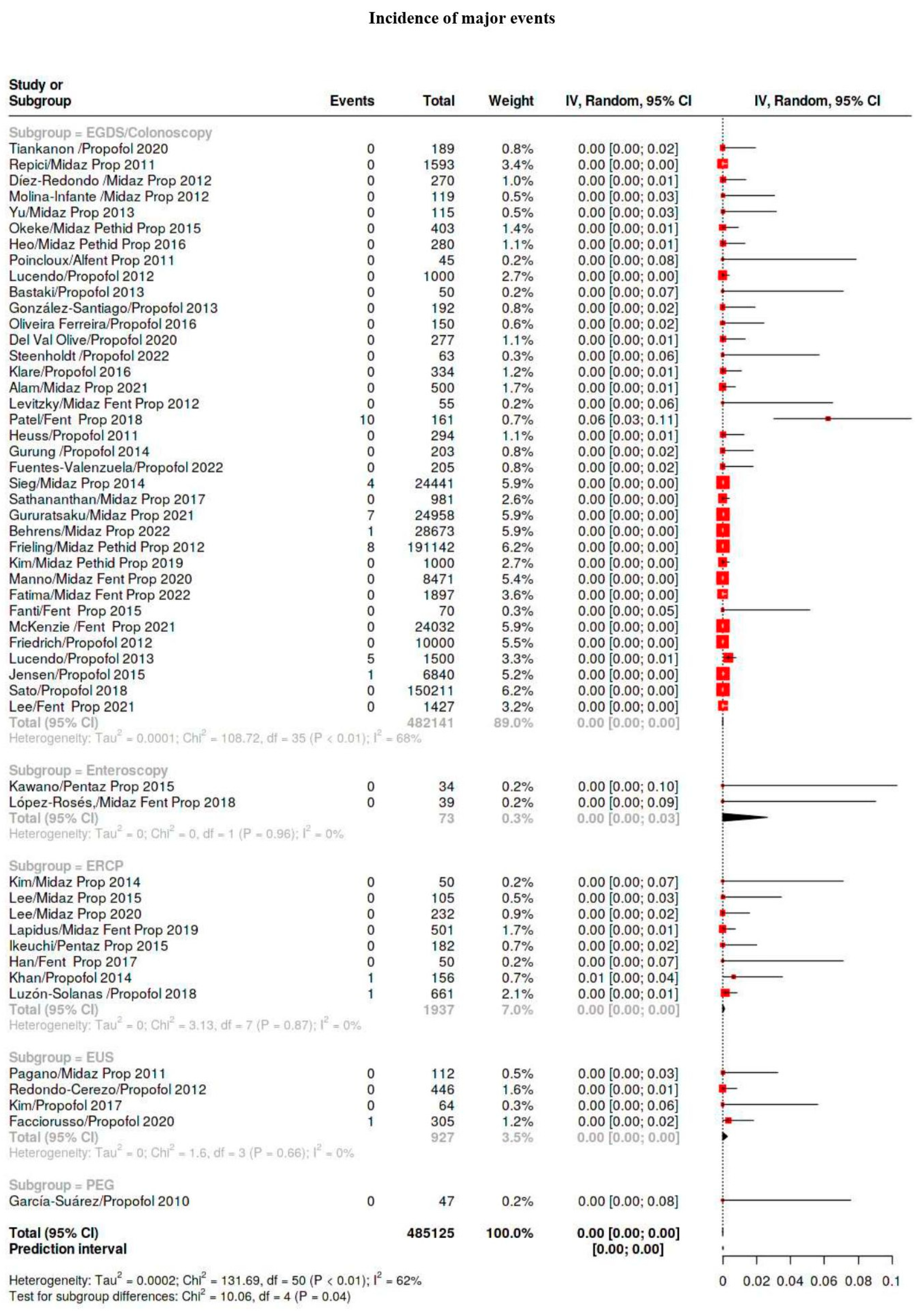
3.4. Outcomes
3.5. Subgroup Analysis
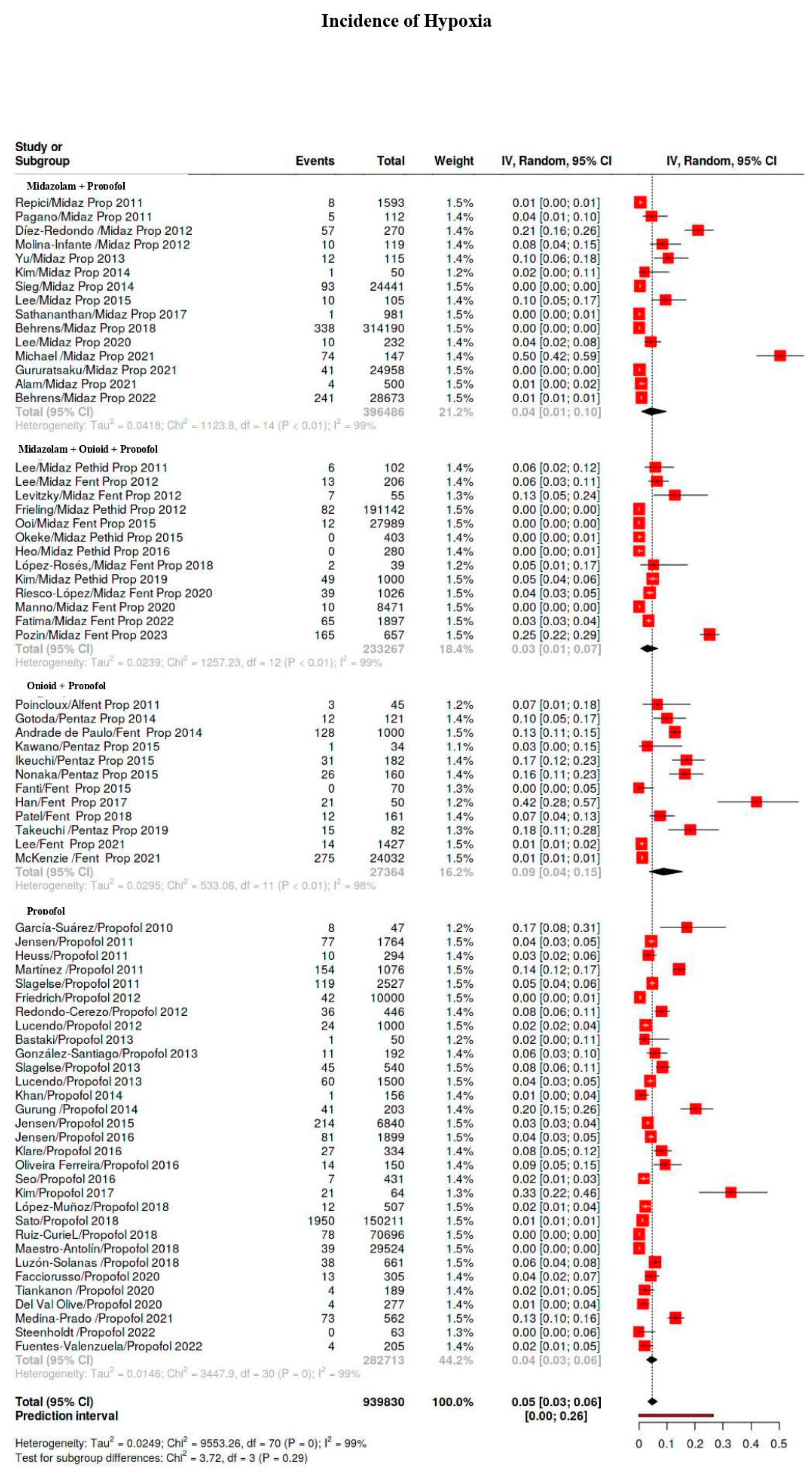
3.5.1. Hypoxia Classes
3.5.2. Sedation Regimen
3.5.3. Procedure Type
3.5.4. First-Level vs. Second-Level Procedures
3.5.5. ASA Classes
3.5.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrini, F.; Giarratano, A.; Giaccone, P.; De Robertis, E.; Monzani, R.; Rossi, M.; Tritapepe, L.; Pasquale, L.; Conigliaro, R.; Fanti, L.; et al. Analgo-Sedazione in Endoscopia Digestiva. Verso un Approccio Multidisciplinare per la Qualitàè la Sicurezza: La Posizione Inter-Societaria SIAARTI-SIED per un Percorso di Buona Pratica Clinica. 2020. Available online: https://www.siaarti.it/news/338126 (accessed on 11 January 2022).
