Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI
Abstract
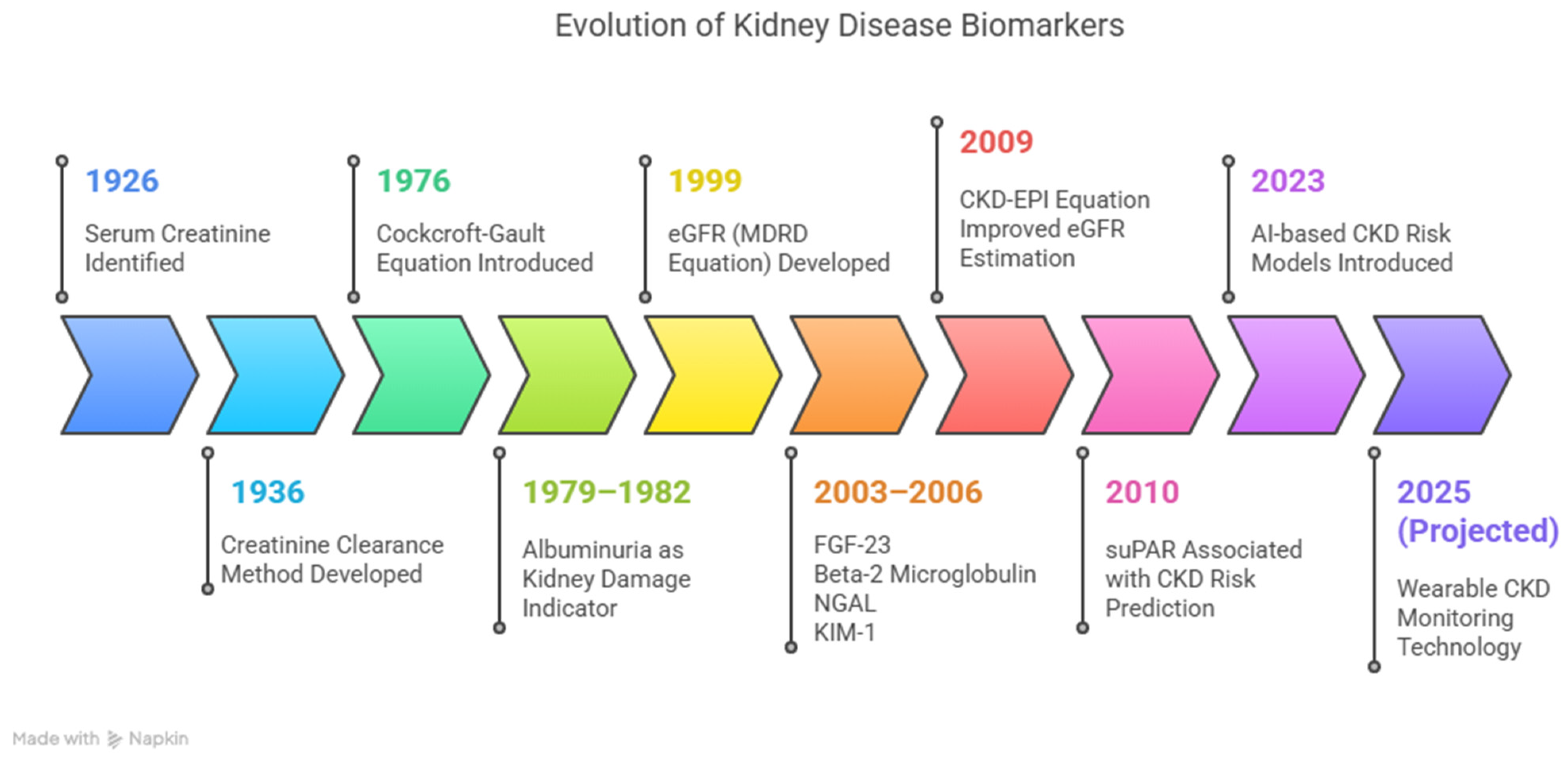
1. Introduction
2. Overview of Conventional CKD Diagnostics
2.1. Serum Creatinine and eGFR
2.2. Urinalysis and Urine Microscopy Exam
2.3. Proteinuria
2.4. Renal Imaging
2.5. Limitations and Emerging Perspectives
3. Rationale for Novel Biomarkers and Advanced Diagnostics
3.1. Unmet Needs in Early Detection
3.2. Improved Risk Stratification
3.3. Potential for Personalized Medicine
3.4. Applications in Prevention and Monitoring
3.5. Artificial Intelligence (AI) and CKD Diagnostics
4. Emerging Biomarkers in CKD
4.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
4.1.1. Diagnostic and Prognostic Utility in AKI
4.1.2. Role in CKD Progression and Risk Stratification
4.1.3. Implications in Diabetic Kidney Disease (DKD)
4.1.4. Applications in Personalized Medicine
4.2. Cystatin C
4.2.1. Integration into Clinical Practice and Foundational Evidence
4.2.2. Applications in Specific Conditions
4.2.3. Clinical Implications of Cystatin C
4.3. Kidney Injury Molecule-1 (KIM-1)
4.3.1. Diagnostic Utility in Acute Kidney Injury (AKI)
4.3.2. Prognostic Value in CKD
4.3.3. Applications in Specific Clinical Contexts
4.3.4. Clinical Implications of KIM-1
4.4. Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR)
4.4.1. Diagnostic and Prognostic Value in CKD
4.4.2. suPAR in Inflammation and Kidney Pathophysiology
4.4.3. suPAR in Multi-Biomarker Panels
4.4.4. Clinical Implications and Future Directions of suPAR
4.5. Other Promising Biomarkers
4.5.1. Beta-2-Microglobulin (B2M) and Beta-Trace Protein (BTP)
4.5.2. Normoalbuminuric Markers
4.5.3. Fibrosis-Related Markers
4.5.4. Novel Biomarker Combinations
4.5.5. Proenkephalin (PENK), Fibroblast Growth Factor-23 (FGF-23), and Dickkopf-3 (DKK3)
4.5.6. Clinical Implications and Future Directions of Additional Biomarkers
5. Multi-Omics Approaches in CKD
5.1. Genomics
5.1.1. Genome-Wide Polygenic Score (GPS) and Genetic Risk Prediction
5.1.2. Genomic Insights into CKD Pathophysiology
5.1.3. Gene Polymorphisms and CKD Risk
5.1.4. Integrative Multi-Omics Approaches
5.1.5. Non-Invasive Genetic Biomarkers
5.1.6. Future Directions in CKD Genomics
5.2. Transcriptomics & Epigenetics
5.2.1. Role of Transcriptomics and Epigenetics in CKD
5.2.2. MicroRNA Profiling as Biomarkers
5.2.3. Epigenetic Mechanisms and Their Clinical Implications
5.2.4. Integration into Multi-Omics Platforms
5.2.5. Future Directions in Transcriptomics & Epigenetics
5.3. Proteomics
5.3.1. Urinary Proteomics in CKD Diagnosis
5.3.2. Technological Advances in Proteomics
5.3.3. Future Directions in Proteomics
5.4. Metabolomics
5.4.1. Emerging Metabolomic Biomarkers in CKD
5.4.2. Metabolomics in CKD Risk Stratification
5.4.3. Integration of Metabolomics into Multi-Omics Frameworks
5.4.4. Future Directions and Clinical Implementation
6. Advances in Imaging-Based Diagnosis
6.1. Functional and Structural Imaging in CKD
6.2. AI-Driven Imaging and Predictive Modeling
6.3. Future Directions and Clinical Implications Imaging-Based Diagnostics
7. Digital Health and Artificial Intelligence in CKD
7.1. AI-Driven Biomarker Integration
7.2. Telehealth and Remote Monitoring
7.3. Innovations in Mobile Health and Decision Support
7.4. Challenges and Ethical Considerations
7.5. Future Directions and Clinical Implications of Digital Health & AI
8. Clinical Implementation and Guidelines
8.1. Biomarker Integration into Clinical Workflows
8.2. Genomic Tools and Personalized Medicine in CKD
8.3. Economic Considerations and Cost-Effectiveness
8.4. Barriers to Adoption and Future Directions
9. Limitations and Controversies in CKD Diagnostics
9.1. Heterogeneity in Biomarker Studies
9.2. Lack of Standardization in Biomarker Measurements
9.3. Overdiagnosis and Unnecessary Interventions
9.4. Economic Implications and Cost-Effectiveness
10. Future Perspectives
10.1. Integration of Genomic and Epigenetic Biomarkers
10.2. Digital Biomarkers and AI Innovations
10.3. Emerging Technologies and Smart Diagnostics
