Fully Automated Photoplethysmography-Based Wearable Atrial Fibrillation Screening in a Hospital Setting
Abstract
:1. Introduction
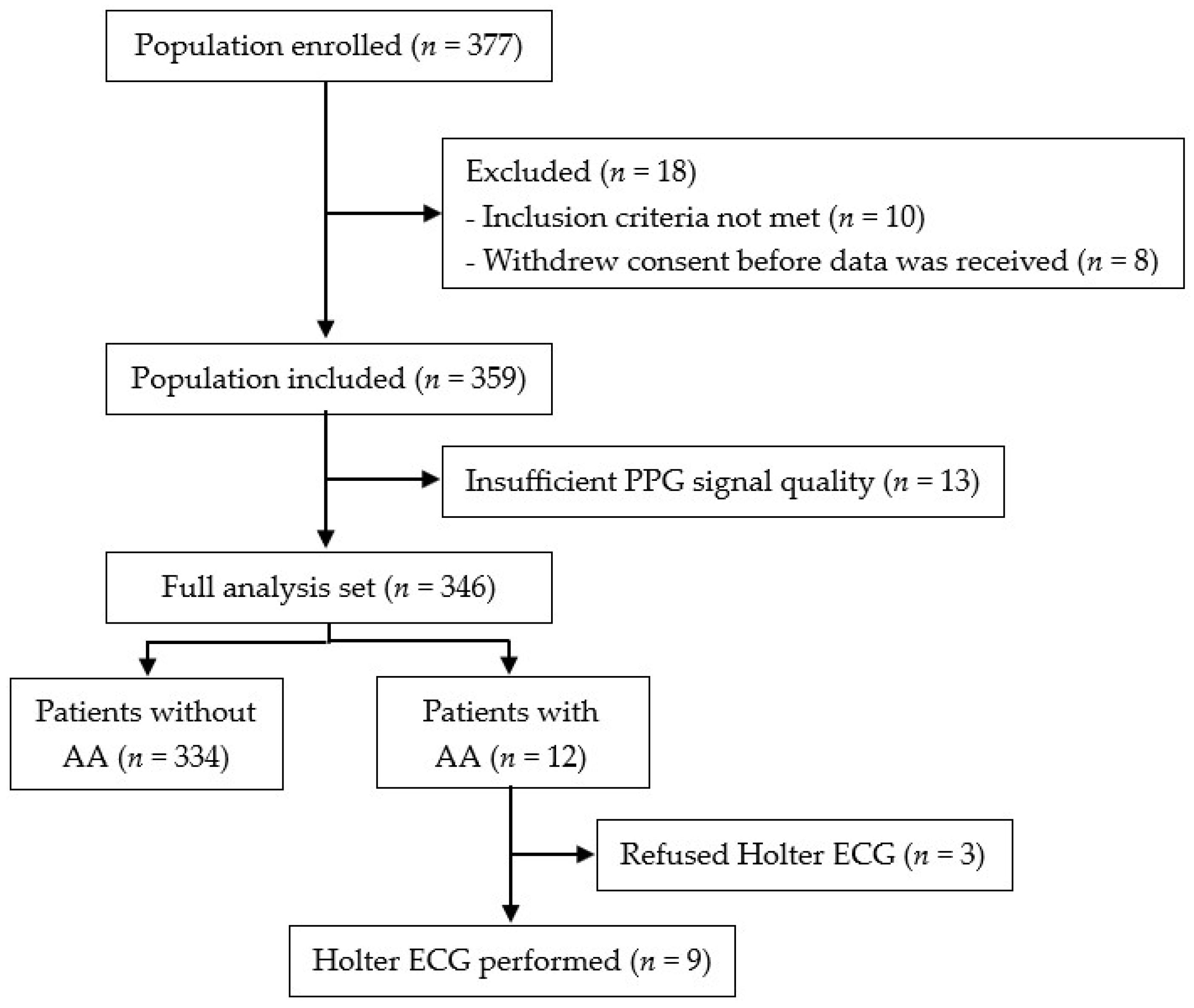
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
2.3. Ethics Approval and Patient Consent
2.4. Investigational Products
2.5. Intervention and Data Flow