- Axon, A. The use of propofol by gastroenterologists: Medico-legal issues. Digestion 2010, 82, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Suzuki, H. Propofol for gastrointestinal endoscopy. United Eur. Gastroenterol. J. 2018, 6, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, R.; Fanti, L.; Manno, M.; Brosolo, P. Italian Society of Digestive Endoscopy (SIED) position paper on the non-anaesthesiologist administration of propofol for gastrointestinal endoscopy. Dig. Liver Dis. 2017, 49, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Goudra, B.G.; Singh, P.M.; Gouda, G.; Borle, A.; Gouda, D.; Dravida, A.; Chandrashakhara, V. Safety of Non-anesthesia Provider- Administered Propofol (NAAP) Sedation in Advanced Gastrointestinal Endoscopic Procedures: Comparative Meta-Analysis of Pooled Results. Dig. Dis. Sci. 2015, 60, 2612–2627. [Google Scholar] [CrossRef]
- Daza, J.F.; Tan, C.M.; Fielding, R.J.; Brown, A.; Farrokhyar, F.; Yang, I. Propofol administration by endoscopists versus anesthesiologists in gastrointestinal endoscopy: A systematic review and meta-analysis of patient safety outcomes Can. J. Surg. 2018, 61, 226–236. [Google Scholar] [CrossRef]
- Wadhwa, V.; Issa, D.; Garg, S.; Lopez, R.; Sanaka, M.R.; Vargo, J.J. Similar Risk of Cardiopulmonary Adverse Events Between Propofol and Traditional Anesthesia for Gastrointestinal Endoscopy: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 194–206. [Google Scholar] [CrossRef]
- Frieling, T.; Heise, J.; Kreysel, C.; Kuhlen, R.; Schepke, M. Sedation-associated complications in endoscopy—Prospective multicentre survey of 191,142 patients. Z. Gastroenterol. 2013, 51, 568–572. [Google Scholar]
- Ruiz-Curiel, R.E.; Bonilla, H.Y.; Baptista, A.; Bronstein, M. Sedation with propofol in digestive endoscopy administered by gastroenterologists. Experience in a Venezuelan hospital. Rev. Espanola Enfermedades Dig. 2018, 110, 246–249. [Google Scholar] [CrossRef]
- Sato, M.; Horiuchi, A.; Tamaki, M.; Ichise, Y.; Kajiyama, M.; Yamamoto, Y.; Tanaka, N. Safety and Effectiveness of Nurse-Administered Propofol Sedation in Outpatients Undergoing Gastrointestinal Endoscopy. Clin. Gastroenterol. Hepatol. 2019, 17, 1098–1104. [Google Scholar] [CrossRef]
- Ooi, M.; Thomson, A. Morbidity and mortality of endoscopist-directed nurse-administered propofol sedation (EDNAPS) in a tertiary referral center. Endosc. Int. Open 2015, 3, E393–E397. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 May 2021).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 2008, 37, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Afreixo, V.; Cruz, S.; Freitas, A.; Hernandez, M.A. Meta-Analysis of a Very Low Proportion Through Adjusted Wald Confidence Intervals. Open Access Biostat. Bioinform. 2019, 2, 10–31031. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Society of Anesthesiologists. Statement on ASA Physical Status Classification System. Available online: https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system (accessed on 24 August 2024).
- Akyuz, U.; Pata, C.; Senkal, V.; Erzin, Y. Is propofol sedation with midazolam induction safe during endoscopic procedures without anesthesiologist? Hepatogastroenterology 2010, 57, 685–687. [Google Scholar]
- García-Suárez, C.; López-Rosés, L.; Olivencia, P.; Lancho, A.; González-Ramírez, A.; Santos, E.; Carral, D.; Castro, E.; Avila, S. Sedation with propofol controlled by endoscopists during percutaneous endoscopic gastrostomy. Rev. Esp. De Enfermedades Dig. 2010, 102, 249–256. [Google Scholar] [CrossRef]
- Poincloux, L.; Laquière, A.; Bazin, J.E.; Monzy, F.; Artigues, F.; Bonny, C.; Abergel, A.; Dapoigny, M.; Bommelaer, G. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Dig. Liver Dis. 2011, 43, 553–558. [Google Scholar] [CrossRef]
- Repici, A.; Pagano, N.; Hassan, C.; Carlino, A.; Rando, G.; Strangio, G.; Romeo, F.; Zullo, A.; Ferrara, E.; Vitetta, E.; et al. Balanced propofol sedation administered by nonanesthesiologists: The first Italian experience. World J. Gastroenterol. 2011, 17, 3818–3823. [Google Scholar] [CrossRef]
- Lee, C.K.; Lee, S.H.; Chung, I.K.; Lee, T.H.; Park, S.H.; Kim, E.O.; Lee, S.H.; Kim, H.S.; Kim, S.J. Balanced propofol sedation for therapeutic GI endoscopic procedures: A prospective, randomized study. Gastrointest. Endosc. 2011, 73, 206–214. [Google Scholar] [CrossRef]
- Pagano, N.; Arosio, M.; Romeo, F.; Rando, G.; Del Conte, G.; Carlino, A.; Strangio, G.; Vitetta, E.; Malesci, A.; Repici, A. Balanced Propofol Sedation in Patients Undergoing EUS-FNA: A Pilot Study to Assess Feasibility and Safety. Diagn. Ther. Endosc. 2011, 2011, 542159. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Vilmann, P.; Horsted, T.; Hornslet, P.; Bodtger, U.; Banning, A.; Hammering, A. Nurse-administered propofol sedation for endoscopy: A risk analysis during an implementation phase. Endoscopy 2011, 43, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Heuss, L.T.; Hanhart, A.; Dell-Kuster, S.; Zdrnja, K.; Ortmann, M.; Beglinger, C.; Bucher, H.C.; Degen, L. Propofol sedation alone or in combination with pharyngeal lidocaine anesthesia for routine upper GI endoscopy: A randomized, double-blind, placebo-controlled, non-inferiority trial. Gastrointest. Endosc. 2011, 74, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.F.; Aparicio, J.R.; Ruiz, F.; Gómez-Escolar, L.; Mozas, I.; Casellas, J.A. Safety of continuous propofol sedation for endoscopic procedures in elderly patients. Rev. Esp. De Enfermedades Dig. 2011, 103, 76–82. [Google Scholar] [CrossRef]