10.4. Metabolomics and Multi-Omics Synergy
10.5. Age-Specific Research Needs and Health Disparities
10.6. Long-Term Research Directions
11. Conclusions
Key Points
- Emerging biomarkers such as NGAL, cystatin C, suPAR, TIMP-2, and IGFBP7 demonstrate superior sensitivity and specificity compared to traditional markers, enabling earlier CKD detection and improved patient stratification.
- Multi-omics approaches integrating genomics, proteomics, metabolomics, and transcriptomics significantly enhance diagnostic accuracy and facilitate personalized CKD management strategies.
- AI-driven predictive models offer powerful, real-time risk assessment tools, enabling earlier identification of high-risk CKD patients compared to conventional approaches.
- Significant hurdles remain regarding biomarker standardization, extensive clinical validation, and integration into routine clinical workflows, underscoring the necessity for ongoing research.
- Future research must prioritize refining and validating biomarker panels, standardizing assays, and clearly defining clinical guidelines to fully leverage these diagnostic advancements for improved CKD outcomes.
Funding
Conflicts of Interest
References
- Canki, E.; Kho, E.; Hoenderop, J.G.J. Urinary biomarkers in kidney disease. Clin. Chim. Acta 2024, 555, 117798. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Chronic Kidney Disease: Early Detection, Mechanisms, and Therapeutic Implications. J. Pers. Med. 2023, 13, 1447. [Google Scholar] [CrossRef]
- Swaminathan, S.M.; Rao, I.R.; Shenoy, S.V.; Prabhu, A.R.; Mohan, P.B.; Rangaswamy, D.; Bhojaraja, M.V.; Nagri, S.K.; Nagaraju, S.P. Novel biomarkers for prognosticating diabetic kidney disease progression. Int. Urol. Nephrol. 2023, 55, 913–928. [Google Scholar] [CrossRef]
- Karimi, F.; Moazamfard, M.; Taghvaeefar, R.; Sohrabipour, S.; Dehghani, A.; Azizi, R.; Dinarvand, N. Early Detection of Diabetic Nephropathy Based on Urinary and Serum Biomarkers: An Updated Systematic Review. Adv. Biomed. Res. 2024, 13, 104. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, G.; Shi, X. Advances in the Progression and Prognosis Biomarkers of Chronic Kidney Disease. Front. Pharmacol. 2021, 12, 785375. [Google Scholar] [CrossRef]
- Bufkin, K.B.; Karim, Z.A.; Silva, J. Review of the limitations of current biomarkers in acute kidney injury clinical practices. SAGE Open Med. 2024, 12, 20503121241228446. [Google Scholar] [CrossRef]
- Spencer, S.; Desborough, R.; Bhandari, S. Should Cystatin C eGFR Become Routine Clinical Practice? Biomolecules 2023, 13, 1075. [Google Scholar] [CrossRef]
- Mirna, M.; Topf, A.; Wernly, B.; Rezar, R.; Paar, V.; Jung, C.; Salmhofer, H.; Kopp, K.; Hoppe, U.C.; Schulze, P.C.; et al. Novel biomarkers in patients with chronic kidney disease: An analysis of patients enrolled in the gckd-study. J. Clin. Med. 2020, 9, 886. [Google Scholar] [CrossRef]
- Abbasi, F.; Moosaie, F.; Khaloo, P.; Firouzabadi, F.D.; Abhari, S.M.F.; Atainia, B.; Ardeshir, M.; Nakhjavani, M.; Esteghamati, A. Neutrophil Gelatinase-Associated Lipocalin and Retinol-Binding Protein-4 as Biomarkers for Diabetic Kidney Disease. Kidney Blood Press. Res. 2020, 45, 222–232. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Beirão, I.; Alves, R.; Belo, L.; Santos-Silva, A. New potential biomarkers for chronic kidney disease management—A review of the literature. Int. J. Mol. Sci. 2021, 22, 43. [Google Scholar] [CrossRef]
- Tummalapalli, L.; Nadkarni, G.N.; Coca, S.G. Biomarkers for predicting outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 480–486. [Google Scholar] [CrossRef]
- Chen, D.C.; Potok, O.A.; Rifkin, D.; Estrella, M.M. Advantages, Limitations, and Clinical Considerations in Using Cystatin C to Estimate GFR. Kidney360 2022, 3, 1807–1814. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Cook, D.L.; Rivera, I.M.; Eisenhower, D.D. Chronic Kidney Disease Affects 47 Million People in the Chronic Kidney Disease: Detection and Evaluation. 2017. [Online]. Available online: www.aafp.org/afp (accessed on 13 February 2025).