2.6. Algorithm
2.7. Terminology
2.8. Interpretation of ECG
2.9. Outcomes
2.10. Compliance
2.11. Follow-Up and Adverse Events
2.12. Statistical Analysis
3. Results and Discussion
3.1. Patient Characteristics
3.2. PPG Analysis
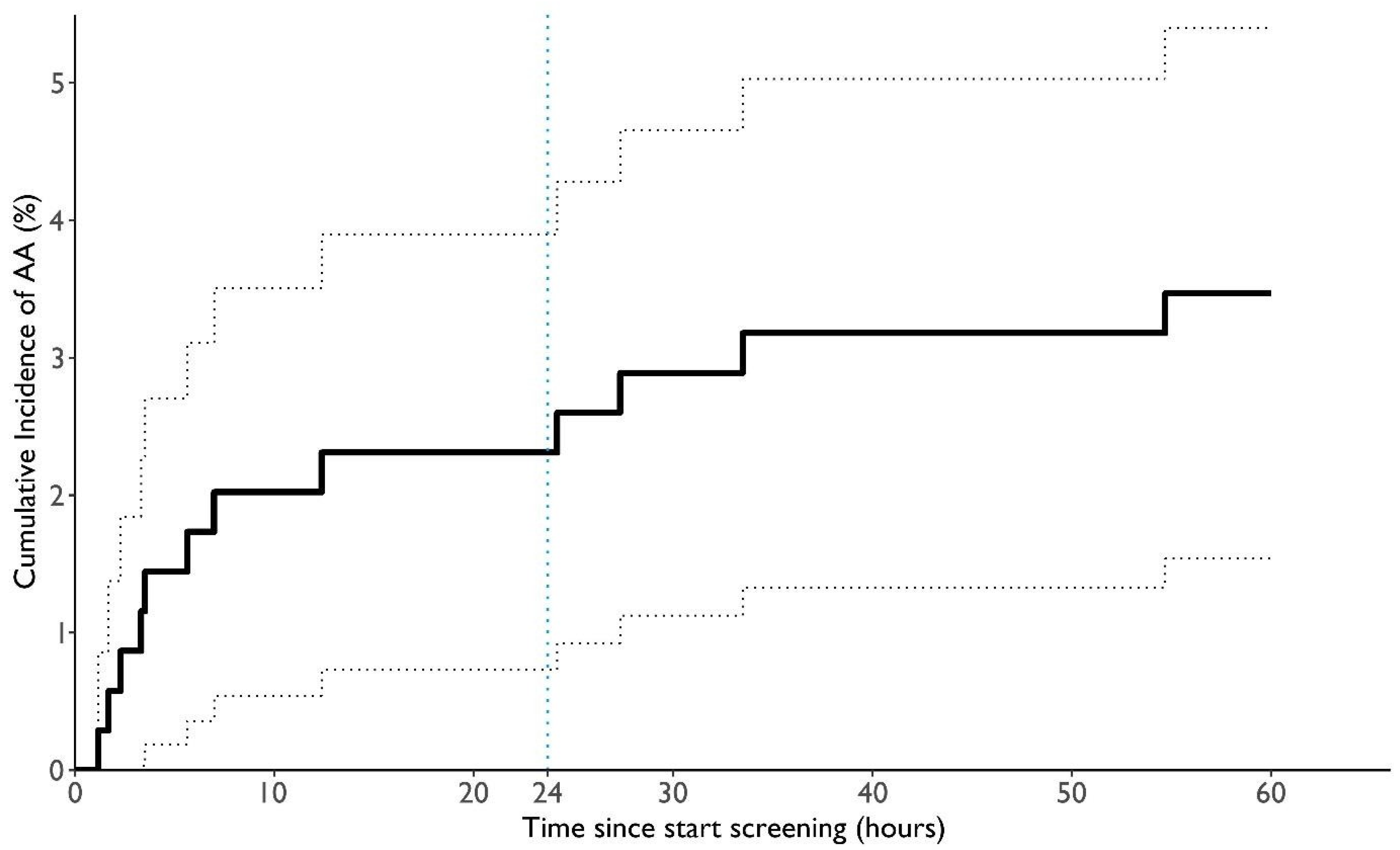
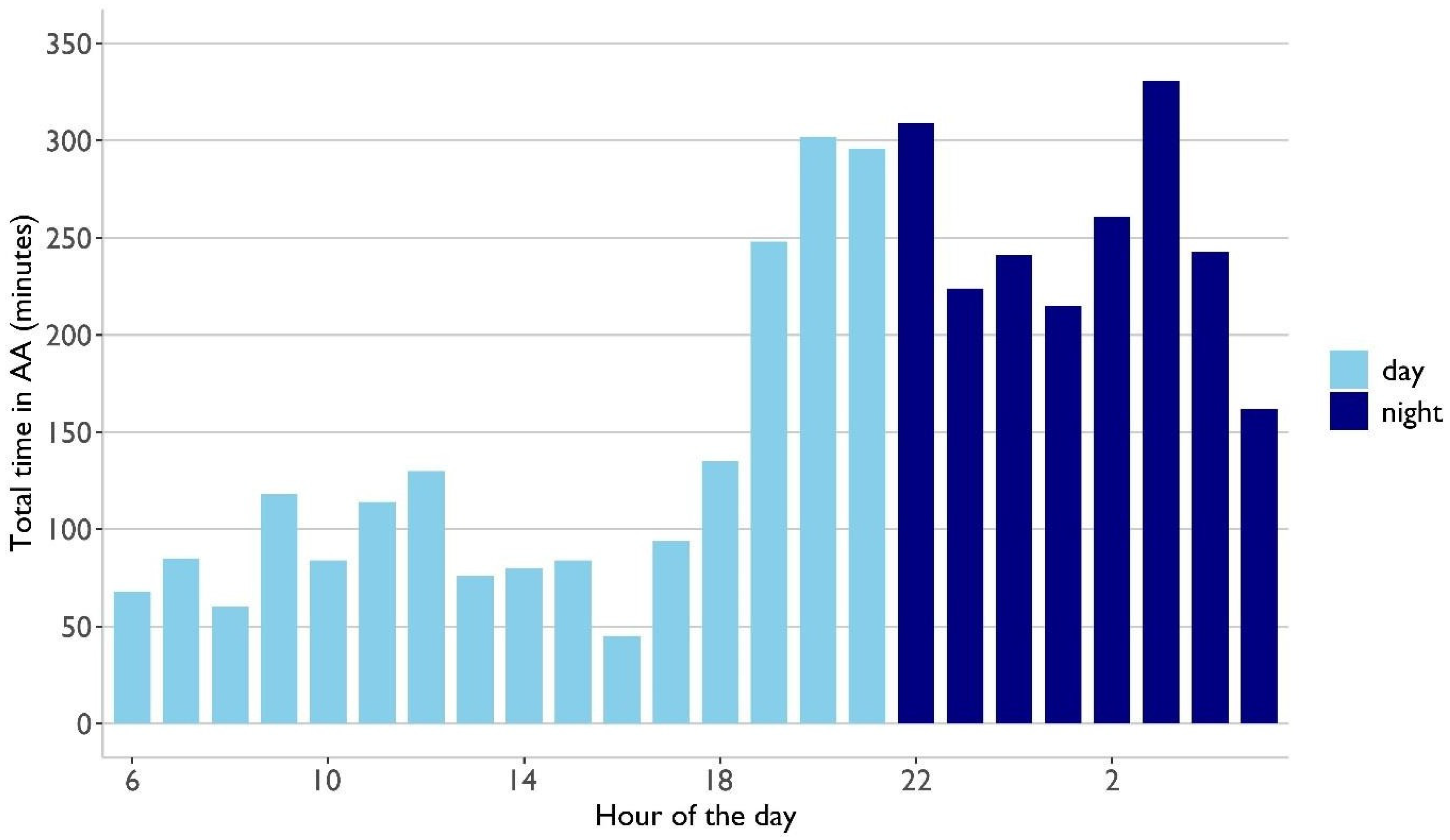
3.3. ECG Analysis
3.4. Compliance
3.5. Adverse Events and Deaths
3.6. Discussion
- The automated screening infrastructure functioned reliably, ensuring continuous data acquisition, processing, and visualization. No data were lost, demonstrating seamless system integration within a hospital environment.
