Detection Rates of PSMA-PET Radiopharmaceuticals in Recurrent Prostate Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Search Results
3.2. Patient Populations
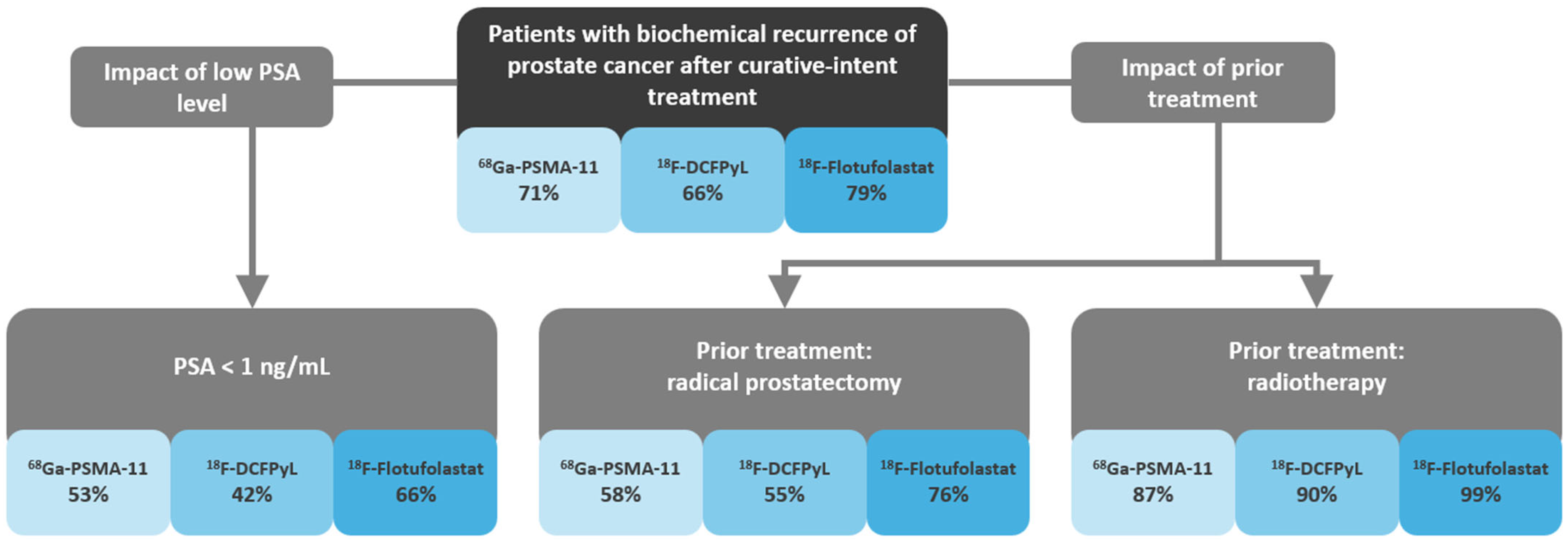
3.3. Detection Rates
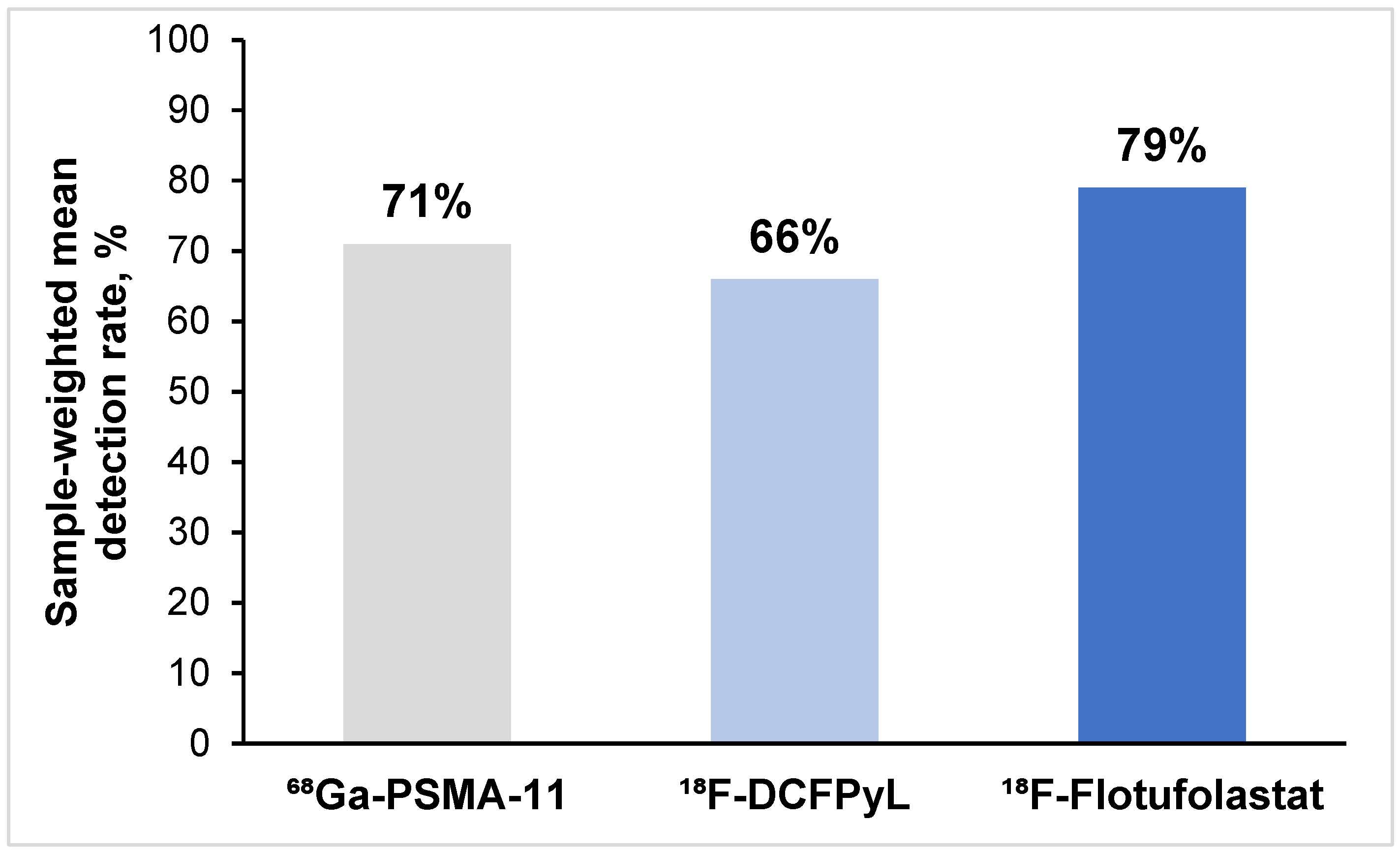
3.3.1. Overall Patient-Level Detection Rates
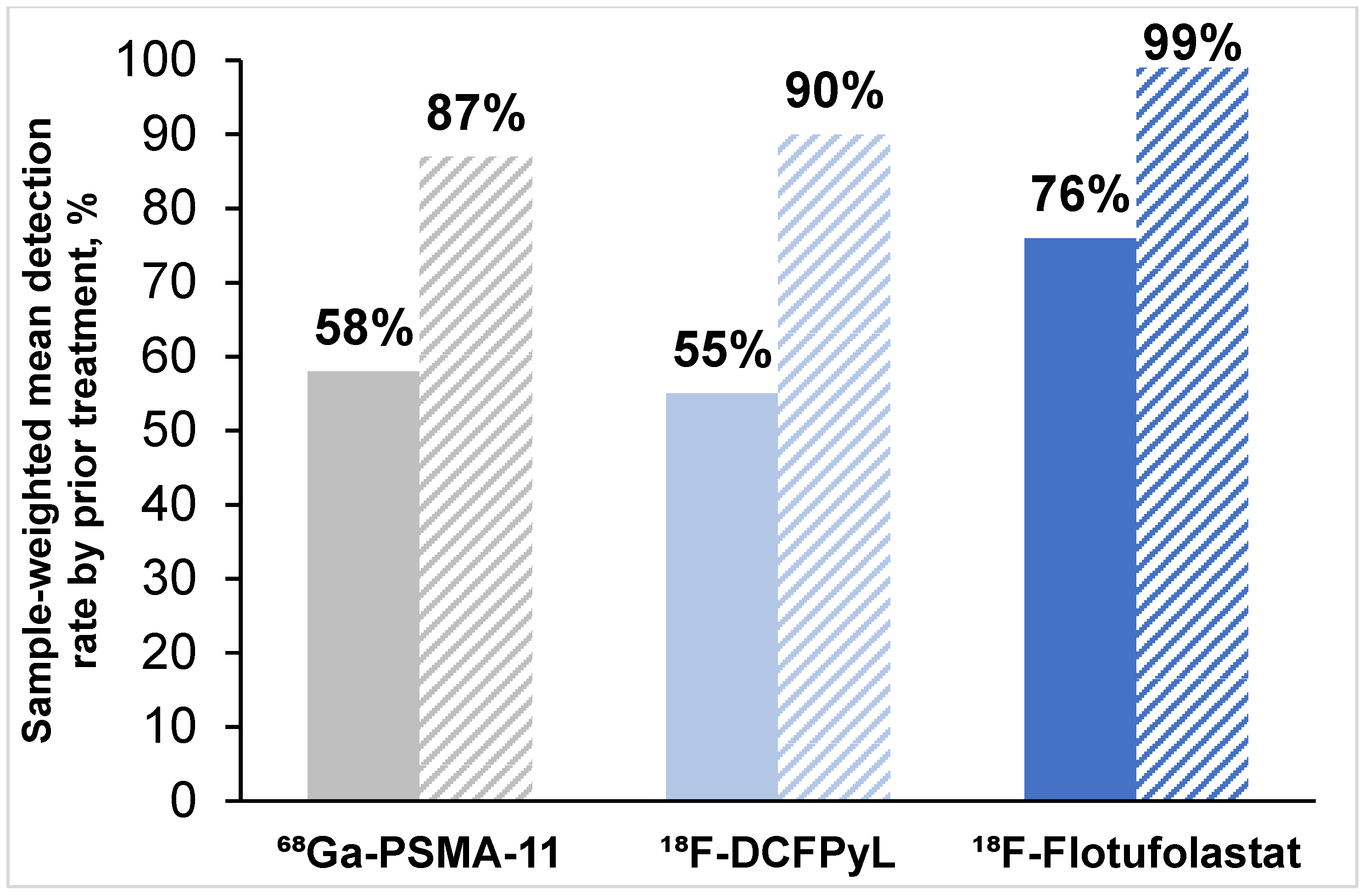
3.3.2. Patient-Level Detection Rates Stratified by Prior Treatment
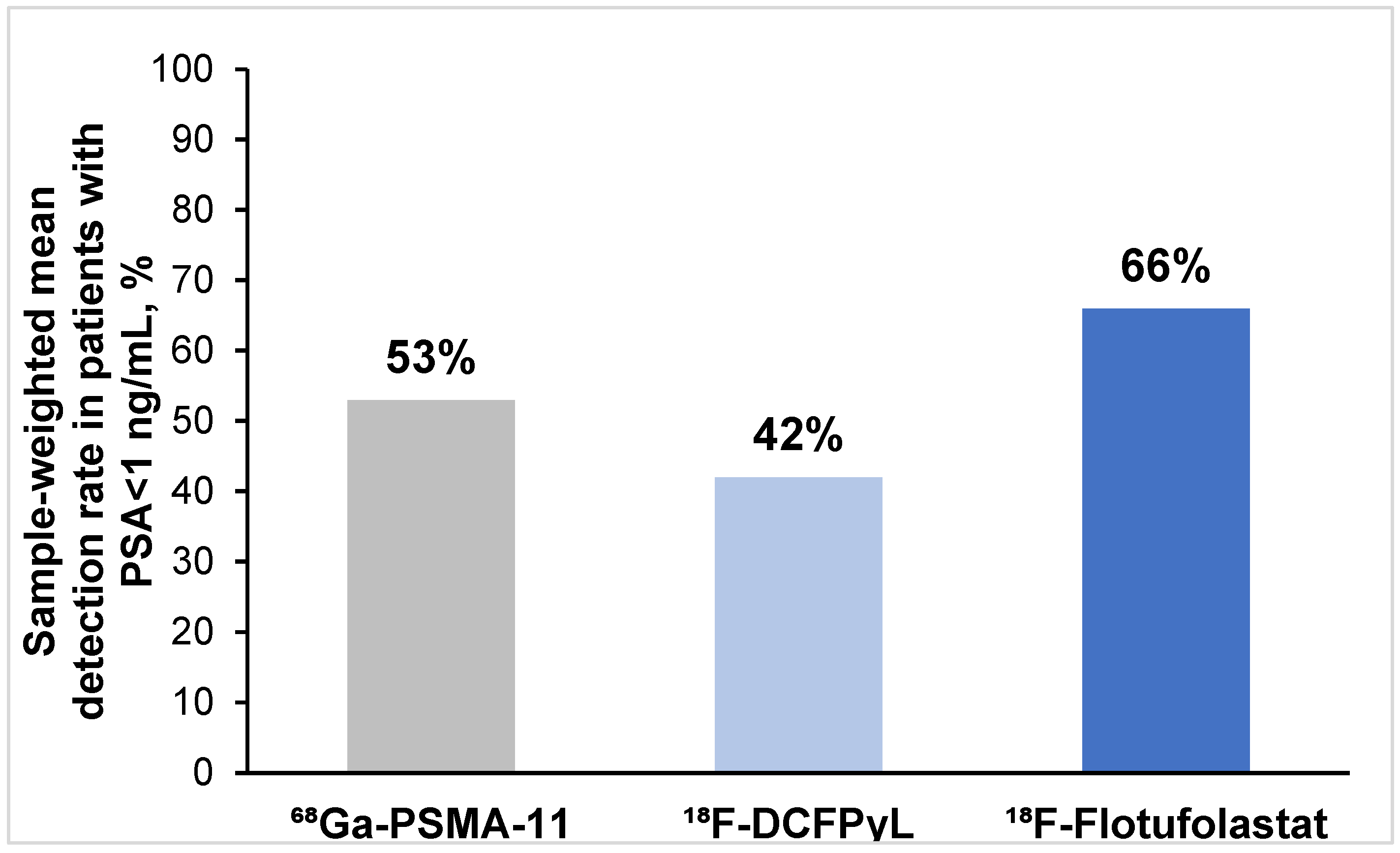
3.3.3. Patient-Level Detection Rates Stratified by PSA Levels
3.4. Positive Predictive Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Tilki, D.; van den Bergh, R.C.N.; Briers, E.; Erberli, D.; De Meerleer, G.; Santis, M.D.; Farolfi, A.; Gandaglia, G.; Gillessen, S.; et al. EAU—EANM—ESTRO—ESUR—ISUP—SIOG guidelines on prostate cancer. In EAU Guidelines Edn, Proceedings of the EAU Annual Congress, Paris, France, 5–8 April 2024; EAU: London, UK, 2024; ISBN 978-94-92671-23-3. [Google Scholar]
- Morgan, T.M.; Boorjian, S.A.; Buyyounouski, M.K.; Chapin, B.F.; Chen, D.Y.T.; Cheng, H.H.; Chou, R.; Jacene, H.A.; Kamran, S.C.; Kim, S.K.; et al. Salvage therapy for prostate cancer: AUA/ASTRO/SUO Guideline Part I: Introduction and treatment decision-making at the time of suspected biochemical recurrence after radical prostatectomy. J. Urol. 2024, 211, 509–517. [Google Scholar] [CrossRef] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 3.2024. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 8 May 2025).