- Slagelse, C.; Vilmann, P.; Hornslet, P.; Hammering, A.; Mantoni, T. Nurse-administered propofol sedation for gastrointestinal endoscopic procedures: First Nordic results from implementation of a structured training program. Scand. J. Gastroenterol. 2011, 46, 1503–1509. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, C.K.; Park, S.H.; Lee, S.H.; Chung, I.K.; Choi, H.J.; Cha, S.W.; Moon, J.H.; Cho, Y.D.; Hwangbo, Y.; et al. Balanced propofol sedation versus propofol monosedation in therapeutic pancreaticobiliary endoscopic procedures. Dig. Dis. Sci. 2012, 57, 2113–2121. [Google Scholar] [CrossRef]
- Díez-Redondo, P.; Gil-Simón, P.; Alcaide-Suárez, N.; Atienza-Sánchez, R.; Barrio-Andrés, J.; De-la-Serna-Higuera, C.; Pérez-Miranda, M. Comparación entre la insuflación con aire ambiente o con dióxido de carbono durante la colonoscopia en pacientes sedados con propofol [Comparison between insufflation with air or carbon dioxide during the colonoscopy in sedated patients with propofol]. Rev. Esp. Enferm Dig. 2012, 104, 411–417. [Google Scholar] [CrossRef]
- Friedrich, K.; Stremmel, W.; Sieg, A. Endoscopist-administered propofol sedation is safe—A prospective evaluation of 10,000 patients in an outpatient practice. J. Gastrointestin Liver Dis. 2012, 21, 259–263. [Google Scholar]
- Redondo-Cerezo, E.; Sánchez-Robaina, A.; Cara, J.G.; Ojeda-Hinojosa, M.; Matas-Cobos, A.; Capilla, A.D.; de Hierro Ruíz, M.L.; Pleguezuelo-Díaz, J.; de Teresa, J. Gastroenterologist-guided sedation with propofol for endoscopic ultrasonography in average-risk and high-risk patients: A prospective series. Eur. J. Gastroenterol. Hepatol. 2012, 24, 506–512. [Google Scholar] [CrossRef]
- Levitzky, B.E.; Lopez, R.; Dumot, J.A.; Vargo, J.J. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: A randomized clinical trial. Endoscopy 2012, 44, 13–20. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Olveira, A.; Friginal-Ruiz, A.B.; Guagnozzi, D.; Angueira, T.; Fernández-Fuente, M.; Cruz-Campos, M.; Serrano-Valverde, M.; Sánchez-Cazalilla, M.; Tenias, J.M.; et al. Nonanesthesiologist-administered propofol sedation for colonoscopy is safe and effective: A prospective Spanish study over 1000 consecutive exams. Eur. J. Gastroenterol. Hepatol. 2012, 24, 787–792. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Dueñas-Sadornil, C.; Mateos-Rodriguez, J.M.; Perez-Gallardo, B.; Vinagre-Rodríguez, G.; Hernandez-Alonso, M.; Fernandez-Bermejo, M.; Gonzalez-Huix, F. Nonanesthesiologist-administered propofol versus midazolam and propofol, titrated to moderate sedation, for colonoscopy: A randomized controlled trial. Dig. Dis. Sci. 2012, 57, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, M.; Douzinas, E.E.; Fotis, T.G.; Bakos, D.S.; Mitsos, A.P.; Argyra, E.; Konstantinou, M.I.; Soultati, A.S.; Kapritsou, M.; Katostaras, T.; et al. A randomized double-blind trial of anesthesia provided for colonoscopy by university-degreed anesthesia nurses in Greece: Safety and efficacy. Gastroenterol. Nurs. 2013, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, J.M.; Martín-Noguerol, E.; Vinagre-Rodríguez, G.; Hernández-Alonso, M.; Dueñas-Sadornil, C.; Pérez-Gallardo, B.; Mateos-Rodríguez, J.M.; Fernández-Bermejo, M.; Robledo-Andrés, P.; Molina-Infante, J. Intermittent boluses versus pump continuous infusion for endoscopist-directed propofol administration in colonoscopy. Rev. Esp. Enferm Dig. 2013, 105, 378–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slagelse, C.; Vilmann, P.; Hornslet, P.; Jørgensen, H.L.; Horsted, T.I. The role of capnography in endoscopy patients undergoing nurse-administered propofol sedation: A randomized study. Scand. J. Gastroenterol. 2013, 48, 1222–1230. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, Á.; González-Castillo, S.; Angueira, T.; Guagnozzi, D.; Fernández-Fuente, M.; Serrano-Valverde, M.; Sanchez-Cazalilla, M.; Chumillas, O.; Fernandez-Ordonez, M.; et al. Same-day bidirectional endoscopy with nonanesthesiologist administration of propofol: Safety and cost-effectiveness compared with separated exams. Eur. J. Gastroenterol. Hepatol. 2014, 26, 301–308. [Google Scholar] [CrossRef]
- Yu, Y.H.; Han, D.S.; Kim, H.S.; Kim, E.K.; Eun, C.S.; Yoo, K.S.; Shin, W.J.; Ryu, S. Efficacy of bispectral index monitoring during balanced propofol sedation for colonoscopy: A prospective, randomized controlled trial. Dig. Dis. Sci. 2013, 58, 3576–3583. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, M.H.; Jeong, S.U.; Lee, B.U.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K. Comparison between Midazolam Used Alone and in Combination with Propofol for Sedation during Endoscopic Retrograde Cholangiopancreatography. Clin. Endosc. 2014, 47, 94–100. [Google Scholar] [CrossRef]