- Cavanaugh, C.; Perazella, M.A. Urine Sediment Examination in the Diagnosis and Management of Kidney Disease: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 258–272. [Google Scholar] [CrossRef]
- Arceo, E.S.; Dizon, G.A.; Tiongco, R.E.G. Serum cystatin C as an early marker of nephropathy among type 2 diabetics: A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Singh, R.J. Utilities of traditional and novel biomarkers in the management of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2019, 57, 215–226. [Google Scholar] [CrossRef]
- Carter, J.L.; Parker, C.T.; E Stevens, P.; Eaglestone, G.; Knight, S.; Farmer, C.K.T.; Lamb, E.J. Biological variation of plasma and urinary markers of acute kidney injury in patients with chronic kidney disease. Clin. Chem. 2016, 62, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, J.I.; Farag, Y.M.K.; Durthaler, J. Albuminuria: An Underappreciated Risk Factor for Cardiovascular Disease. J. Am. Heart Assoc. 2024, 13, e030131. [Google Scholar] [CrossRef]
- Yaqoob, K.; Naderi, H.; Thomson, R.J.; Aksentijevic, D.; Jensen, M.T.; Munroe, P.B.; E Petersen, S.; Aung, N.; Yaqoob, M.M. Prognostic impact of albuminuria in early-stage chronic kidney disease on cardiovascular outcomes: A cohort study. Heart 2025. ahead of print. [Google Scholar] [CrossRef]
- Fleig, S.; Magnuska, Z.A.; Koczera, P.; Salewski, J.; Djudjaj, S.; Schmitz, G.; Kiessling, F. Advanced ultrasound methods to improve chronic kidney disease diagnosis. npj Imaging 2024, 2, 22. [Google Scholar] [CrossRef]
- Chaykovska, L.; Heunisch, F.; von Einem, G.; Alter, M.L.; Hocher, C.-F.; Tsuprykov, O.; Dschietzig, T.; Kretschmer, A.; Hocher, B. Urinary Vitamin D binding protein and KIM-1 are potent new biomarkers of major adverse renal events in patients undergoing coronary angiography. PLoS ONE 2016, 11, e0145723. [Google Scholar] [CrossRef]
- Liu, C.; Debnath, N.; Mosoyan, G.; Chauhan, K.; Vasquez-Rios, G.; Soudant, C.; Menez, S.; Parikh, C.R.; Coca, S.G. Systematic Review and Meta-Analysis of Plasma and Urine Biomarkers for CKD Outcomes. J. Am. Soc. Nephrol. 2022, 33, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Mizdrak, M.; Kumrić, M.; Kurir, T.T.; Božić, J. Emerging Biomarkers for Early Detection of Chronic Kidney Disease. J. Pers. Med. 2022, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Thielemans, R.; Speeckaert, R.; Delrue, C.; De Bruyne, S.; Oyaert, M.; Speeckaert, M.M. Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases. Diagnostics 2023, 13, 3077. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Wahab, A.M.; El Sayed Zaki, M.; Motawea, M. MicroRNA-451 as an Early Predictor of Chronic Kidney Disease in Diabetic Nephropathy. Int. J. Nephrol. 2020, 2020, 8075376. [Google Scholar] [CrossRef]
- Abdou, A.E.; Anani, H.A.; Ibrahim, H.F.; Ebrahem, E.E.; Seliem, N.; Youssef, E.M.; Ghoraba, N.M.; Hassan, A.S.; Ramadan, M.A.A.; Mahmoud, E.; et al. Urinary IgG, serum CX3CL1 and miRNA-152-3p: As predictors of nephropathy in Egyptian type 2 diabetic patients. Tissue Barriers 2022, 10, 1994823. [Google Scholar] [CrossRef]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef]
- Gembillo, G.; Siligato, R.; Santoro, D. Personalized Medicine in Kidney Disease. J. Pers. Med. 2023, 13, 1501. [Google Scholar] [CrossRef]
- Gocze, I.; Koch, M.; Renner, P.; Zeman, F.; Graf, B.M.; Dahlke, M.H.; Nerlich, M.; Schlitt, H.J.; Kellum, J.A.; Bein, T. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS ONE 2015, 10, e0120863. [Google Scholar] [CrossRef]
- Engelman, D.T.; Crisafi, C.; Germain, M.; Greco, B.; Nathanson, B.H.; Engelman, R.M.; Schwann, T.A. Using urinary biomarkers to reduce acute kidney injury following cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 1235–1246.e2. [Google Scholar] [CrossRef]
- El Ghoul, B.; Squalli, T.; Servais, A.; Elie, C.; Meas-Yedid, V.; Trivint, C.; Vanmassenhove, J.; Grunfeld, J.P.; Olivo-Marin, J.C.; Thervet, E.; et al. Urinary procollagen III aminoterminal propeptide (PIIINP): A fibrotest for the nephrologist. Clin. J. Am. Soc. Nephrol. 2010, 5, 205–210. [Google Scholar] [CrossRef]
- Pan, Q.; Tong, M. Artificial intelligence in predicting chronic kidney disease prognosis. A systematic review and meta-analysis. Ren. Fail. 2024, 46, 2435483. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Islam, M.M.; Poly, T.N.; Weng, Y.C. Artificial Intelligence in Kidney Disease: A Comprehensive Study and Directions for Future Research. Diagnostics 2024, 14, 397. [Google Scholar] [CrossRef]
- Engström, J.; Koozi, H.; Didriksson, I.; Larsson, A.; Friberg, H.; Frigyesi, A.; Spångfors, M. Plasma neutrophil gelatinase-associated lipocalin independently predicts dialysis need and mortality in critical COVID-19. Sci. Rep. 2024, 14, 6695. [Google Scholar] [CrossRef] [PubMed]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.; Choi, I.J.; Lee, D.; Ahn, Y.; Kim, M.J.; Jeon, D.S. Predictive and Prognostic Value of Serum Neutrophil Gelatinase-Associated Lipocalin for Contrast-Induced Acute Kidney Injury and Long-Term Clinical Outcomes after Percutaneous Coronary Intervention. J. Clin. Med. 2022, 11, 5971. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Bourgonje, M.F.; Kieneker, L.M.; Gemert, S.l.B.-V.; Gordijn, S.J.; Hidden, C.; Nilsen, T.; Gansevoort, R.T.; Mulder, D.J.; et al. Plasma Neutrophil Gelatinase-Associated Lipocalin Associates with New-Onset Chronic Kidney Disease in the General Population. Biomolecules 2023, 13, 338. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Marker of Kidney Damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- He, P.; Bai, M.; Hu, J.P.; Dong, C.; Sun, S.; Huang, C. Significance of Neutrophil Gelatinase-Associated Lipocalin as a Biomarker for the Diagnosis of Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2020, 45, 497–509. [Google Scholar] [CrossRef]