- FDA. Highlights of Prescribing Information: PYLARIFY® (Piflufolastat F 18) Injection. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf (accessed on 8 May 2025).
- FDA. Highlights of Prescribing Information: Gallium Ga 68 PSMA-11 Injection. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212642s000lbl.pdf (accessed on 8 May 2025).
- FDA. Highlights of Prescribing Information: POSLUMA (Flotufolastat F 18) Injection. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216023s000lbl.pdf (accessed on 8 May 2025).
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomäcker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Iravani, A.; Hofman, M.S.; Hicks, R.J. Intra-individual comparison of 68Ga-PSMA-11 and 18F-DCFPyL normal-organ biodistribution. Cancer Imaging 2019, 19, 23. [Google Scholar] [CrossRef]
- Kuo, P.H.; Hermsen, R.; Penny, R.; Postema, E.J. Quantitative and qualitative assessment of urinary activity of 18F-flotufolastat-PET/CT in patients with prostate cancer: A post hoc analysis of the LIGHTHOUSE and SPOTLIGHT studies. Mol. Imaging Biol. 2024, 26, 53–60. [Google Scholar] [CrossRef]
- Jani, A.B.; Ravizzini, G.C.; Gartrell, B.A.; Siegel, B.A.; Twardowski, P.; Saltzstein, D.; Fleming, M.T.; Chau, A.; Davis, P.; Chapin, B.F.; et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in men with suspected prostate cancer recurrence: Results from a phase 3, prospective, multicenter study (SPOTLIGHT). J. Urol. 2023, 210, 299–311. [Google Scholar] [CrossRef]
- Jani, A.B.; Ravizzini, G.C.; Gartrell, B.A.; Siegel, B.A.; Twardowski, P.; Saltzstein, D.; Fleming, M.T.; Chau, A.; Davis, P.; Chapin, B.F.; et al. Reply by authors. J. Urol. 2023, 210, 310–311. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Akdemir, E.N.; Tuncel, M.; Akyol, F.; Bilen, C.Y.; Baydar, D.E.; Karabulut, E.; Ozen, H.; Caglar, M. 68Ga-labelled PSMA ligand HBED-CC PET/CT imaging in patients with recurrent prostate cancer. World J. Urol. 2019, 37, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Mingels, C.; Zacho, H.D.; Lanz, S.; Schöder, H.; Rominger, A.; Zwahlen, M.; Afshar-Oromieh, A. Comparing the clinical performance and cost efficacy of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 in the diagnosis of recurrent prostate cancer: A Markov chain decision analysis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.R.; Kishan, A.U.; Booker, K.M.; Grogan, T.R.; Elashoff, D.; Lam, E.C.; Clark, K.J.; Steinberg, M.L.; Fendler, W.P.; Hope, T.A.; et al. Impact of prostate-specific membrane antigen positron emission tomography/computed tomography on prostate cancer salvage radiotherapy management: Results from a prospective multicenter randomized phase 3 trial (PSMA-SRT NCT03582774). Eur. Urol. 2024, 86, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Boreta, L.; Gadzinski, A.J.; Wu, S.Y.; Xu, M.; Greene, K.; Quanstrom, K.; Nguyen, H.G.; Carroll, P.R.; Hope, T.A.; Feng, F.Y. Location of recurrence by gallium-68 PSMA-11 PET scan in prostate cancer patients eligible for salvage radiotherapy. Urology 2019, 129, 165–171. [Google Scholar] [CrossRef]
- Brito, A.E.T.; Mourato, F.A.; de Oliveira, R.P.M.; Leal, A.L.G.; Filho, P.J.A.; de Filho, J.L.L. Evaluation of whole-body tumor burden with 68Ga-PSMA PET/CT in the biochemical recurrence of prostate cancer. Ann. Nucl. Med. 2019, 33, 344–350. [Google Scholar] [CrossRef]
- Burgard, C.; Hoffmann, M.A.; Frei, M.; Buchholz, H.-G.; Khreish, F.; Marlowe, R.J.; Schreckenberger, M.; Ezziddin, S.; Rosar, F. Detection efficacy of 68Ga-PSMA-11 PET/CT in biochemical recurrence of prostate cancer with very low PSA levels: A 7-year, two-center “real-world” experience. Cancers 2023, 15, 1376. [Google Scholar] [CrossRef]