- Gotoda, T.; Kusano, C.; Nonaka, M.; Fukuzawa, M.; Kono, S.; Suzuki, S.; Sato, T.; Tsuji, Y.; Itoi, T.; Moriyasu, F. Non-anesthesiologist administrated propofol (NAAP) during endoscopic submucosal dissection for elderly patients with early gastric cancer. Gastric Cancer 2014, 17, 686–691. [Google Scholar] [CrossRef]
- Sieg, A.; bng-Study-Group Beck, S.; Scholl, S.G.; Heil, F.J.; Gotthardt, D.N.; Stremmel, W.; Rex, D.K.; Friedrich, K. Safety analysis of endoscopist-directed propofol sedation: A prospective, national multicenter study of 24,441 patients in German outpatient practices. J. Gastroenterol. Hepatol. 2014, 29, 517–523. [Google Scholar] [CrossRef]
- Khan, H.A.; Umar, M.; Nisar, G.; Bilal, M.; Umar, S. Safety of non-anaesthesiologist-administered propofol sedation in ERCP. Arab. J. Gastroenterol. 2014, 15, 32–35. [Google Scholar] [CrossRef]
- Gurung, R.B.; Purbe, B.; Malla, B.; Dhungel, A.; Yogol, S.; Poudel, A.; Konwor, K.; Byanju, S. Safety profile and patient satisfaction of the routine use of propofol in gastrointestinal endoscopy. Kathmandu Univ. Med. J. 2014, 12, 101–105. [Google Scholar] [CrossRef] [PubMed]
- de Paulo, G.A.; Martins, F.P.; Macedo, E.P.; Gonçalves, M.E.; Mourão, C.A.; Ferrari, A.P. Sedation in gastrointestinal endoscopy: A prospective study comparing nonanesthesiologist-administered propofol and monitored anesthesia care. Endosc. Int. Open 2015, 3, E7–E13. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Okada, H.; Iwamuro, M.; Kouno, Y.; Miura, K.; Inokuchi, T.; Kanzaki, H.; Hori, K.; Harada, K.; Hiraoka, S.; et al. An effective and safe sedation technique combining target-controlled infusion pump with propofol, intravenous pentazocine, and bispectral index monitoring for peroral double-balloon endoscopy. Digestion 2015, 91, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, J.H.; Lee, H.S.; Kim, K.B.; Lee, I.K.; Cha, E.J.; Shin, Y.D.; Park, N.; Park, S.M. Efficacy and safety of a patient-positioning device (EZ-FIX) for endoscopic retrograde cholangiopancreatography. World J. Gastroenterol. 2015, 21, 5995–6000. [Google Scholar] [CrossRef]
- Ikeuchi, N.; Itoi, T.; Gotoda, T.; Kusano, C.; Kono, S.; Kamada, K.; Tsuchiya, T.; Tominaga, N.; Mukai, S.; Moriyasu, F. Feasibility of non-anesthesiologist-administered propofol sedation for emergency endoscopic retrograde cholangiopancreatography. Gastroenterol. Res. Pract. 2015, 2015, 685476. [Google Scholar] [CrossRef]
- Jensen, J.T.; Møller, A.; Hornslet, P.; Konge, L.; Vilmann, P. Moderate and deep nurse-administered propofol sedation is safe. Dan Med. J. 2015, 62, A5049. [Google Scholar]
- Nonaka, M.; Gotoda, T.; Kusano, C.; Fukuzawa, M.; Itoi, T.; Moriyasu, F. Safety of gastroenterologist-guided sedation with propofol for upper gastrointestinal therapeutic endoscopy in elderly patients compared with younger patients. Gut Liver 2015, 9, 38–42. [Google Scholar] [CrossRef][Green Version]
- Fanti, L.; Gemma, M.; Agostoni, M.; Rossi, G.; Ruggeri, L.; Azzolini, M.L.; Dabizzi, E.; Beretta, L.; Testoni, P.A. Target Controlled Infusion for non-anaesthesiologist propofol sedation during gastrointestinal endoscopy: The first double blind randomized controlled trial. Dig. Liver Dis. 2015, 47, 566–571. [Google Scholar] [CrossRef]
- Okeke, F.C.; Shaw, S.; Hunt, K.K.; Korsten, M.A.; Rosman, A.S. Safety of Propofol Used as a Rescue Agent During Colonoscopy. J. Clin. Gastroenterol. 2016, 50, e77–e80. [Google Scholar] [CrossRef]
- Heo, J.; Jung, M.K.; Lee, H.S.; Cho, C.M.; Jeon, S.W.; Kim, S.K.; Jeon, Y.H. Effects of bispectral index monitoring as an adjunct to nurse-administered propofol combined sedation during colonoscopy: A randomized clinical trial. Korean J. Intern. Med. 2016, 31, 260–266. [Google Scholar] [CrossRef]
- Jensen, J.T.; Hornslet, P.; Konge, L.; Møller, A.M.; Vilmann, P. High efficacy with deep nurse-administered propofol sedation for advanced gastroenterologic endoscopic procedures. Endosc. Int. Open 2016, 4, E107–E111. [Google Scholar] [CrossRef] [PubMed]
- Klare, P.; Hartrampf, B.; Haller, B.; Schlag, C.; Geisler, F.; Abdelhafez, M.; Einwächter, H.; Bajbouj, M.; RM, S.; von Delius, S. Magnetic endoscope imaging for routine colonoscopy: Impact on propofol dosage and patient safety—A randomized trial. Endoscopy 2016, 48, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.O.; Torres, J.; Barjas, E.; Nunes, J.; Glória, L.; Ferreira, R.; Rocha, M.; Pereira, S.; Dias, S.; Santos, A.A.; et al. Non-anesthesiologist administration of propofol sedation for colonoscopy is safe in low risk patients: Results of a noninferiority randomized controlled trial. Endoscopy 2016, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.I.; Ryu, J.Y.; Kang, S.S.; Lee, J.S.; Kim, H.S.; Jang, M.K.; Kim, H.Y.; Shin, W.G. Safety of Target-Controlled Propofol Infusion by Gastroenterologists in Patients Undergoing Endoscopic Resection. Dig. Dis. Sci. 2016, 61, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, D.; Young, E.; Nind, G.; George, B.; Ashby, A.; Drummond, S.; Redel, K.; Green, N.; Singh, R. Assessing the safety of physician-directed nurse-administered propofol sedation in low-risk patients undergoing endoscopy and colonoscopy. Endosc. Int. Open 2017, 5, E110–E115. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, T.H.; Park, S.H.; Cho, Y.S.; Lee, Y.N.; Jung, Y.; Choi, H.J.; Chung, I.K.; Cha, S.W.; Moon, J.H.; et al. Efficacy of midazolam- versus propofol-based sedations by non-anesthesiologists during therapeutic endoscopic retrograde cholangiopancreatography in patients aged over 80 years. Dig. Endosc. 2017, 29, 369–376. [Google Scholar] [CrossRef]