- The system identified absolute arrhythmias in 3.5% of hospitalized patients, showcasing the potential of automated real-time monitoring.
- High patient acceptance was observed, with nearly 90% compliance.
- Automated analysis revealed that arrhythmic burden was 1.3 times higher at night, emphasizing the advantage of continuous tracking, particularly at night.
3.7. Limitations
3.8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| AA | absolute arrythmia |
| PPG | photoplethysmography |
| CHA2DS-VASc | congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age 65 to 74 years, sex category (female) |
| CI | confidence interval |
| Ref. | reference |
| ECG | electrocardiogram |
| SR | sinus rhythm |
| EHRA | European Heart Rhythm Association |
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide Epidemiology of Atrial Fibrillation. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Magnuson, A.; Bryngelsson, I.L.; Frøbert, O.; Henriksson, K.M.; Edvardsson, N.; Poçi, D. All-cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: A Swedish nationwide long-term case–control study. Eur. Heart J. 2013, 34, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Blomstrom Lundqvist, C.; Lip, G.Y.H.; Kirchhof, P. What are the costs of atrial fibrillation? Europace 2011, 13 (Suppl. S2), ii9–ii12. [Google Scholar] [CrossRef] [PubMed]
- Svennberg, E.; Friberg, L.; Frykman, V.; Al-Khalili, F.; Engdahl, J.; Rosenqvist, M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): A multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021, 398, 1498–1506. [Google Scholar] [CrossRef]
- Potpara, T.; Dagres, N.; Arbelo, E.; Boriani, G.; Castella, M.; Dan, G.A. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): Supplementary Data. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Rienstra, M.; Lubitz, S.A.; Mahida, S.; Magnani, J.W.; Fontes, J.D.; Sinner, M.F.; Ellinor, P.; Van Gelder, I.C.; Benjamin, E.J. Symptoms and functional status of patients with atrial fibrillation: State of the art and future research opportunities. Circulation 2012, 125, 2933–2943. [Google Scholar] [CrossRef]
- Healey, J.S.; Lopes, R.D.; Granger, C.B.; Alings, M.; Rivard, L.; McIntyre, W.F.; Atar, D.; Birnie, D.H.; Boriani, G.; Camm, A.J.; et al. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. N. Engl. J. Med. 2024, 390, 107–117. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Côté, R.; et al. Atrial Fibrillation in Patients with Cryptogenic Stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef]
- Reiffel, J.A.; Verma, A.; Kowey, P.R.; Halperin, J.L.; Gersh, B.J.; Wachter, R.; Pouliot, E.; Ziegler, P.D. Incidence of Previously Undiagnosed Atrial Fibrillation Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL AF Study. JAMA Cardiol. 2017, 2, 1120–1127. [Google Scholar] [CrossRef]
- Chua, S.K.; Chen, L.C.; Lien, L.M.; Lo, H.M.; Liao, Z.Y.; Chao, S.P.; Chuang, C.-Y.; Chiu, C.-Z. Comparison of Arrhythmia Detection by 24-Hour Holter and 14-Day Continuous Electrocardiography Patch Monitoring. Acta Cardiol. Sin. 2020, 36, 251–259. [Google Scholar] [PubMed]
- Bonomi, A.G.; Schipper, F.; Eerikäinen, L.M.; Margarito, J.; van Dinther, R.; Muesch, G.; de Morree, H.M.; Aarts, R.M.; Babaeizadeh, S.; McManus, D.D.; et al. Atrial Fibrillation Detection Using a Novel Cardiac Ambulatory Monitor Based on Photo-Plethysmography at the Wrist. J. Am. Heart Assoc. 2018, 7, e009351. [Google Scholar] [CrossRef] [PubMed]