- Gottlieb, E.R.; Estiverne, C.; Tolan, N.V.; Melanson, S.E.F.; Mendu, M.L. Estimated GFR With Cystatin C and Creatinine in Clinical Practice: A Retrospective Cohort Study. Kidney Med. 2023, 5, 100600. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Lees, J.S.; Rutherford, E.; Stevens, K.I.; Chen, D.C.; Scherzer, R.; Estrella, M.M.; Sullivan, M.K.; Ebert, N.; Mark, P.B.; Shlipak, M.G. Assessment of Cystatin C Level for Risk Stratification in Adults with Chronic Kidney Disease. JAMA Netw. Open 2022, 5, e2238300. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Katz, R.; Sarnak, M.J.; Ix, J.; Fried, L.F.; De Boer, I.; Palmas, W.; Siscovick, D.; Levey, A.S.; Shlipak, M.G. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J. Am. Soc. Nephrol. 2011, 22, 147–155. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Gao, B.; Wang, J.; Yang, C.; Zhao, M.H.; Zhang, L. The Difference Between Cystatin C- and Creatinine-Based Estimated Glomerular Filtration Rate and Risk of Diabetic Microvascular Complications Among Adults With Diabetes: A Population-Based Cohort Study. Diabetes Care 2024, 47, 873–880. [Google Scholar] [CrossRef]
- Helmersson-Karlqvist, J.; Lipcsey, M.; Ärnlöv, J.; Bell, M.; Ravn, B.; Dardashti, A.; Larsson, A. Cystatin C predicts long term mortality better than creatinine in a nationwide study of intensive care patients. Sci. Rep. 2021, 11, 5882. [Google Scholar] [CrossRef]
- Geng, J.; Qiu, Y.; Qin, Z.; Su, B. The value of kidney injury molecule 1 in predicting acute kidney injury in adult patients: A systematic review and Bayesian meta-analysis. J. Transl. Med. 2021, 19, 105. [Google Scholar] [CrossRef]
- Lan, H.; Liu, X.; Yang, D.; Zhang, D.; Wang, L.; Hu, L. Comparing diagnostic accuracy of biomarkers for acute kidney injury after major surgery: A PRISMA systematic review and network meta-analysis. Medicine 2023, 102, e35284. [Google Scholar] [CrossRef]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Brilland, B.; Boud’Hors, C.; Wacrenier, S.; Blanchard, S.; Cayon, J.; Blanchet, O.; Piccoli, G.B.; Henry, N.; Djema, A.; Coindre, J.-P.; et al. Kidney injury molecule 1 (KIM-1): A potential biomarker of acute kidney injury and tubulointerstitial injury in patients with ANCA-glomerulonephritis. Clin. Kidney J. 2023, 16, 1521–1533. [Google Scholar] [CrossRef]
- Schulz, C.-A.; Engström, G.; Nilsson, J.; Almgren, P.; Petkovic, M.; Christensson, A.; Nilsson, P.M.; Melander, O.; Orho-Melander, M. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol. Dial. Transplant. 2020, 35, 265–273. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef]
- Garg, V.; Kumar, M.; Mahapatra, H.S.; Chitkara, A.; Gadpayle, A.K.; Sekhar, V. Novel urinary biomarkers in pre-diabetic nephropathy. Clin. Exp. Nephrol. 2015, 19, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Schrauben, S.J.; Shou, H.; Zhang, X.; Anderson, A.H.; Bonventre, J.V.; Chen, J.; Coca, S.; Furth, S.L.; Greenberg, J.H.; Gutierrez, O.M.; et al. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. J. Am. Soc. Nephrol. 2021, 32, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sudhini, Y.R.; Wei, C.; Reiser, J. suPAR: An Inflammatory Mediator for Kidneys. Kidney Dis. 2022, 8, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.S.; Sever, S.; Ko, Y.-A.; Trachtman, H.; Awad, M.; Wadhwani, S.; Altintas, M.M.; Wei, C.; Hotton, A.L.; French, A.L.; et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N. Engl. J. Med. 2015, 373, 1916–1925. [Google Scholar] [CrossRef]
- Hayek, S.S.; Landsittel, D.P.; Wei, C.; Zeier, M.; Yu, A.S.; Torres, V.E.; Roth, S.; Pao, C.S.; Reiser, J. Soluble urokinase plasminogen activator receptor and decline in kidney function in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2019, 30, 1305–1313. [Google Scholar] [CrossRef]
- Jhee, J.H.; Nam, B.Y.; Lee, C.J.; Park, J.T.; Han, S.H.; Kang, S.; Park, S.; Yoo, T. Soluble urokinase-type plasminogen activator receptor, changes of 24-hour blood pressure, and progression of chronic kidney disease. J. Am. Heart Assoc. 2021, 10, e017225. [Google Scholar] [CrossRef]
- O’Connor, L.M.; O’Connor, B.A.; Lim, S.B.; Zeng, J.; Lo, C.H. Integrative multi-omics and systems bioinformatics in translational neuroscience: A data mining perspective. J. Pharm. Anal. 2023, 13, 836–850. [Google Scholar] [CrossRef]
- Yadav, N.; Narang, J.; Chhillar, A.K.; Rana, J.S.; Siddique, M.U.M.; Kenawy, E.-R.; Alkahtani, S.; Ahsan, M.N.; Nayak, A.K.; Hasnain, S. Diagnostic methods employing kidney biomarkers clinching biosensors as promising tools. Sens. Int. 2024, 5, 100253. [Google Scholar] [CrossRef]
- Barton, K.T.; Kakajiwala, A.; Dietzen, D.J.; Goss, C.W.; Gu, H.; Dharnidharka, V.R. Using the newer Kidney Disease: Improving global outcomes criteria, beta-2-microglobulin levels associate with severity of acute kidney injury. Clin. Kidney J. 2018, 11, 797–802. [Google Scholar] [CrossRef]
- Inker, L.A.; Tighiouart, H.; Coresh, J.; Foster, M.C.; Anderson, A.H.; Beck, G.J.; Contreras, G.; Greene, T.; Karger, A.B.; Kusek, J.W.; et al. GFR Estimation Using β-Trace Protein and β2-Microglobulin in CKD. Am. J. Kidney Dis. 2016, 67, 40–48. [Google Scholar] [CrossRef]