- Kim, M.G.; Park, S.W.; Kim, J.H.; Lee, J.; Kae, S.H.; Jang, H.J.; Koh, D.H.; Choi, M.H. Etomidate versus propofol sedation for complex upper endoscopic procedures: A prospective double-blinded randomized controlled trial. Gastrointest. Endosc. 2017, 86, 452–461. [Google Scholar] [CrossRef]
- Behrens, A.; Kreuzmayr, A.; Manner, H.; Koop, H.; Lorenz, A.; Schaefer, C.; Plauth, M.; Jetschmann, J.U.; Von Tirpitz, C.; Ewald, M.; et al. Acute sedation-associated complications in GI endoscopy (ProSed 2 Study): Results from the prospective multicentre electronic registry of sedation-associated complications. Gut 2019, 68, 445–452. [Google Scholar] [CrossRef]
- López Muñoz, C.; Sanchez Yague, A.; Canca Sanchez, J.C.; Reinaldo-Lapuerta, J.A.; Moya Suarez, A.B. Quality of sedation with propofol administered by non-anesthetists in a digestive endoscopy unit: The results of a one year experience. Rev. Esp. Enferm Dig. 2018, 110, 231–236. [Google Scholar] [CrossRef]
- Patel, J.; Fang, J.; Taylor, L.J.; Adler, D.G.; Gawron, A.J. Safety and efficacy of non-anesthesiologist administration of propofol sedation during esophagogastroduodenoscopy in the intensive care unit. Endosc. Int. Open 2019, 7, E625–E629. [Google Scholar] [CrossRef]
- Maestro Antolín, S.; Moreira Da Silva, B.; Santos Santamarta, F.; Germade, A.; Pérez Citores, L.; Santamaria, A.; Bonoso Criado, R.; Madrigal, R.E.; Saracibar, E.; Barcenilla Laguna, J.; et al. Severe cardiorespiratory complications derived from propofol sedation monitored by an endoscopist. Rev. Esp. Enferm Dig. 2018, 110, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Luzón Solanas, L.; Ollero Domenche, L.; Sierra Moros, E.M.; Val-Perez, J.; Soria-San Teodoro, M.T.; Gimenez-Julvez, T.; Uribarrena-Amezaga, R. The safety of deep sedation with propofol controlled by the endoscopist in endoscopic retrograde cholangiopancreatography (ERCP): A prospective study in a tertiary hospital. Rev. Esp. Enferm Dig. 2018, 110, 217–222. [Google Scholar] [CrossRef] [PubMed]
- López Rosés, L.; Alvarez, B.; González Ramírez, A.; López Baz, A.; Fernández López, A.; Alonso, S.; Dacal, A.; Marti, E.; Albines, G.; Fernandez-Molina, J.; et al. Viability of single balloon enteroscopy performed under endoscopist-directed sedation. Rev. Esp. Enferm Dig. 2018, 110, 240–245. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Kim, J.H. Low-dose midazolam and propofol use for conscious sedation during diagnostic endoscopy. Kaohsiung J. Med. Sci. 2019, 35, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Yamaguchi, D.; Yamaguchi, N.; Ikeda, K.; Yoshioka, W.; Fukuda, H.; Morisaki, T.; Ario, K.; Tsunada, S.; Katsuki, R.; et al. Propofol Sedation in the Endoscopy Room versus Operation Room during Endoscopic Submucosal Dissection for Gastric Tumors: A Propensity Score-Matching Analysis. Digestion 2020, 101, 450–457. [Google Scholar] [CrossRef]
- Lapidus, A.; Gralnek, I.M.; Suissa, A.; Yassin, K.; Khamaysi, I. Safety and efficacy of endoscopist-directed balanced propofol sedation during endoscopic retrograde cholangiopancreatography. Ann. Gastroenterol. 2019, 32, 303–311. [Google Scholar] [CrossRef]
- Lee, J.G.; Yoo, K.S.; Byun, Y.J. Continuous infusion versus intermittent bolus injection of propofol during endoscopic retrograde cholangiopancreatography. Korean J. Intern. Med. 2020, 35, 1338–1345. [Google Scholar] [CrossRef]
- Facciorusso, A.; Turco, A.; Barnabà, C.; Longo, G.; Dipasquale, G.; Muscatiello, N. Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis. Diagnostics 2020, 10, 791. [Google Scholar] [CrossRef]
- Riesco-López, J.M.; Rizo-Pascual, J.; Díaz-Sánchez, A.; Manzano-Fernández, R.; Martín-Saborido, C.; Varillas-Delgado, D.; Rivero-Fernández, M.; González-Alonso, R.; Moya-Valverde, E.; García-Fernández, P.; et al. Endoscopist-directed propofol is more efficient than anesthesiologist-administered propofol in patients at low-intermediate anesthetic risk. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1440–1446. [Google Scholar] [CrossRef]
- Tiankanon, K.; Mekaroonkamol, P.; Pittayanon, R.; Kongkam, P.; Gonlachanvit, S.; Rerknimitr, R. Nurse Administered Propofol Sedation (NAPS) versus On-call Anesthesiologist Administered Propofol Sedation (OAPS) in Elective Colonoscopy. J. Gastrointest. Liver Dis. 2020, 29, 579–585. [Google Scholar] [CrossRef]
- Del Val Oliver, B.; González Valverde, F.M.; Del Valle Ruiz, S.R. Safety of propofol sedation administered by an endoscopy team for outpatient colonoscopy. Rev. Esp. Enferm Dig. 2021, 113, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Manno, M.; Deiana, S.; Gabbani, T.; Gazzi, M.; Pignatti, A.; Becchi, E.; Ottaviani, L.; Vavassori, S.; Sacchi, E.; Hassan, C.; et al. Implementation of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) sedation training course in a regular endoscopy unit. Endoscopy 2021, 53, 65–71. [Google Scholar] [PubMed]