- Caroli, P.; Sandler, I.; Matteucci, F.; De Giorgi, U.; Uccelli, L.; Celli, M.; Foca, F.; Barone, D.; Romeo, A.; Sarnelli, A.; et al. 68Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: Prospective results in 314 patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2035–2044. [Google Scholar] [CrossRef]
- Cerci, J.J.; Fanti, S.; Lobato, E.E.; Kunikowska, J.; Alonso, O.; Medina, S.; Novruzov, F.; Lengana, T.; Granados, C.; Kumar, R.; et al. Diagnostic performance and clinical impact of 68Ga-PSMA-11 PET/CT imaging in early relapsed prostate cancer after radical therapy: A prospective multicenter study (IAEA-PSMA Study). J. Nucl. Med. 2022, 63, 240–247. [Google Scholar] [CrossRef]
- Christensen, M.T.; Jochumsen, M.R.; Klingenberg, S.; Sorensen, K.D.; Borre, M.; Bouchelouche, K. Evaluation of predictors of biochemical recurrence in prostate cancer patients, as detected by 68Ga-PSMA PET/CT. Diagnostics 2022, 12, 195. [Google Scholar] [CrossRef]
- Einspieler, I.; Rauscher, I.; Düwel, C.; Krönke, M.; Rischpler, C.; Habl, G.; Dewes, S.; Ott, A.; Wester, H.-J.; Schwaiger, M.; et al. Detection efficacy of hybrid 68Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by Phoenix Criteria. J. Nucl. Med. 2017, 58, 1081–1087. [Google Scholar] [CrossRef]
- Emmett, L.; Tang, R.; Nandurkar, R.H.; Hruby, G.; Roach, P.J.; Watts, J.A.; Cusick, T.; Kneebone, A.; Ho, B.; Chan, L.; et al. 3-Year freedom from progression after 68Ga-PSMA PET/CT-triaged management in men with biochemical recurrence after radical prostatectomy: Results of a prospective multicenter trial. J. Nucl. Med. 2020, 61, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Farolfi, A.; Ceci, F.; Castellucci, P.; Graziani, T.; Siepe, G.; Lambertini, A.; Schiavina, R.; Lodi, F.; Morganti, A.G.; Fanti, S. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA < 0.5 ng/mL. Efficacy and impact on treatment strategy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Fourquet, A.; Lahmi, L.; Rusu, T.; Belkacemi, Y.; Crehange, G.; de la Taille, A.; Fournier, G.; Cussenot, O.; Gauthé, M. Restaging the biochemical recurrence of prostate cancer with [68Ga]Ga-PSMA-11 PET/CT: Diagnostic performance and impact on patient disease management. Cancers 2021, 13, 1594. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.T.; Radtke, J.P.; Afshar-Oromieh, A.; Roethke, M.C.; Hadaschik, B.; Gleave, M.; Bonekamp, D.; Kopka, K.; Eder, M.; Heusser, T.; et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: Comparison with mpMRI integrated in simultaneous PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 776–787. [Google Scholar] [CrossRef]
- Hamed, M.A.G.; Basha, M.A.A.; Ahmed, H.; Obaya, A.A.; Afifi, A.H.M.; Abdelbary, E.H. 68Ga-PSMA PET/CT in patients with rising prostatic-specific antigen after definitive treatment of prostate cancer: Detection efficacy and diagnostic accuracy. Acad. Radiol. 2019, 26, 450–460. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Buchholz, H.-G.; Wieler, H.J.; Müller-Hübenthal, J.; Trampert, L.; Richardsen, I.; Schreckenberger, M. Diagnostic performance of 68Ga-PSMA-11 positron-emission-tomography/computed-tomography in a large cohort of patients with biochemical recurrence of prostate carcinoma. Health Phys. 2020, 119, 141–147. [Google Scholar] [CrossRef]
- Lawal, I.O.; Lengana, T.; Popoola, G.O.; Orunmuyi, A.T.; Kgatle, M.M.; Mokoala, K.M.G.; Sathekge, M.M. Pattern of prostate cancer Recurrence assessed by 68Ga-PSMA-11 PET/CT in men treated with primary local therapy. J. Clin. Med. 2021, 10, 3883. [Google Scholar] [CrossRef]
- McCarthy, M.; Francis, R.; Tang, C.; Watts, J.; Campbell, A. A multicenter prospective clinical trial of 68Gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: Oligometastatic rate and distribution compared with standard imaging. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 801–808. [Google Scholar] [CrossRef]