- Benito, S.; Unceta, N.; Maciejczyk, M.; Sánchez-Ortega, A.; Taranta-Janusz, K.; Szulimowska, J.; Zalewska, A.; Andrade, F.; Gómez-Caballero, A.; Dubiela, P.; et al. Revealing novel biomarkers for diagnosing chronic kidney disease in pediatric patients. Sci. Rep. 2024, 14, 11549. [Google Scholar] [CrossRef] [PubMed]
- Villalvazo, P.; Villavicencio, C.; de Rivera, M.G.; Fernandez-Fernandez, B.; Ortiz, A. Systems biology and novel biomarkers for the early detection of diabetic kidney disease. Nephron 2024, 149, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yi, B.; Liu, Y.; Wang, J.; Dai, Q.; Huang, Y.; Li, Y.C.; Zhang, H. Urinary NGAL and RBP Are Biomarkers of Normoalbuminuric Renal Insufficiency in Type 2 Diabetes Mellitus. J. Immunol. Res. 2019, 2019, 5063089. [Google Scholar] [CrossRef]
- Ix, J.H.; Katz, R.; Bansal, N.; Foster, M.; Weiner, D.E.; Tracy, R.; Jotwani, V.; Hughes-Austin, J.; McKay, D.; Gabbai, F.; et al. Urine Fibrosis Markers and Risk of Allograft Failure in Kidney Transplant Recipients: A Case-Cohort Ancillary Study of the FAVORIT Trial. Am. J. Kidney Dis. 2017, 69, 410–419. [Google Scholar] [CrossRef]
- Rende, U.; Guller, A.; Goldys, E.M.; Pollock, C.; Saad, S. Diagnostic and prognostic biomarkers for tubulointerstitial fibrosis. J. Physiol. 2023, 601, 2801–2826. [Google Scholar] [CrossRef]
- Mao, H.; Chen, L.; Wu, W.; Zhang, L.; Li, X.; Chen, Y.; Huang, Z.; Ou, S. Noninvasive Assessment of Renal Fibrosis of Chronic Kidney Disease in Rats by [68Ga]Ga-FAPI-04 Small Animal PET/CT and Biomarkers. Mol. Pharm. 2023, 20, 2714–2725. [Google Scholar] [CrossRef]
- Mansour, S.G.; Puthumana, J.; Coca, S.G.; Gentry, M.; Parikh, C.R. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: A systematic review. BMC Nephrol. 2017, 18, 72. [Google Scholar] [CrossRef]
- Connolly, M.; Kinnin, M.; McEneaney, D.; Menown, I.; Kurth, M.; Lamont, J.; Morgan, N.; Harbinson, M. Prediction of contrast induced acute kidney injury using novel biomarkers following contrast coronary angiography. QJM Int. J. Med. 2018, 111, 103–110. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Xiang, Y.; Ma, B.; Li, H.; Li, Y.; Shi, Y.; Li, S.; Bai, Y. Role of MCP-1 as an inflammatory biomarker in nephropathy. Front. Immunol. 2023, 14, 1303076. [Google Scholar] [CrossRef]
- Khan, A.; Turchin, M.C.; Patki, A.; Srinivasasainagendra, V.; Shang, N.; Nadukuru, R.; Jones, A.C.; Malolepsza, E.; Dikilitas, O.; Kullo, I.J.; et al. Genome-wide polygenic score to predict chronic kidney disease across ancestries. Nat. Med. 2022, 28, 1412–1420. [Google Scholar] [CrossRef]
- Franceschini, N.; Feldman, D.L.; Berg, J.S.; Besse, W.; Chang, A.R.; Dahl, N.K.; Gbadegesin, R.; Pollak, M.R.; Rasouly, H.M.; Smith, R.J.; et al. Advancing Genetic Testing in Kidney Diseases: Report From a National Kidney Foundation Working Group. Am. J. Kidney Dis. 2024, 84, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, J.; Mallett, A.J. Exploring the impact and utility of genomic sequencing in established CKD. Clin. Kidney J. 2024, 17, sfae043. [Google Scholar] [CrossRef] [PubMed]
- Mallawaarachchi, A.C.; Fowles, L.; Wardrop, L.; Wood, A.; O’Shea, R.; Biros, E.; Harris, T.; Alexander, S.I.; Bodek, S.; Boudville, N.; et al. Genomic Testing in Patients with Kidney Failure of an Unknown Cause: A National Australian Study. Clin. J. Am. Soc. Nephrol. 2024, 19, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, D.; Krishnamoorthy, E.; Mariappanadar, V.; Viswanathan, V.; Ramkumar, K.M. Increased levels of circulating (TNF-α) is associated with (-308G/A) promoter polymorphism of TNF-α gene in Diabetic Nephropathy. Int. J. Biol. Macromol. 2018, 107, 2113–2121. [Google Scholar] [CrossRef]
- Kumari, M.; Mohan, A.; Ecelbarger, C.M.; Gupta, A.; Prasad, N.; Tiwari, S. miR-451 Loaded Exosomes Are Released by the Renal Cells in Response to Injury and Associated With Reduced Kidney Function in Human. Front. Physiol. 2020, 11, 234. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Kokori, E.; Ogieuhi, I.J.; Babalola, A.E.; Ayodeji, K.B.; Bisola, M.-A.I.; Oluwatomiwa, A.V.; Ishola, I.V. Genomic insights into renal diseases: Advancements and implications. Egypt. J. Intern. Med. 2024, 36, 73. [Google Scholar] [CrossRef]
- Schlosser, P.; Tin, A.; Matias-Garcia, P.R.; Thio, C.H.L.; Joehanes, R.; Liu, H.; Weihs, A.; Yu, Z.; Hoppmann, A.; Grundner-Culemann, F.; et al. Meta-analyses identify DNA methylation associated with kidney function and damage. Nat. Commun. 2021, 12, 7174. [Google Scholar] [CrossRef]
- Wing, M.R.; Ramezani, A.; Gill, H.S.; Devaney, J.M.; Raj, D.S. Epigenetics of Progression of Chronic Kidney Disease: Fact or Fantasy? Semin. Nephrol. 2013, 33, 363–374. [Google Scholar] [CrossRef]
- Ben-Dov, I.Z.; Tan, Y.-C.; Morozov, P.; Wilson, P.D.; Rennert, H.; Blumenfeld, J.D.; Tuschl, T. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: Description of miRNA profiles at baseline. PLoS ONE 2014, 9, e86856. [Google Scholar] [CrossRef]
- Hill, C.; Avila-Palencia, I.; Maxwell, A.P.; Hunter, R.F.; McKnight, A.J. Harnessing the Full Potential of Multi-Omic Analyses to Advance the Study and Treatment of Chronic Kidney Disease. Front. Nephrol. 2022, 2, 923068. [Google Scholar] [CrossRef]