- Michael, F.A.; Peveling-Oberhag, J.; Herrmann, E.; Zeuzem, S.; Bojunga, J.; Friedrich-Rust, M. Evaluation of the Integrated Pulmonary Index® during non-anesthesiologist sedation for percutaneous endoscopic gastrostomy. J. Clin. Monit. Comput. 2021, 35, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nagra, N.; La Selva, D.; Kozarek, R.A.; Ross, A.; Weigel, W.; Beecher, R.; Chiorean, M.; Gluck, M.; Boden, E.; et al. Nurse-Administered Propofol Continuous Infusion Sedation for Gastrointestinal Endoscopy in Patients Who Are Difficult to Sedate. Clin. Gastroenterol. Hepatol. 2021, 19, 180–188. [Google Scholar] [CrossRef]
- Gururatsakul, M.; Lee, R.; Ponnuswamy, S.K.; Gilhotra, R.; McGowan, C.; Whittaker, D.; Ombiga, J.; Boyd, P. Prospective audit of the safety of endoscopist-directed nurse-administered propofol sedation in an Australian referral hospital. J. Gastroenterol. Hepatol. 2021, 36, 490–497. [Google Scholar] [CrossRef]
- Alam, L.; Khattak, M.A.; Alam, M. Safety of balanced propofol and midazolam in upper gastrointestinal endoscopy for sedation in cirrhotic patients. J. Pak. Med. Assoc. 2021, 71, 64–68. [Google Scholar]
- Medina-Prado, L.; Sempere, J.M.; Bozhychko, M.; Mangas-Sanjuán, C.; Gómez, F.R.; Tormo, J.R.; Valde, J.A.; Lucía, M.P.; Juan, M.S.; Maryana, B.; et al. Safety of endoscopist-administered deep sedation with propofol in ASA III patients. Rev. Esp. Enferm Dig. 2022, 114, 468–473. [Google Scholar] [CrossRef]
- McKenzie, P.; Fang, J.; Davis, J.; Qiu, Y.; Zhang, Y.; Adler, D.G.; Gawron, A.J. Safety of endoscopist-directed nurse-administered balanced propofol sedation in patients with severe systemic disease (ASA class III). Gastrointest. Endosc. 2021, 94, 124–130. [Google Scholar] [CrossRef]
- Steenholdt, C.; Jensen, J.T.; Brynskov, J.; Møller, A.M.; Limschou, A.C.; Konge, L.; Vilmann, P. Patient Satisfaction of Propofol Versus Midazolam and Fentanyl Sedation During Colonoscopy in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 559–568. [Google Scholar] [CrossRef]
- Fuentes-Valenzuela, E.; Pérez-Arenas, E.; de Benito Sanz, M.; Chavarría, C.; Peña, A.M.; López, A.C.; Tejedor-Tejada, J.; Gómez, B.B.; Sánchez-Ocaña, R.; Albillos-Blanco, M.; et al. Prospective cohort study to evaluate premedication with simethicone and n-acetilcysteine for upper diagnostic gastrointestinal endoscopy. Rev. Esp. Enferm Dig. 2023, 115, 10–15. [Google Scholar]
- Behrens, A.; Ell, C.; Studiengruppe, A.P.; Frieling, T.; Labenz, J.; May, A.; Ell, C.; Offenbach, S.K.; Ellrichmann, M.; Schilling, D.; et al. Safety of endoscopist-guided sedation in a low-risk collective. Z. Für Gastroenterol. 2023, 61, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Imperiale, T. Safety Profile of Endoscopist-directed Balanced Propofol Sedation for Procedural Sedation: An Experience at a Hospital-based Endoscopy Unit. J. Clin. Gastroenterol. 2022, 56, e209–e215. [Google Scholar] [CrossRef] [PubMed]
- Pozin, I.E.; Zabida, A.; Nadler, M.; Zahavi, G.; Orkin, D.; Berkenstadt, H. Respiratory complications during recovery from gastrointestinal endoscopies performed by gastroenterologists under moderate sedation. Clin. Endosc. 2023, 56, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E.; Feiner, J.R.; Lipnick, M.S.; Batchelder, P.; MacLeod, D.B.; Severinghaus, J.W. Effects of acute, profound hypoxia on healthy humans: Implications for safety of tests evaluating pulse oximetry or tissue oximetry performance. Anesth. Analg. 2017, 124, 146–153. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Sidhu, R.; Tham, T.; Tziatzios, G.; Guy, C.; Messmann, H.; Arvanitakis, M.; Hassan, C.; Bisschops, R.; Gralnek, I.M. Sedation practices in Gastrointestinal Endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) survey. Endoscopy 2024, 56, 964–974. [Google Scholar] [CrossRef]
- Aguirre, J.A.; Etzensperger, F.; Brada, M.; Guzzella, S.; Saporito, A.; Blumenthal, S.; Bühler, P.; Borgeat, A. The beach chair position for shoulder surgery in intravenous general anesthesia and controlled hypotension: Impact on cerebral oxygenation, cerebral blood flow and neurobehavioral outcome. J. Clin. Anesth. 2019, 53, 40–48. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [Google Scholar] [CrossRef]
- Cohen, L.B.; DeLegge, M.H.; Aisenberg, J.; Brill, J.V.; Inadomi, J.M.; Kochman, M.L.; Piorkowski, J.D. AGA Institute review of endoscopic sedation. Gastroenterology 2007, 133, 675–701. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Riphaus, A.; Schreiber, F.; Vilmann, P.; Beilenhoff, U.; Aparicio, J.R.; Vargo, J.J.; Manolaraki, M.; Wientjes, C.; Rácz, I.; et al. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline—Updated June 2015. Endoscopy 2015, 47, 1175–1189. [Google Scholar] [CrossRef]