- Miksch, J.; Bottke, D.; Krohn, T.; Thamm, R.; Bartkowiak, D.; Solbach, C.; Bolenz, C.; Beer, M.; Wiegel, T.; Beer, A.J.; et al. Interobserver variability, detection rate, and lesion patterns of 68Ga-PSMA-11-PET/CT in early-stage biochemical recurrence of prostate cancer after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2339–2347. [Google Scholar] [CrossRef]
- Müller, J.; Ferraro, D.A.; Muehlematter, U.J.; Schüler, H.I.G.; Kedzia, S.; Eberli, D.; Guckenberger, M.; Kroeze, S.G.C.; Sulser, T.; Schmid, D.M.; et al. Clinical impact of 68Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Pinot, F.; Le Pennec, R.; Abgral, R.; Blanc-Béguin, F.; Hennebicq, S.; Schick, U.; Valeri, A.; Fournier, G.; Le Roux, P.-Y.; Salaun, P.-Y.; et al. PSMA-11 PET/CT for detection of recurrent prostate cancer in patients with negative choline PET/CT. Clin. Genitourin. Cancer 2023, 21, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, I.; Düwel, C.; Haller, B.; Rischpler, C.; Heck, M.M.; Gschwend, J.E.; Schwaiger, M.; Maurer, T.; Eiber, M. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur. Urol. 2018, 73, 656–661. [Google Scholar] [CrossRef]
- Rauscher, I.; Kroenke, M.; König, M.; Gafita, A.; Maurer, T.; Horn, T.; Schiller, K.; Weber, W.; Eiber, M. Matched-pair comparison of 68Ga-PSMA-11 and 18F-PSMA-1007 PET/CT: Frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2020, 61, 51–57. [Google Scholar] [CrossRef]
- Raveenthiran, S.; Yaxley, J.; Gianduzzo, T.; Kua, B.; McEwan, L.; Wong, D.; Tsang, G.; MacKean, J. The use of 68Ga-PET/CT PSMA to determine patterns of disease for biochemically recurrent prostate cancer following primary radiotherapy. Prostate Can. Prostate Dis. 2019, 22, 385–390. [Google Scholar] [CrossRef]
- Seniaray, N.; Verma, R.; Khanna, S.; Belho, E.; Pruthi, A.; Mahajan, H. Localization and restaging of carcinoma prostate by 68Gallium prostate-specific membrane antigen positron emission tomography computed tomography in patients with biochemical recurrence. Indian J. Urol. 2020, 36, 191–199. [Google Scholar] [CrossRef]
- Verburg, F.A.; Pfister, D.; Heidenreich, A.; Vogg, A.; Drude, N.I.; Vöö, S.; Mottaghy, F.M.; Behrendt, F.F. Extent of disease in recurrent prostate cancer determined by [68Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 397–403. [Google Scholar] [CrossRef]
- Garcia-Zoghby, L.; Lucas-Lucas, C.; Amo-Salas, M.; Soriano-Castrejon, A.M.; Garcia-Vicente, A.M. Head-to-head comparison of [18F]F-choline and imaging of prostate-specific membrane antigen, using [18F]DCFPyL PET/CT, in patients with biochemical recurrence of prostate cancer. Curr. Oncol. 2023, 30, 6271–6288. [Google Scholar] [CrossRef]
- Li, E.V.; Bennett, R.; Ho, A.; Wong, C.; Mahenthiran, A.K.; Kumar, S.K.S.R.; Sun, Z.; Savas, H.; Rowe, S.P.; Schaeffer, E.M.; et al. Clinical factors associated with suspicious 18F-DCFPyL prostate-specific membrane antigen positron emission tomography activity in patients initially managed with radical prostatectomy including prostate-specific antigen < 0.5 ng/mL. J. Urol. 2025, 213, 183–191. [Google Scholar] [CrossRef]
- Meijer, D.; Jansen, B.H.E.; Wondergem, M.; Bodar, Y.J.L.; Srbljin, S.; Vellekoop, A.E.; Keizer, B.; van der Zant, F.M.; Hoekstra, O.S.; Nieuwenhuijzen, J.A.; et al. Clinical verification of 18F-DCFPyL PET-detected lesions in patients with biochemically recurrent prostate cancer. PLoS ONE 2020, 15, e0239414. [Google Scholar] [CrossRef]
- Mena, E.; Rowe, S.P.; Shih, J.H.; Lindenberg, L.; Turkbey, B.; Fourquet, A.; Lin, F.I.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. Predictors of 18F-DCFPyL PET/CT positivity in patients with biochemical recurrence of prostate cancer after local therapy. J. Nucl. Med. 2022, 63, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR phase III, multicenter study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Oprea-Lager, D.-E.; Gontier, E.; García-Cañamaque, L.; Gauthé, M.; Olivier, P.; Mitjavila, M.; Tamayo, P.; Robin, P.; Vicente, A.M.G.; Bouyeure, A.-C.; et al. [18F]DCFPyL PET/CT versus [18F]fluoromethylcholine PET/CT in biochemical recurrence of prostate cancer (PYTHON): A prospective, open label, cross-over, comparative study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Bénard, F. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J. Nucl. Med. 2019, 60, 1587–1593. [Google Scholar] [CrossRef]