- Reznichenko, A.; Nair, V.; Eddy, S.; Fermin, D.; Tomilo, M.; Slidel, T.; Ju, W.; Henry, I.; Badal, S.S.; Wesley, J.D.; et al. Unbiased kidney-centric molecular categorization of chronic kidney disease as a step towards precision medicine. Kidney Int. 2024, 105, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Catanese, L.; Siwy, J.; Mischak, H.; Wendt, R.; Beige, J.; Rupprecht, H. Recent Advances in Urinary Peptide and Proteomic Biomarkers in Chronic Kidney Disease: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 9156. [Google Scholar] [CrossRef] [PubMed]
- Makhammajanov, Z.; Kabayeva, A.; Auganova, D.; Tarlykov, P.; Bukasov, R.; Turebekov, D.; Kanbay, M.; Molnar, M.Z.; Kovesdy, C.P.; Abidi, S.H.; et al. Candidate protein biomarkers in chronic kidney disease: A proteomics study. Sci. Rep. 2024, 14, 14014. [Google Scholar] [CrossRef] [PubMed]
- Dubin, R.F.; Deo, R.; Ren, Y.; Wang, J.; Zheng, Z.; Shou, H.; Go, A.S.; Parsa, A.; Lash, J.P.; Rahman, M.; et al. Proteomics of CKD progression in the chronic renal insufficiency cohort. Nat. Commun. 2023, 14, 6340. [Google Scholar] [CrossRef]
- Geng, T.-T.; Chen, J.-X.; Lu, Q.; Wang, P.-L.; Xia, P.-F.; Zhu, K.; Li, Y.; Guo, K.-Q.; Yang, K.; Liao, Y.-F.; et al. Nuclear Magnetic Resonance–Based Metabolomics and Risk of CKD. Am. J. Kidney Dis. 2023, 83, 9–17. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Ieong, C.A.; Tou, T.; Tsai, T.; Zhu, H.; Liu, Z.; Liu, P. A Metabolomics study of metabolites associated with the glomerular filtration rate. BMC Nephrol. 2023, 24, 105. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Nimer, R.M.; Sumaily, K.M.; Alabdaljabar, M.S.; Jacob, M.; Sabi, E.M.; Hussein, M.H.; Rahman, A.A. Metabolomics profiling distinctively identified end-stage renal disease patients from chronic kidney disease patients. Sci. Rep. 2023, 13, 6161. [Google Scholar] [CrossRef]
- Ragi, N.; Sharma, K. Deliverables from Metabolomics in Kidney Disease: Adenine, New Insights, and Implication for Clinical Decision-Making. Am. J. Nephrol. 2024, 55, 421–438. [Google Scholar] [CrossRef]
- Wen, D.; Zheng, Z.; Surapaneni, A.; Yu, B.; Zhou, L.; Zhou, W.; Xie, D.; Shou, H.; Avila-Pacheco, J.; Kalim, S.; et al. Metabolite profiling of CKD progression in the chronic renal insufficiency cohort study. JCI Insight 2022, 7, e161696. [Google Scholar] [CrossRef]
- Khan, K.; Zameer, F.; Jain, P.; Kr, R.; Niranjan, V.; S, M.; H, R.; Padyana, S. Artificial Intelligence in Revolutionizing Kidney Care and Beyond: Kid-AI Revolution. J. Bio-X Res. 2024, 7, 0022. [Google Scholar] [CrossRef]
- Bodard, S.; Cornelis, F.H. Non-invasive functional MRI techniques for early detection of kidney injury in chronic kidney disease: A positive step forward. Ann. Transl. Med. 2024, 12, 80. [Google Scholar] [CrossRef]
- Bais, T.; Geertsema, P.; Knol, M.G.; van Gastel, M.D.; de Haas, R.J.; Meijer, E.; Gansevoort, R.T.; on behalf of the DIPAK Consortium. Validation of the Mayo Imaging Classification System for Predicting Kidney Outcomes in ADPKD. Clin. J. Am. Soc. Nephrol. 2024, 19, 591–601. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Huang, H.; Jiao, L.; Zheng, S.; Ying, Y.; Li, Q. AI-based segmentation of renal enhanced CT images for quantitative evaluate of chronic kidney disease. Sci. Rep. 2024, 14, 16890. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, X.; Meng, L.; Chen, Y.; Zhao, Q.; Cheng, S.; Zhang, W.; Cheng, T.; Wang, C.; Shi, Z.; et al. Screening chronic kidney disease through deep learning utilizing ultra-wide-field fundus images. Npj Digit. Med. 2024, 7, 275. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, H.; Deng, T.; Tang, S.; Yuan, X.; Tang, W.; Xie, Y.; Ge, H.; Wang, X.; Zhou, Q.; et al. Role of artificial intelligence in kidney disease. Int. J. Med Sci. 2020, 17, 970–984. [Google Scholar] [CrossRef]
- Isaza-Ruget, M.A.; Yomayusa, N.; González, C.A.; H., C.A.; de Oro V., F.A.; Cely, A.; Murcia, J.; Gonzalez-Velez, A.; Robayo, A.; Colmenares-Mejía, C.C.; et al. Predicting chronic kidney disease progression with artificial intelligence. BMC Nephrol. 2024, 25, 148. [Google Scholar] [CrossRef]
- Martin-Cleary, C.; Sanz, A.B.; Avello, A.; Sanchez-Niño, M.D.; Ortiz, A. NephroCheck at 10: Addressing unmet needs in AKI diagnosis and risk stratification. Clin. Kidney J. 2023, 16, 1359–1366. [Google Scholar] [CrossRef]
- Ellis, T.; Kwon, A.J.; Hong, M.Y. The Effectiveness of Telehealth Intervention on Chronic Kidney Disease Management in Adults: A Systematic Review. Mayo Clin. Proc. Digit. Health 2024, 3, 100181. [Google Scholar] [CrossRef]
- Lightfoot, C.J.; Wilkinson, T.J.; Sohansoha, G.K.; Gillies, C.L.; Vadaszy, N.; Ford, E.C.; Davies, M.J.; Yates, T.; Smith, A.C.; Graham-Brown, M.P.M. The effects of a digital health intervention on patient activation in chronic kidney disease. npj Digit. Med. 2024, 7, 318. [Google Scholar] [CrossRef]
- Tangri, N.; Ferguson, T.W. Role of artificial intelligence in the diagnosis and management of kidney disease: Applications to chronic kidney disease and acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2022, 31, 283–287. [Google Scholar] [CrossRef]
- Gupta, A.; Sontakke, T.; Acharya, S.; Kumar, S. A Comprehensive Review of Biomarkers for Chronic Kidney Disease in Older Individuals: Current Perspectives and Future Directions. Cureus 2024, 16, e70262. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Roadmap for Accelerating the Development of Biomarkers for Acute Kidney Injury. 2022. [Online]. Available online: www.kidneyhealthinitiative.org (accessed on 17 March 2025).