- Kerker, A.; Hardt, C.; Schlief, H.-E.; Dumoulin, F.L. Combined sedation with midazolam/propofol for gastrointestinal endoscopy in elderly patients. BMC Gastroenterol. 2010, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.; Schmitt, T.H.; Gültekin, T.; Caspary, W.F.; Wehrmann, T. Sedation with propofol plus midazolam versus propofol alone for interventional endoscopic procedures: A prospective, randomized study. Aliment. Pharmacol. Ther. 2000, 14, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Wadhwa, V.; Thaker, A.; Chuttani, R.; Pleskow, D.K.; Barnett, S.R.; Leffler, D.A.; Berzin, T.M.; Sethi, N.; Sawhney, M.S. Propofol versus traditional sedative agents for advanced endoscopic procedures: A meta-analysis. Dig. Endosc. 2014, 26, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.-L.; Bai, Y.; Bian, J.-J.; Wen, P.-S.; Li, J.-B.; Deng, X.-M. Propofol vs traditional sedative agents for endoscopic retrograde cholangiopancreatography: A meta-analysis. World J. Gastroenterol. 2011, 17, 3538–3543. [Google Scholar] [CrossRef]
- Gotoda, T.; Akamatsu, T.; Abe, S.; Shimatani, M.; Nakai, Y.; Hatta, W.; Hosoe, N.; Miura, Y.; Miyahara, R.; Yamaguchi, D.; et al. Guidelines for sedation in gastroenterological endoscopy (second edition). Dig. Endosc. 2021, 33, 21–53. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Riphaus, A.; Beilenhoff, U.; Vilmann, P.; Hornslet, P.; Aparicio, J.; Dinis-Ribeiro, M.; Giostra, E.; Ortmann, M.; Knape, J.; et al. European curriculum for sedation training in gastrointestinal endoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA). Endoscopy 2013, 45, 496–504. [Google Scholar] [CrossRef]
- Vargo, J.J.; DeLegge, M.H.; Feld, A.D.; Gerstenberger, P.D.; Kwo, P.Y.; et al.; American Association for Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association Institute; American Society for Gastrointestinal Endoscopy; Society for Gastroenterology Nurses and Associates Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest. Endosc. 2012, 76, e1–e25. [Google Scholar] [CrossRef]
- Schilling, D.; Leicht, K.; Beilenhoff, U.; Waechter, E.K.; Kallinowski, B.; Labenz, J.; Weiß, C.; Büttner, S.; Riphaus, A. Impact of S3 training courses “Sedation and Emergency Management in Endoscopy for Endoscopy Nurses and Assisting Personnel” on the process and structure quality in gastroenterological endoscopy in practices and clinics: Results of a nationwide survey. Z. Gastroenterol. 2013, 51, 619–627. [Google Scholar] [CrossRef]

| Study | Year | Design | No. Patients | Procedures | Medication Used | Administrator | Way of Administration | ASA |
|---|---|---|---|---|---|---|---|---|
| Akyuz [19] | 2010 | Retro | 2918 | EGDS Colon ERCP DBE | Mida Propofol | NuES | Bolus | I–IV |
| García-Suárez [20] | 2010 | Prosp | 47 | PEG | Propofol | NuES | Bolus | III–IV |
| Poincloux [21] | 2011 | RCT | 45 | Colon | Alfent Propofol | Endoscopist | Bolus | I/II |
| Repici [22] | 2011 | Prosp | 1593 | Colon | Mida Propofol | Endoscopist | Bolus | I/II |
| Lee [23] | 2011 | RCT | 102 | EGDS ERCP | Mida Pethid Propofol | Nurse | Bolus | I–IV |
| Pagano [24] | 2011 | Prosp | 112 | EUS | Mida Propofol | Endoscopist | Bolus | I/II |
| Jensen [25] | 2011 | Prosp | 1764 | EGDS Colon EUS ERCP DBE | Propofol | NuES | Bolus | I–IV |
| Heuss [26] | 2011 | RCT | 294 | EGDS | Propofol | Nurse | Bolus | I–IV |
| Martínez [27] | 2011 | Prosp | 1076 | EGDS Colon EUS | Propofol | Endoscopist | TCI | I–IV |
| Slagelse [28] | 2011 | Prosp | 2527 | EGDS Colon EUS ERCP DBE | Propofol | Nurse | Bolus | I–IV |
| Lee [29] | 2012 | RCT | 206 | EUS ERCP | Mida Fent Propofol | NuES | Bolus | I–IV |
| Díez-Redondo [30] | 2012 | RCT | 270 | Colon | Mida Propofol | Endoscopist | Bolus | NA |
| Friedrich [31] | 2012 | Prosp | 10,000 | EGDS Colon | Propofol | Nurse or Endoscopist | Bolus | NA |
| Redondo-Cerezo [32] | 2012 | Prosp | 446 | EUS | Propofol | NuES | Bolus | I–IV |
| Levitzky [33] | 2012 | RCT | 55 | EGDS | Mida Fent Propofol | Endoscopist | Bolus | I–IV |
| Lucendo [34] | 2012 | Prosp | 1000 | Colon | Propofol | NuES | Bolus | I/II |
| Molina-Infante [35] | 2012 | RCT | 119 | Colon | Mida Propofol | NuES | Bolus | I–IV |
| Frieling [8] | 2012 | Prosp | 191,142 | EGDS Colon | Mida Pethid Propofol | Nurse or Endoscopist | Bolus | NA |
| Bastaki [36] | 2013 | RCT | 50 | Colon | Propofol | Nurse | Bolus | I–II |
| González-Santiago [37] | 2013 | RCT | 192 | Colon | Propofol | Nurse | Bolus/TCI | I–IV |
| Slagelse [38] | 2013 | RCT | 540 | EGDS Colon | Propofol | Nurse | Bolus | I–IV |
| Lucendo [39] | 2013 | Prosp | 1500 | EGDS Colon | Propofol | NuES | Bolus | I–IV |
| Yu [40] | 2013 | RCT | 115 | Colon | Mida Propofol | NuES | Bolus | I–IV |
| Kim [41] | 2014 | Retro | 50 | ERCP | Mida Propofol | NuES | Bolus | I/II |
| Gotoda [42] | 2014 | Retro | 121 | EGDS (ESD) | Pentaz Propofol | Endoscopist | TCI | I–IV |
| Sieg [43] | 2014 | Prosp | 24,441 | EGDS Colon | Mida Propofol | NuES | Bolus | NA |
| Khan [44] | 2014 | Prosp | 156 | ERCP | Propofol | Endoscopist | Bolus | I–IV |
| Gurung [45] | 2014 | Prosp | 203 | EGDS | Propofol | NuES | Bolus | NA |
| Andrade de Paulo [46] | 2014 | Prosp | 1000 | EGDS Colon | Fent Propofol | Nurse | Bolus | I–II |
| Kawano [47] | 2015 | Prosp | 34 | Entero | Penthaz Propofol | Endoscopist | TCI | NA |
| Lee [48] | 2015 | RCT | 105 | ERCP | Mida Propofol | NuES | Bolus | I–IV |
| Ikeuchi [49] | 2015 | Prosp | 182 | ERCP | Penthaz Propofol | Endoscopist | Bolus/TCI | I–IV |
| Jensen [50] | 2015 | Retro | 6840 | EGDS Colon | Propofol | NuEs | Bolus | I–IV |
| Ooi [11] | 2015 | Prosp | 27,989 | EGDS Colon PEG | Mida Fent Propofol | NuES | Bolus | NA |