- Sigurdson, S.; al Salman, K.; Mesci, A.; Dayes, I.; Quan, K.; Goldberg, M.; Schnarr, K.; Shayegan, B.; Bauman, G.; Zukotynski, K.; et al. Patterns of failure with 18F-DCFPyL PSMA-PET/CT in the post-prostatectomy setting: A regional cohort analysis. Can. Urol. Assoc. J. 2024, 19, 17–24. [Google Scholar] [CrossRef]
- Rauscher, I.; Karimzadeh, A.; Schiller, K.; Horn, T.; D’alessandria, C.; Franz, C.; Wörther, H.; Nguyen, N.; Combs, S.E.; Weber, W.A.; et al. Detection efficacy of 18F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. J. Nucl. Med. 2021, 62, 1719–1726. [Google Scholar] [CrossRef]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Lowentritt, B.H.; Jani, A.B.; Helfand, B.T.; Uchio, E.M.; Morris, M.A.; Michalski, J.M.; Chau, A.; Davis, P.; Chapin, B.F.; Schuster, D.M. Impact of clinical factors on 18F-flotufolastat detection rates in men with recurrent prostate cancer: Exploratory analysis of the phase 3 SPOTLIGHT Study. Adv. Rad. Oncol. 2024, 9, 101532. [Google Scholar] [CrossRef]
- Wurzer, A.; Di Carlo, D.; Schmidt, A.; Beck, R.; Eiber, M.; Schwaiger, M.; Wester, H.-J. Radiohybrid ligands: A novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA-inhibitors. J. Nucl. Med. 2020, 61, 735–742. [Google Scholar] [CrossRef]
- Lowentritt, B.H.; Chau, A.; Davis, P. How standard of truth methodology impacts diagnostic PSMA-targeting radiopharmaceutical evaluation: Learnings from the Phase 3 SPOTLIGHT study. Diagnostics 2025, 15, 473. [Google Scholar] [CrossRef]
| Article | Risk of Bias | |||
|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | |
| Akdemir, 2019 [16] | LOW | LOW | HIGH | HIGH |
| Alberts, 2022 [17] | LOW | LOW | HIGH | HIGH |
| Armstrong, 2024 [18] | LOW | LOW | HIGH | HIGH |
| Boreta, 2019 [19] | LOW | LOW | HIGH | HIGH |
| Brito, 2019 [20] | LOW | LOW | HIGH | HIGH |
| Burgard, 2023 [21] | UNCLEAR | LOW | HIGH | HIGH |
| Caroli, 2018 [22] | LOW | LOW | HIGH | HIGH |
| Cerci, 2022 [23] | UNCLEAR | LOW | HIGH | HIGH |
| Christensen, 2022 [24] | LOW | LOW | HIGH | HIGH |
| Einspieler, 2017 [25] | LOW | LOW | HIGH | HIGH |
| Emmett, 2020 [26] | UNCLEAR | LOW | HIGH | HIGH |
| Farolfi, 2019 [27] | UNCLEAR | LOW | HIGH | HIGH |
| Fendler, 2019 [28] | LOW | LOW | HIGH | HIGH |
| Fourquet, 2021 [29] | LOW | LOW | HIGH | HIGH |
| Freitag, 2017 [30] | LOW | LOW | HIGH | HIGH |
| Hamed, 2019 [31] | LOW | LOW | HIGH | HIGH |
| Hoffmann, 2020 [32] | LOW | LOW | HIGH | HIGH |
| Lawal, 2021 [33] | UNCLEAR | LOW | HIGH | HIGH |
| McCarthy, 2019 [34] | UNCLEAR | LOW | HIGH | HIGH |
| Miksch, 2020 [35] | UNCLEAR | LOW | HIGH | HIGH |
| Müller, 2019 [36] | LOW | LOW | HIGH | HIGH |
| Pinot, 2023 [37] | LOW | LOW | HIGH | HIGH |
| Rauscher, 2018 [38] | UNCLEAR | LOW | HIGH | HIGH |
| Rauscher, 2020 [39] | LOW | LOW | HIGH | HIGH |
| Raveenthiran, 2019 [40] | LOW | LOW | HIGH | HIGH |
| Seniaray, 2020 [41] | LOW | LOW | HIGH | HIGH |
| Verburg, 2016 [42] | LOW | LOW | HIGH | HIGH |
| García-Zoghby, 2023 [43] | UNCLEAR | LOW | HIGH | HIGH |
| Li, 2025 [44] | UNCLEAR | LOW | HIGH | HIGH |
| Meijer, 2020 [45] | LOW | LOW | HIGH | HIGH |
| Mena, 2022 [46] | UNCLEAR | LOW | HIGH | HIGH |
| Morris, 2021 [47] | UNCLEAR | LOW | HIGH | HIGH |
| Oprea-Lager, 2023 [48] | LOW | LOW | HIGH | HIGH |
| Rousseau, 2019 [49] | LOW | LOW | HIGH | HIGH |
| Sigurdson, 2024 [50] | LOW | LOW | HIGH | HIGH |