- Lopes, M.B.; Coletti, R.; Duranton, F.; Glorieux, G.; Campos, M.A.J.; Klein, J.; Ley, M.; Perco, P.; Sampri, A.; Tur-Sinai, A. The Omics-Driven Machine Learning Path to Cost-Effective Precision Medicine in Chronic Kidney Disease. Proteomics 2025, 25, e202400108. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Andreoletti, M.; Haller, L.; Vayena, E.; Blasimme, A. Mapping the ethical landscape of digital biomarkers: A scoping review. PLoS Digit. Health 2024, 3, e0000519. [Google Scholar] [CrossRef]
- Abraham, A.G.; Xu, Y.; Roem, J.L.; Greenberg, J.H.; Weidemann, D.K.; Sabbisetti, V.S.; Bonventre, J.V.; Denburg, M.; Warady, B.A.; Furth, S.L. Variability in CKD Biomarker Studies: Soluble Urokinase Plasminogen Activator Receptor (suPAR) and Kidney Disease Progression in the Chronic Kidney Disease in Children (CKiD) Study. Kidney Med. 2021, 3, 712–721.e1. [Google Scholar] [CrossRef]
- Yeo, S.C.; Wang, H.; Ang, Y.G.; Lim, C.K.; Ooi, X.Y. Cost-effectiveness of screening for chronic kidney disease in the general adult population: A systematic review. Clin. Kidney J. 2024, 17, sfad137. [Google Scholar] [CrossRef]
- Zafarnejad, R.; Chen, Q.; Griffin, P.M. Cost-effectiveness of screening for chronic kidney disease using a cumulative eGFR-based statistic. PLoS ONE 2024, 19, e0299401. [Google Scholar] [CrossRef]
- Ali, H.; Vairo, F.P.E.; AlSahow, A.; Abu-Farha, M. Editorial: Advances in chronic kidney disease diagnosis and therapy. Front. Med. 2023, 10, 1209571. [Google Scholar] [CrossRef]
- Jawad, K.M.T.; Verma, A.; Amsaad, F.; Ashraf, L. AI-Driven Predictive Analytics Approach for Early Prognosis of Chronic Kidney Disease Using Ensemble Learning and Explainable AI. arXiv 2024, arXiv:2406.06728. [Google Scholar]
- Stewart, S.; Kalra, P.A.; Blakeman, T.; Kontopantelis, E.; Cranmer-Gordon, H.; Sinha, S. Chronic kidney disease: Detect, diagnose, disclose—A UK primary care perspective of barriers and enablers to effective kidney care. BMC Med. 2024, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.A.; Gonzalez-King, H.; Mellergaard, M.; Nair, S.; Salomon, C.; Handberg, A. Comprehensive strategy for identifying extracellular vesicle surface proteins as biomarkers for chronic kidney disease. Front. Physiol. 2024, 15, 1328362. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, X.M.; Huang, C.; Pollock, C.A. MicroRNA as novel biomarkers and therapeutic targets in diabetic kidney disease: An update. FASEB Bioadv. 2019, 1, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Zierer, J.; Würtz, P.; Haller, T.; Metspalu, A.; Gieger, C.; Thorand, B.; Meisinger, C.; Waldenberger, M.; Raitakari, O.; et al. Circulating metabolic biomarkers of renal function in diabetic and non-diabetic populations. Sci. Rep. 2018, 8, 15249. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, U.; Chiwhane, A.; Acharya, S.; Daiya, V.; Kasat, P.R.; Sachani, P.; Mapari, S.A.; Bedi, G.N. A Comprehensive Review of Advanced Biomarkers for Chronic Kidney Disease in Older Adults: Current Insights and Future Directions. Cureus 2024, 16, e70413. [Google Scholar] [CrossRef]
| Biomarker | Strengths | Limitations |
|---|---|---|
| Serum Creatinine | Widely available, inexpensive | Late-stage detection, affected by muscle mass, diet, age, and sex |
| eGFR (Creatinine-based) | Standardized, globally used | Influenced by age, sex, muscle mass, and metabolic factors, reducing accuracy in certain populations |
| Proteinuria (ACR included) | Predicts CKD progression, cardiovascular risk | Highly variable due to hydration, physical activity, and transient conditions |
| Urinalysis | Detects hematuria and proteinuria | Detects abnormalities but cannot determine CKD cause or stage |
| Urine Microscopy | Identifies RBC, WBC, and casts for CKD assessment | Requires expertise for accurate interpretation, variability in readings |
| Renal Ultrasound | Identifies structural abnormalities, obstruction | Cannot assess early CKD or renal function directly |
| Advanced Renal Imaging (CEUS, SWE) | Improves fibrosis and microvascular damage detection | Limited availability, requires specialized training |
| Biomarker | Mechanism | Clinical Applications | Limitations | Validation Status |
|---|---|---|---|---|
| NGAL | Released from tubular epithelial cells in response to injury | Early detection of AKI, CKD progression marker | Affected by systemic inflammation, not specific to kidney injury | Widely studied, available for clinical use for AKI |
| KIM-1 | Upregulated in proximal tubular cells after injury | Sensitive marker for AKI, predictor of CKD progression | Requires standardization of assay cutoffs, limited availability in routine practice | Under validation, used in nephrotoxicity studies |
| suPAR | Inflammatory biomarker linked to podocyte dysfunction and fibrosis | Predicts CKD onset and progression, associated with cardiovascular risk | Influenced by systemic inflammation, lacks standardized cutoffs | Increasing clinical interest, studies support risk prediction |
| B2M | Freely filtered and reabsorbed in tubules; marker of filtration function | Alternative to creatinine-based eGFR, CKD severity indicator | Elevated in inflammatory conditions, not kidney-specific | Limited routine use, under evaluation |
| BTP | Low-molecular-weight protein used for GFR estimation | Enhances CKD staging, complements B2M for filtration assessment | Requires further validation for clinical adoption | Emerging, gaining interest for eGFR refinement |
| FGF-23 | Regulator of phosphate homeostasis, linked to CKD-mineral bone disorder | Predictor of CKD progression and cardiovascular morbidity | Variability in assay results, influenced by dietary phosphate | Increasing use in research and risk assessment |