| Nonaka [51] | 2015 | Prosp | 160 | ERCP EGDS (ESD) | Pentaz Propofol | Endoscopist | Bolus | I–IV |
| Fanti [52] | 2015 | RCT | 70 | EGDS Colon | Fent Propofol | Endoscopist | TCI | I/II |
| Okeke [53] | 2015 | Retro | 403 | Colon | Mida Pethid Propofol | Endoscopist | Bolus | NA |
| Heo [54] | 2016 | RCT | 280 | Colon | Mida Pethid Propofol | Nurse | Bolus | I–IV |
| Jensen [55] | 2016 | Prosp | 1899 | ERCP EUS DBE | Propofol | Nurse | Bolus | I–IV |
| Klare [56] | 2016 | RCT | 334 | Colon | Propofol | Endoscopist | Bolus | I–IV |
| Oliveira Ferreira [57] | 2016 | RCT | 150 | Colon | Propofol | NuES | Bolus | I/II |
| Seo [58] | 2016 | Retro | 431 | EGDS Colon (EMR/ESD) | Propofol | NuES | Bolus/TCI | I–IV |
| Sathananthan [59] | 2017 | Prosp | 981 | EGDS Colon | Mida Propofol | NuES | Bolus | I–IV |
| Han [60] | 2017 | RCT | 50 | ERCP | Fent Propofol | Nurse | Bolus | I–IV |
| Kim [61] | 2017 | RCT | 64 | EUS | Propofol | NuES | Bolus | I/II |
| Behrens [62] | 2018 | Prosp | 314,190 | EGDS Colon EUS ERCP Entero | Mida Propofol | Nurse or Endoscopist | NA | I–IV |
| López-Muñoz [63] | 2018 | Prosp | 507 | EGDS Colon ERCP EUSDBE | Propofol | NuES | NA | NA |
| Sato [10] | 2018 | Prosp | 150,211 | EGDS Colon | Propofol | Nurse | Bolus | I/II |
| Patel [64] | 2018 | Retro | 161 | EGDS | Fent Propofol | NuES | Bolus | I–IV |
| Ruiz-Curiel [9] | 2018 | Retro | 70,696 | EGDS Colon ERCP EUS | Propofol | NuES | Bolus | NA |
| Maestro-Antolín [65] | 2018 | Retro | 29,524 | EGDS Colon ERCP EUS | Propofol | Endoscopist | Bolus/TCI | I–IV |
| Luzón-Solanas [66] | 2018 | Prosp | 661 | ERCP | Propofol | Endoscopist | TCI | I–IV |
| López-Rosés [67] | 2018 | Prosp | 39 | Entero | Mida Fent Propofol | NuES | TCI | I–IV |
| Kim [68] | 2019 | Retro | 1000 | EGDS Colon | Mida Pethid Propofol | Nurse or Endoscopist | Bolus | I/II |
| Takeuchi [69] | 2019 | Retro | 82 | EGDS (ESD) | Pentaz Propofol | Endoscopist | TCI | I–IV |
| Lapidus [70] | 2019 | Retro | 501 | ERCP | Mida Fent Propofol | Endoscopist | Bolus | I–IV |
| Lee [71] | 2020 | RCT | 232 | ERCP | Mida Propofol | NuES | Bolus/TCI | NA |
| Facciorusso [72] | 2020 | Prosp | 305 | EUS | Propofol | Nurse or Endoscopist | Bolus | I–IV |
| Riesco-López [73] | 2020 | Prosp | 1026 | EGDS Colon EUS | Mida Fent Propofol | NuES | Bolus | I–IV |
| Tiankanon [74] | 2020 | Retro | 189 | Colon | Propofol | Nurse | TCI | I/II |
| Del Val Oliver [75] | 2020 | Retro | 277 | Colon | Propofol | NuES | NA | I/II |
| Manno [76] | 2020 | Prosp | 8471 | EGDS Colon | Mida Fent Propofol | NuES | Bolus | I–IV |
| Michael [77] | 2021 | RCT | 147 | PEG | Mida Propofol | NuES | Bolus | NA |
| Lee [78] | 2021 | Retro | 1427 | EGDS Colon | Fent Propofol | NuES | TCI | I–IV |
| Gururatsaku [79] | 2021 | Prosp | 24,958 | EGDS Colon | Mida Propofol | Nurse | Bolus | I–IV |
| Alam [80] | 2021 | Prosp | 500 | EGDS | Mida Propofol | Nurse or Endoscopist | Bolus | I–IV |
| Medina-Prado [81] | 2021 | Prosp | 562 | EGDS Colon EUS | Propofol | Endoscopist | TCI | I–IV |
| McKenzie [82] | 2021 | Retro | 24,032 | EGDS Colon | Fent Propofol | NuES | Bolus | I–IV |
| Steenholdt [83] | 2022 | RCT | 63 | Colon | Propofol | NuES | Bolus | I–II |
| Fuentes-Valenzuela [84] | 2022 | Prosp | 205 | EGDS | Propofol | Endoscopist | NA | NA |
| Behrens [85] | 2022 | RCT | 28,673 | EGDS Colon | Mida Propofol | Nurse or Endoscopist | Bolus | I/II |
| Fatima [86] | 2022 | Retro | 1897 | EGDS Colon | Mida Fent Propofol | NuES | Bolus | I–IV |
| Pozin [87] | 2023 | Retro | 657 | EGDS Colon EUS ERCP DBE | Mida Fent Propofol | NuES | Bolus |
| Adverse Event | Events per Patients | Estimate per 1000 | 95%CI | I2 (%) |
|---|---|---|---|---|
| Hypoxia | 5101/939,830 | 40 | 34–47 | 99 |
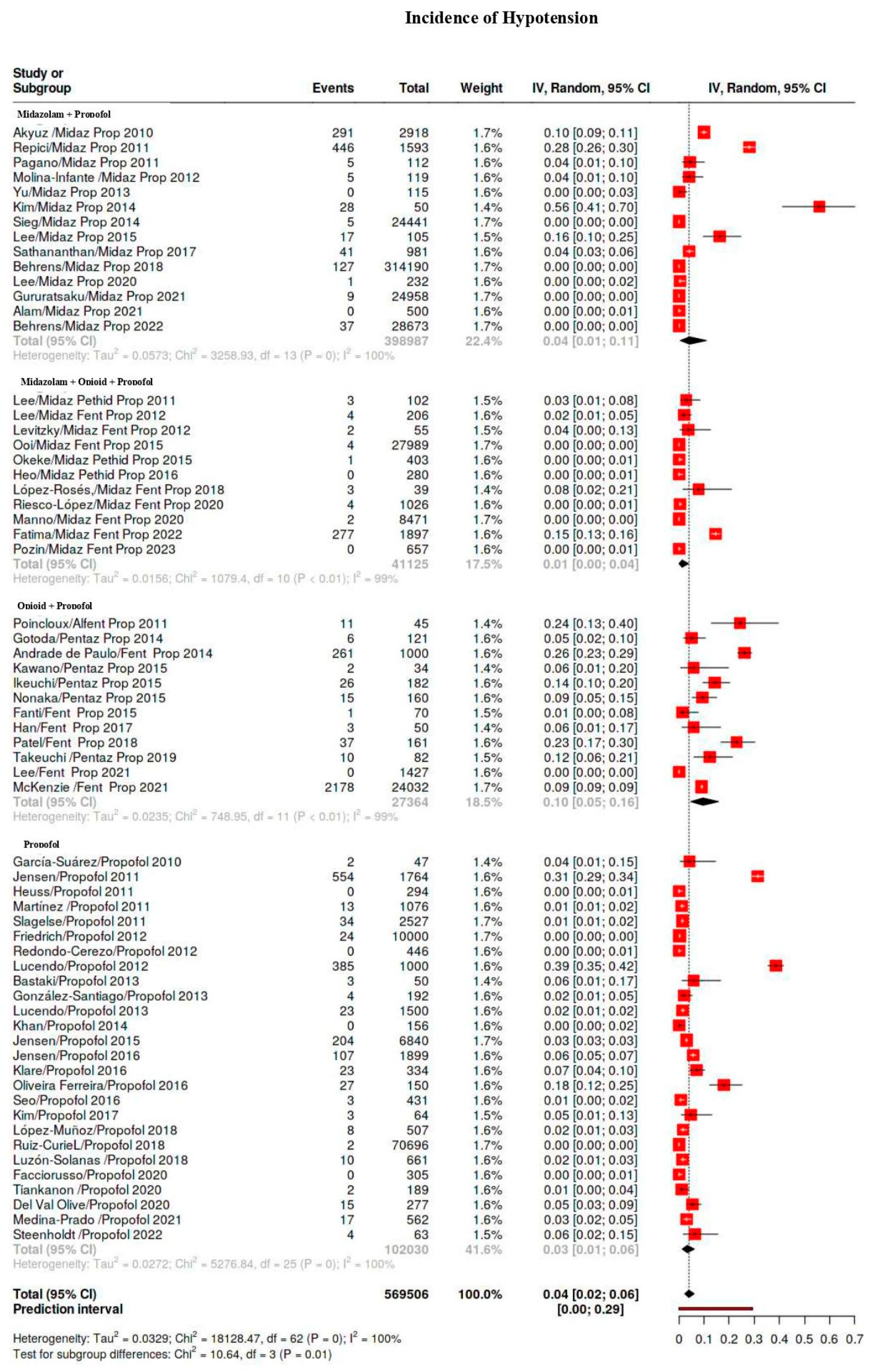
| Hypotension | 5329/569,506 | 38 | 28–50 | 99 |
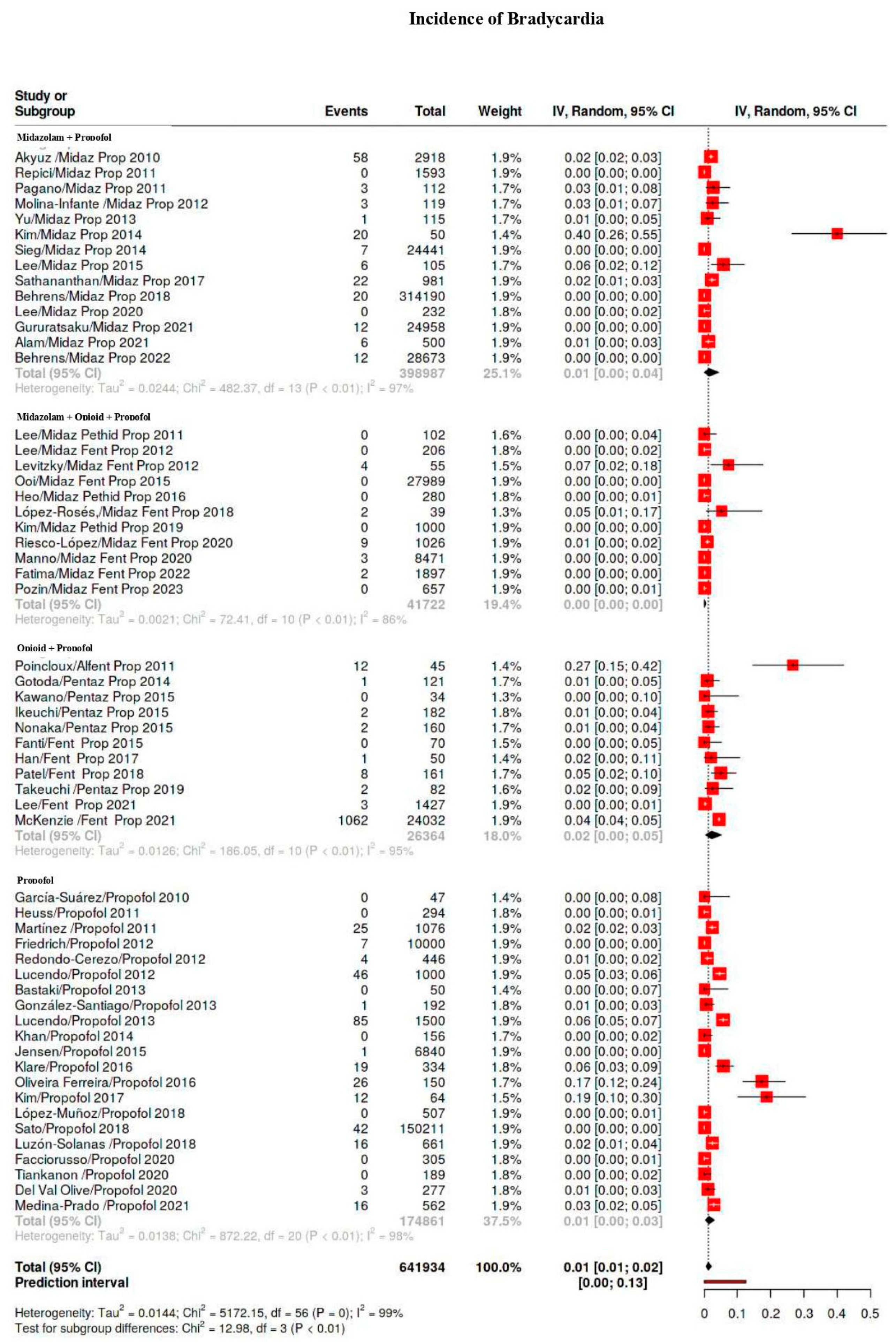
| Bradicardia | 1586/641,934 | 9 | 6–13 | 98 |
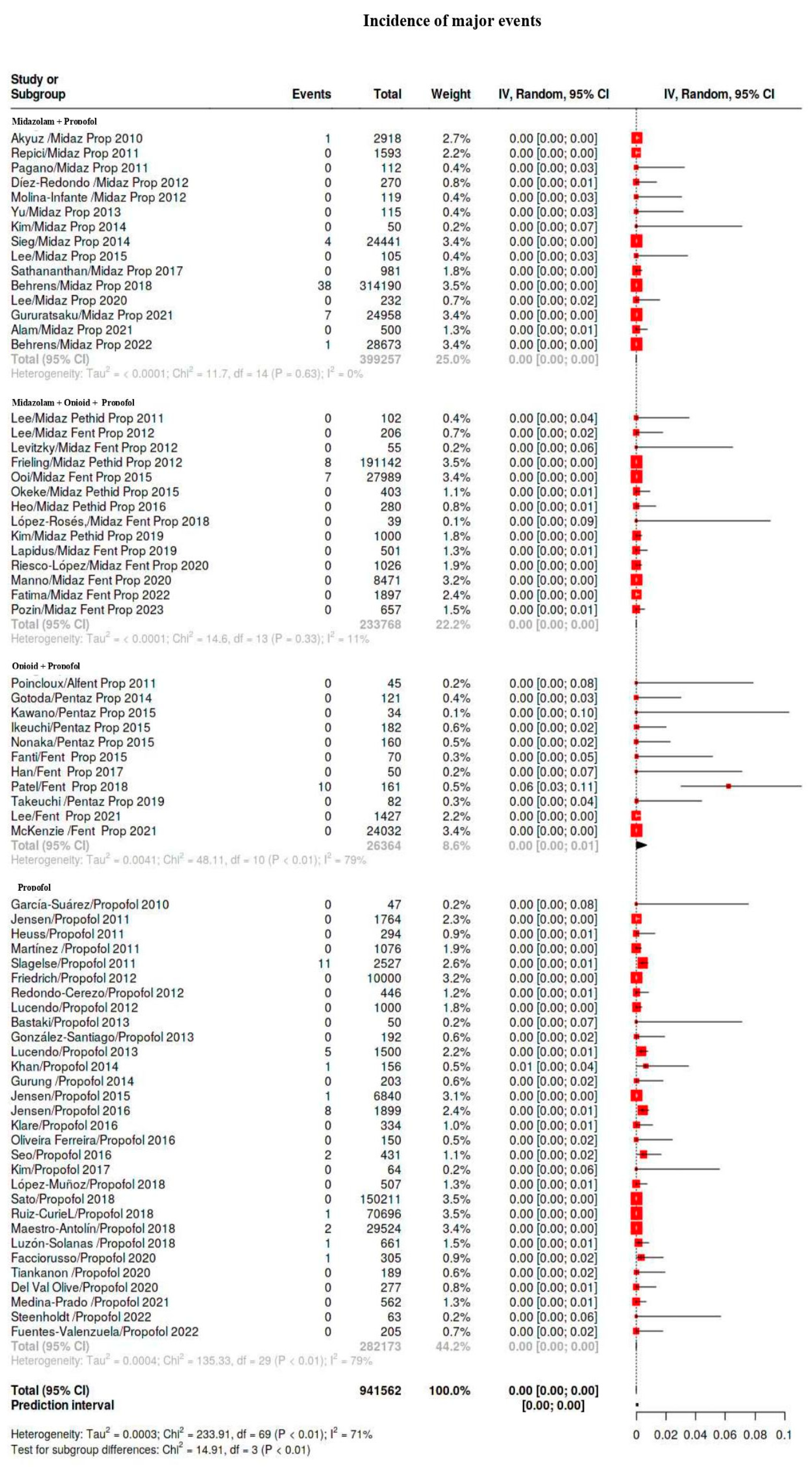
| Major | 109/941,562 | 0.12 | 0.04–0.16 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigò, F.; Gottin, M.; Conigliaro, R. The Incidence of Adverse Events in Adults Undergoing Procedural Sedation with Propofol Administered by Non-Anesthetists: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1234. https://doi.org/10.3390/diagnostics15101234
Pigò F, Gottin M, Conigliaro R. The Incidence of Adverse Events in Adults Undergoing Procedural Sedation with Propofol Administered by Non-Anesthetists: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(10):1234. https://doi.org/10.3390/diagnostics15101234
Chicago/Turabian StylePigò, Flavia, Matteo Gottin, and Rita Conigliaro. 2025. "The Incidence of Adverse Events in Adults Undergoing Procedural Sedation with Propofol Administered by Non-Anesthetists: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 10: 1234. https://doi.org/10.3390/diagnostics15101234
APA StylePigò, F., Gottin, M., & Conigliaro, R. (2025). The Incidence of Adverse Events in Adults Undergoing Procedural Sedation with Propofol Administered by Non-Anesthetists: A Systematic Review and Meta-Analysis. Diagnostics, 15(10), 1234. https://doi.org/10.3390/diagnostics15101234