- Dörr, M.; Nohturfft, V.; Brasier, N.; Bosshard, E.; Djurdjevic, A.; Gross, S.; Raichle, C.J.; Rhinisperger, M.; Stöckli, R.; Eckstein, J. The WATCH AF Trial: SmartWATCHes for Detection of Atrial Fibrillation. JACC Clin. Electrophysiol. 2019, 5, 199–208. [Google Scholar] [CrossRef]
- Krivoshei, L.; Weber, S.; Burkard, T.; Maseli, A.; Brasier, N.; Kühne, M.; Conen, D.; Huebner, T.; Seeck, A.; Eckstein, J. Smart detection of atrial fibrillation. EP Eur. 2017, 19, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Koenig, N.; Seeck, A.; Eckstein, J.; Mainka, A.; Huebner, T.; Voss, A.; Weber, S. Validation of a New Heart Rate Measurement Algorithm for Fingertip Recording of Video Signals with Smartphones. Telemed. e-Health 2016, 22, 631–636. [Google Scholar] [CrossRef]
- Wasserlauf, J.; You, C.; Patel, R.; Valys, A.; Albert, D.; Passman, R. Smartwatch Performance for the Detection and Quantification of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2019, 12, e006834. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- van Vliet, M.; Aalberts, J.J.J.; Hamelinck, C.; Hauer, A.D.; Hoftijzer, D.; Monnink, S.H.J.; Schipper, J.C.; Constandse, J.C.; Peters, N.S.; Lip, G.Y.H.; et al. Ambulatory atrial fibrillation detection and quantification by wristworn AI device compared to standard holter monitoring. NPJ Digit. Med. 2025, 8, 177. [Google Scholar] [CrossRef]
- Sibomana, O.; Hakayuwa, C.M.; Obianke, A.; Gahire, H.; Munyantore, J.; Chilala, M.M. Diagnostic accuracy of ECG smart chest patches versus PPG smartwatches for atrial fibrillation detection: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2025, 25, 132. [Google Scholar] [CrossRef]
- Chang, P.C.; Wen, M.S.; Chou, C.C.; Wang, C.C.; Hung, K.C. Atrial fibrillation detection using ambulatory smartwatch photoplethysmography and validation with simultaneous holter recording. Am. Heart J. 2022, 247, 55–62. [Google Scholar] [CrossRef]
- Charlton, P.H.; Kyriacou, P.A.; Mant, J.; Marozas, V.; Chowienczyk, P.; Alastruey, J. Wearable Photoplethysmography for Cardiovascular Monitoring. Proc. IEEE Inst. Electr. Electron. Eng. 2022, 110, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Faranesh, A.Z.; Selvaggi, C.; Atlas, S.J.; McManus, D.D.; Singer, D.E.; Pagoto, S.; McConnell, M.V.; Pantelopoulos, A.; Foulkes, A.S. Detection of Atrial Fibrillation in a Large Population Using Wearable Devices: The Fitbit Heart Study. Circulation 2022, 146, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, H.; Zhang, H.; Liu, T.; Liang, Z.; Xia, Y.; Yan, L.; Xing, Y.; Shi, H.; Li, S.; et al. Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 2365–2375. [Google Scholar] [CrossRef]
- Avram, R.; Ramsis, M.; Cristal, A.D.; Nathan, V.; Zhu, L.; Kim, J.; Kuang, J.; Gao, A.; Vittinghoff, E.; Rohdin-Bibby, L.; et al. Validation of an algorithm for continuous monitoring of atrial fibrillation using a consumer smartwatch. Heart Rhythm. 2021, 18, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Brasier, N.; Geissmann, L.; Käch, M.; Mutke, M.; Hoelz, B.; De Ieso, F.; Eckstein, J. Device- and Analytics-Agnostic Infrastructure for Continuous Inpatient Monitoring: A Technical Note. Digit. Biomark. 2020, 4, 62–68. [Google Scholar] [CrossRef]
- Fabritz, L.; Connolly, D.L.; Czarnecki, E.; Dudek, D.; Guasch, E.; Haase, D.; Huebner, T.; Zlahoda-Huzior, A.; Jolly, K.; Kirchhof, P.; et al. Smartphone and wearable detected atrial arrhythmias in Older Adults: Results of a fully digital European Case finding study. Eur. Heart J. Digit. Health 2022, 3, 610–625. [Google Scholar] [CrossRef]