| Jani, 2023 [11] | LOW | LOW | HIGH | HIGH |
| Rauscher, 2021 [51] | LOW | LOW | HIGH | HIGH |
| Article | Radiopharmaceutical Evaluated | Number of Patients in the Relevant Cohort | Median PSA, ng/mL | Overall Patient-Level Detection Rate a, % | Proportion of Patients Who Had Undergone Radical Prostatectomy, % |
|---|---|---|---|---|---|
| Akdemir, 2019 [16] | 68Ga-PSMA-11 | 121 | 3.9 | 76 | 47 |
| Alberts, 2022 [17] | 68Ga-PSMA-11 | 122 | 2.8 | 87 | 79 |
| Armstrong, 2024 [18] | 68Ga-PSMA-11 | 102 | 0.2 | 38 | 100 |
| Boreta, 2019 [19] | 68Ga-PSMA-11 | 125 | 0.4 | 53 | 100 |
| Brito, 2019 [20] | 68Ga-PSMA-11 | 100 | 1.7 | 72 | 69 |
| Burgard, 2023 [21] | 68Ga-PSMA-11 | 115 | 0.1 | 25 | 100 |
| Caroli, 2018 [22] | 68Ga-PSMA-11 | 314 | 0.8 | 63 | 84 |
| Cerci, 2022 [23] | 68Ga-PSMA-11 | 1004 | NR (mean = 1.6) | 65 | 78 |
| Christensen, 2022 [24] | 68Ga-PSMA-11 | 189 | 10.5 | 55 | 81 |
| Einspieler, 2017 [25] | 68Ga-PSMA-11 | 118 | 6.4 | 91 | 0 |
| Emmett, 2020 [26] | 68Ga-PSMA-11 | 260 | 0.3 | 65 | 100 |
| Farolfi, 2019 [27] | 68Ga-PSMA-11 | 119 | 0.3 | 34 | 100 |
| Fendler, 2019 [28] | 68Ga-PSMA-11 | 635 | 2.1 | 75 | 73 |
| Fourquet, 2021 [29] | 68Ga-PSMA-11 | 294 | NR (mean = 3.0) | 69 | 86 |
| Freitag, 2017 [30] | 68Ga-PSMA-11 | 119 | 1.7 | 78 | 100 |
| Hamed, 2019 [31] | 68Ga-PSMA-11 | 188 | 2.2 | 88 | 38 |
| Hoffmann, 2020 [32] | 68Ga-PSMA-11 | 660 | 10.7 | 76 | 81 |
| Lawal, 2021 [33] | 68Ga-PSMA-11 | 247 | 2.7 | 81 | 64 |
| McCarthy, 2019 [34] | 68Ga-PSMA-11 | 238 | 2.6 | 77 | 64 |
| Miksch, 2020 [35] | 68Ga-PSMA-11 | 116 | NR (mean = 0.3) | 50 | 100 |
| Müller, 2019 [36] | 68Ga-PSMA-11 | 223 | 1.0 | 74 | 98 |
| Pinot, 2023 [37] | 68Ga-PSMA-11 | 159 | 0.8 | 66 | 90 |
| Rauscher, 2018 [38] | 68Ga-PSMA-11 | 272 | 0.5 | 65 | 100 |
| Rauscher, 2020 [39] | 68Ga-PSMA-11 | 102 | 0.9 | 80 | 100 |
| Raveenthiran, 2019 [40] | 68Ga-PSMA-11 | 276 | 3.6 | 86 | 0 |
| Seniaray, 2020 [41] | 68Ga-PSMA-11 | 124 | 1.8 | 70 | 100 |
| Verburg, 2016 [42] | 68Ga-PSMA-11 | 155 | 4.0 | 80 | 64 |
| García-Zoghby, 2023 [43] | 18F-DCFPyL | 138 | NR (mean = 2.8) | 65 | 57 |
| Li, 2025 [44] | 18F-DCFPyL | 415 | 0.4 | 49 | 100 |
| Meijer, 2020 [45] | 18F-DCFPyL | 262 | 2.5 | 86 | 48 |
| Mena, 2022 [46] | 18F-DCFPyL | 245 | 1.6 | 79 | 80 |
| Morris, 2021 [47] | 18F-DCFPyL | 208 | 0.8 | 62 | 85 |
| Oprea-Lager, 2023 [48] | 18F-DCFPyL | 201 | 0.5 | 58 | 73 |
| Rousseau, 2019 [49] | 18F-DCFPyL | 130 | NR (mean = 5.2) | 85 | 72 |
| Sigurdson, 2024 [50] | 18F-DCFPyL | 116 | 0.2 | 52 | 100 |
| Jani, 2023 [11] | 18F-Flotufolastat | 389 | 1.1 | 83 | 79 |
| Rauscher, 2021 [51] | 18F-Flotufolastat | 242 | 0.6 | 73 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rais-Bahrami, S.; Davis, P.; Chau, A.; Galgano, S.J.; Chapin, B.F.; Schuster, D.M.; Turnbull, C.M. Detection Rates of PSMA-PET Radiopharmaceuticals in Recurrent Prostate Cancer: A Systematic Review. Diagnostics 2025, 15, 1224. https://doi.org/10.3390/diagnostics15101224
Rais-Bahrami S, Davis P, Chau A, Galgano SJ, Chapin BF, Schuster DM, Turnbull CM. Detection Rates of PSMA-PET Radiopharmaceuticals in Recurrent Prostate Cancer: A Systematic Review. Diagnostics. 2025; 15(10):1224. https://doi.org/10.3390/diagnostics15101224
Chicago/Turabian StyleRais-Bahrami, Soroush, Phillip Davis, Albert Chau, Samuel J. Galgano, Brian F. Chapin, David M. Schuster, and Catriona M. Turnbull. 2025. "Detection Rates of PSMA-PET Radiopharmaceuticals in Recurrent Prostate Cancer: A Systematic Review" Diagnostics 15, no. 10: 1224. https://doi.org/10.3390/diagnostics15101224
APA StyleRais-Bahrami, S., Davis, P., Chau, A., Galgano, S. J., Chapin, B. F., Schuster, D. M., & Turnbull, C. M. (2025). Detection Rates of PSMA-PET Radiopharmaceuticals in Recurrent Prostate Cancer: A Systematic Review. Diagnostics, 15(10), 1224. https://doi.org/10.3390/diagnostics15101224