| DKK3 | Tubular stress biomarker involved in fibrosis pathways | Early CKD detection, prognostic marker for renal fibrosis | Lacks standardized reference ranges | Early-stage validation, promising for risk stratification |
| PENK | Peptide reflecting real-time GFR | More accurate than creatinine in dynamic kidney function assessment | Limited commercial assays available | Research phase, high potential for precision nephrology |
| MCP-1 | Chemokine involved in monocyte recruitment and renal inflammation | Indicator of fibrosis severity, CKD progression marker | Affected by systemic inflammatory diseases | Experimental, not yet widely adopted |
| PIIINP | Marker of extracellular matrix turnover and collagen deposition | Assesses fibrosis severity, predicts renal allograft dysfunction | Assay standardization needed, requires further validation | Research phase, being explored for transplant monitoring |
| Multi-Biomarker Panels | Integrates multiple biomarkers for improved CKD risk stratification | Enhances predictive accuracy by combining different pathophysiological pathways | Cost and accessibility limitations, lack of established guidelines | Gaining traction, panels under evaluation for clinical implementation |
| Category | Biomarker | Function | Clinical Relevance | Validation Status |
|---|---|---|---|---|
| Genomics | APOL1 Genetic Variants | Associated with podocyte dysfunction and increased CKD risk, particularly in African ancestry populations | Genetic screening for early identification of high-risk individuals | Well-established, used in risk prediction models |
| UMOD Gene Variants | Implicated in CKD progression via sodium transport dysregulation | Potential target for precision medicine in CKD | Under research, potential for therapeutic applications | |
| COL4A5 Variants (Alport Syndrome) | Causes inherited CKD via glomerular basement membrane defects | Genetic diagnosis crucial for early intervention in hereditary CKD | Clinically validated, used for hereditary CKD diagnosis | |
| Transcriptomics & Epigenetics | miRNA-451 | Biomarker for diabetic nephropathy, linked to tubular injury | Highly sensitive and specific for early-stage CKD detection | Early validation, requires large-scale cohort studies |
| miRNA-152-3p | Predictor of CKD progression, associated with eGFR decline | Useful for monitoring disease progression in at-risk populations | Experimental, requires further validation | |
| DNA Methylation at RASAL1 | Epigenetic marker of renal fibrosis | Potential therapeutic target for renal fibrosis interventions | Promising, under investigation for clinical translation | |
| Proteomics | CKD273 Peptide Panel | Predictive panel for CKD progression and diabetic nephropathy | Validated in clinical trials (PRIORITY) for early detection and intervention | Well-validated, incorporated into multi-omics models |
| Plasma Proteomic Markers (B2MG, FETUA, VTDB, AMBP, CERU) | Circulating proteins associated with CKD progression and kidney function decline | Identified in proteomic risk models but requires further validation in large cohorts | Experimental, undergoing validation in CKD cohorts | |
| Urinary Peptide Profiling (CE-MS-Based) | Mass spectrometry-based identification of urinary peptides for CKD risk assessment | Improves early-stage CKD detection and progression monitoring | Increasing clinical use, pending standardization | |
| Metabolomics | Branched-Chain Amino Acids (BCAAs) | Dysregulation linked to muscle catabolism and metabolic imbalance | Potential metabolic intervention target for CKD progression | Under research, potential for clinical application |
| Tryptophan Metabolites (Xanthurenic Acid, Hydroxypicolinic Acid) | Indicators of systemic inflammation and oxidative stress | Identifies high-risk CKD patients for early intervention | Emerging, requires further large-scale validation | |
| Adenine | Novel fibrosis marker, particularly in diabetic kidney disease | Early diagnostic and prognostic marker for CKD | Under investigation, promising for CKD monitoring | |
| Hydroxyasparagine | Marker for kidney function assessment superior to creatinine | Enhances pathway-specific biomarkers in personalized CKD risk assessment | Early-stage research, not yet standardized | |
| Pseudouridine & Homocitrulline | Indicators of glomerular filtration decline | Validated in CRIC, ARIC, and AASK cohorts for CKD risk stratification | Clinically validated in multiple CKD cohorts | |
| Lipid Metabolites (VLDL, HDL, Triglycerides) | Dysregulated lipid metabolism associated with CKD risk | Integrated into predictive models for enhanced CKD risk assessment | Increasingly recognized, requires clinical validation | |
| Multi-Omics Integration | Multi-Biomarker Panels (e.g., suPAR + TNFR-1 + MCP-1) | Combines markers of inflammation, fibrosis, and kidney function | Validated in inflammation-associated CKD risk models; enhances predictive accuracy | Gaining traction, undergoing clinical evaluation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alobaidi, S. Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI. Diagnostics 2025, 15, 1225. https://doi.org/10.3390/diagnostics15101225
Alobaidi S. Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI. Diagnostics. 2025; 15(10):1225. https://doi.org/10.3390/diagnostics15101225
Chicago/Turabian StyleAlobaidi, Sami. 2025. "Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI" Diagnostics 15, no. 10: 1225. https://doi.org/10.3390/diagnostics15101225
APA StyleAlobaidi, S. (2025). Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI. Diagnostics, 15(10), 1225. https://doi.org/10.3390/diagnostics15101225