- Reissenberger, P.; Serfözö, P.; Piper, D.; Juchler, N.; Glanzmann, S.; Gram, J.; Hensler, K.; Tonidandel, H.; Börlin, E.; D’souza, M.; et al. Determine atrial fibrillation burden with a photoplethysmographic mobile sensor: The atrial fibrillation burden trial: Detection and quantification of episodes of atrial fibrillation using a cloud analytics service connected to a wearable with photoplethysmographic sensor. Eur. Heart J. Digit. Health 2023, 4, 402–410. [Google Scholar]
- Birkemeyer, R.; Müller, A.; Wahler, S.; von der Schulenburg, J.M. A cost-effectiveness analysis model of Preventicus atrial fibrillation screening from the point of view of statutory health insurance in Germany. Health Econ. Rev. 2020, 10, 16. [Google Scholar] [CrossRef]
- Wahler, S.; Birkemeyer, R.; Alexopoulos, D.; Siudak, Z.; Müller, A.; von der Schulenburg, J.M. Cost-effectiveness of a photopethysmographic procedure for screening for atrial fibrillation in 6 European countries. Health Econ. Rev. 2022, 12, 17. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical Atrial Fibrillation and the Risk of Stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef]
- Philippsen, T.J.; Christensen, L.S.; Hansen, M.G.; Dahl, J.S.; Brandes, A. Detection of Subclinical Atrial Fibrillation in High-Risk Patients Using an Insertable Cardiac Monitor. JACC Clin. Electrophysiol. 2017, 3, 1557–1564. [Google Scholar] [CrossRef]
- Boriani, G.; Glotzer, T.V.; Santini, M.; West, T.M.; De Melis, M.; Sepsi, M.; Gasparini, M.; Lewalter, T.; Camm, J.A.; Singer, D.E. Device-detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur. Heart J. 2014, 35, 508–516. [Google Scholar] [CrossRef]
- Go, A.S.; Reynolds, K.; Yang, J.; Gupta, N.; Lenane, J.; Sung, S.H.; Harrison, T.N.; Liu, T.I.; Solomon, M.D. Association of Burden of Atrial Fibrillation with Risk of Ischemic Stroke in Adults with Paroxysmal Atrial Fibrillation: The KP-RHYTHM Study. JAMA Cardiol. 2018, 3, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Chung, M.K.; Allen, L.A.; Ezekowitz, M.; Furie, K.L.; McCabe, P.; Noseworthy, P.A.; Perez, M.V.; Turakhia, M.P.; American Heart Association Council on Clinical Cardiology; et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e623–e644. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.C.; Gummidipundi, S.E.; Lee, J.; Hedlin, H.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Gardner, R.M.; Cheung, L.; Hung, G.; et al. Arrhythmias Other Than Atrial Fibrillation in Those with an Irregular Pulse Detected with a Smartwatch: Findings from the Apple Heart Study. Circ. Arrhythmia Electrophysiol. 2021, 14, e010063. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Amino, M.; Adachi, K.; Matsuzaki, A.; Iwata, O.; Yoshioka, K.; Watanabe, E.; Tanabe, T. Circadian distribution of paroxysmal atrial fibrillation in patients with and without structural heart disease in untreated state. Ann. Noninvasive Electrocardiol. 2009, 14, 280–289. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Rajagopal, S.; Prieto-Simón, B.; Pogue, B.W. Recent advances in smart wearable sensors for continuous human health monitoring. Talanta 2024, 272, 125817. [Google Scholar] [CrossRef]


| Manufacturer | Device | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Reference |
|---|---|---|---|---|---|
| Preventicus | Device agnostic: Samsung Gear Fit II, Corsano Cardiowatch 287-1, Polar Verity Sense | 93.7 (CI: 89.8–96.4) | 98.2 (CI: 95.8–99.4) | 97.8 (CI: 94.9–99.3) | [13] |
| Apple | Apple Watch 2 | Not applicable | Not applicable | 84.0 (CI: 76.0–92.0) | [17] |
| Fitbit | Fitbit devices | 67.6 (CI: 62.4–72.6) | 98.4 (CI: 97.3–99.2) | 98.2 (CI: 95.5–99.5) | [22] |
| Huawei | Huawei Watch GT, Honor Watch | Not applicable | Not applicable | 91.6 (CI: 91.5–91.8) | [23] |
| Samsung | Samsung Galaxy Watch Active 2 | 87.8 (CI: 83.6–91.0) | 97.4 (CI: 97.1–97.7) | Not applicable | [24] |
| Product | Vendor/Make | Model/Version | Country |
|---|---|---|---|
| Wristband with PPG Sensor | Corsano Health B.V. | CardioWatch 287 Bracelet | The Netherlands |
| AF Detection Algorithm | Preventicus GmbH | Heartbeats Algorithm v1.1.4 | Germany |
| Device Hub Platform | Leitwert AG | Device Hub Platform | Switzerland |
| Holter ECG Device | GE Healthcare Getemed AG | SEERTM 1000 | Germany |
| Total (n = 346) | Without AA (n = 334) | With AA (n = 12) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 72.0 ± 9.8 | 71.8 ± 9.8 | 76.9 (10.2) | |
| Median (IQR) | 73 (12) | 72.5 (12) | 79.5 (11.5) | 0.63 1 | |
| Range | 32–96 | 32–96 | 58–89 | ||
| Sex | Female | 175 (50.6%) | 170 (50.9%) | 5 (41.7%) | 0.738 2 |
| Male | 171 (49.4%) | 164 (49.1%) | 7 (58.3%) | ||
| BMI | Mean ± SD | 26.7 ± 5.5 | 26.8 ± 5.5 | 26.0 ± 5.2 | 0.708 1 |
| CHA2DS2-VASc score | Mean ± SD | 3.5 ± 1.3 | 3.5 ± 1.3 | 4.0 ± 1.5 | 0.207 1 |
| Median (IQR) | 3 (2) | 3 (2) | 3.5 (2.25) | ||
| Range | 2–9 | 2–9 | 2–6 | ||
| Tattoo in sensor area | Yes | 1 (0.3%) | 1 (0.3%) | 0 | - |
| Skin color Fitzpatrick scale 3 | Mean ± SD | 2.2 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.6 | 0.908 1 |
| Noncompliant Patients (n = 42) | ||
|---|---|---|
| Irregular study termination (n = 20) | Not convinced by the wristband | 6 |
| Reason not provided | 3 | |
| Pacemaker or Holter | 4 | |
| Afraid of PPG sensor | 2 | |
| Health-related (unrelated to the study) | 5 | |
| Regular study termination (n = 22) | Forgot to wear device | 6 |
| Unaware device had to be worn at all times | 4 | |
| Uncomfortable | 5 | |
| Itchiness | 3 | |
| Lost to follow-up | 2 | |
| Deceased | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, K.; Reissenberger, P.; Piper, D.; Koenig, N.; Hoelz, B.; Schlaepfer, J.; Gysler, S.; McCullough, H.; Ramin-Wright, S.; Gabathuler, A.-L.; et al. Fully Automated Photoplethysmography-Based Wearable Atrial Fibrillation Screening in a Hospital Setting. Diagnostics 2025, 15, 1233. https://doi.org/10.3390/diagnostics15101233
Abdelhamid K, Reissenberger P, Piper D, Koenig N, Hoelz B, Schlaepfer J, Gysler S, McCullough H, Ramin-Wright S, Gabathuler A-L, et al. Fully Automated Photoplethysmography-Based Wearable Atrial Fibrillation Screening in a Hospital Setting. Diagnostics. 2025; 15(10):1233. https://doi.org/10.3390/diagnostics15101233
Chicago/Turabian StyleAbdelhamid, Khaled, Pamela Reissenberger, Diana Piper, Nicole Koenig, Bianca Hoelz, Julia Schlaepfer, Simone Gysler, Helena McCullough, Sebastian Ramin-Wright, Anna-Lena Gabathuler, and et al. 2025. "Fully Automated Photoplethysmography-Based Wearable Atrial Fibrillation Screening in a Hospital Setting" Diagnostics 15, no. 10: 1233. https://doi.org/10.3390/diagnostics15101233
APA StyleAbdelhamid, K., Reissenberger, P., Piper, D., Koenig, N., Hoelz, B., Schlaepfer, J., Gysler, S., McCullough, H., Ramin-Wright, S., Gabathuler, A.-L., Khandpur, J., Meier, M., & Eckstein, J. (2025). Fully Automated Photoplethysmography-Based Wearable Atrial Fibrillation Screening in a Hospital Setting. Diagnostics, 15(10), 1233. https://doi.org/10.3390/diagnostics15101233






