Real-World Data: Implementation and Outcomes of Next-Generation Sequencing in the MENA Region
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsimberidou, A.M.; Kahle, M.; Vo, H.H.; Baysal, M.A.; Johnson, A.; Meric-Bernstam, F. Molecular tumour boards—Current and future considerations for precision oncology. Nat. Rev. Clin. Oncol. 2023, 20, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, M.; Hong, Y.S.; Kim, H.S.; Kim, S.T.; Kim, J.; Yun, H.; Yoo, C.; Ahn, H.K.; Kim, H.S.; et al. ERRATUM: Recommendations for the Use of Next-Generation Sequencing and the Molecular Tumor Board for Patients with Advanced Cancer: A Report from KSMO and KCSG Precision Medicine Networking Group. Cancer Res. Treat. 2023, 55, 1061. [Google Scholar] [CrossRef]
- Du, R.; Wang, X.; Ma, L.; Larcher, L.M.; Tang, H.; Zhou, H.; Chen, C.; Wang, T. Adverse reactions of targeted therapy in cancer patients: A retrospective study of hospital medical data in China. BMC Cancer 2021, 21, 206. [Google Scholar] [CrossRef]
- Seebacher, N.A.; Stacy, A.E.; Porter, G.M.; Merlot, A.M. Clinical development of targeted and immune based anti-cancer therapies. J. Exp. Clin. Cancer Res. 2019, 38, 156. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Dhakshanamoorthy, R.; Baskaran, A.; Sabari Krishnan, B.; Maddaly, R. Drug resistance in human cancers—Mechanisms and implications. Life Sci. 2024, 352, 122907. [Google Scholar] [CrossRef]
- Andrei, L.; Kasas, S.; Ochoa Garrido, I.; Stankovic, T.; Suarez Korsnes, M.; Vaclavikova, R.; Assaraf, Y.G.; Pesic, M. Advanced technological tools to study multidrug resistance in cancer. Drug Resist. Updat. 2020, 48, 100658. [Google Scholar] [CrossRef]
- Hou, P.; Wu, C.; Wang, Y.; Qi, R.; Bhavanasi, D.; Zuo, Z.; Dos Santos, C.; Chen, S.; Chen, Y.; Zheng, H.; et al. A Genome-Wide CRISPR Screen Identifies Genes Critical for Resistance to FLT3 Inhibitor AC220. Cancer Res. 2017, 77, 4402–4413. [Google Scholar] [CrossRef]
- Yip, S.; Christofides, A.; Banerji, S.; Downes, M.R.; Izevbaye, I.; Lo, B.; MacMillan, A.; McCuaig, J.; Stockley, T.; Yousef, G.M.; et al. A Canadian guideline on the use of next-generation sequencing in oncology. Curr. Oncol. 2019, 26, e241–e254. [Google Scholar] [CrossRef] [PubMed]
- Gagan, J.; Van Allen, E.M. Next-generation sequencing to guide cancer therapy. Genome Med. 2015, 7, 80. [Google Scholar] [CrossRef]
- Horgan, D.; Hamdi, Y.; Lal, J.A.; Nyawira, T.; Meyer, S.; Kondji, D.; Francisco, N.M.; De Guzman, R.; Paul, A.; Bernard, B.; et al. Framework for Adoption of Next-Generation Sequencing (NGS) Globally in the Oncology Area. Healthcare 2023, 11, 431. [Google Scholar] [CrossRef]
- Gibbs, S.N.; Peneva, D.; Cuyun Carter, G.; Palomares, M.R.; Thakkar, S.; Hall, D.W.; Dalglish, H.; Campos, C.; Yermilov, I. Comprehensive Review on the Clinical Impact of Next-Generation Sequencing Tests for the Management of Advanced Cancer. JCO Precis. Oncol. 2023, 7, e2200715. [Google Scholar] [CrossRef] [PubMed]
- Mosele, M.F.; Westphalen, C.B.; Stenzinger, A.; Barlesi, F.; Bayle, A.; Bieche, I.; Bonastre, J.; Castro, E.; Dienstmann, R.; Kramer, A.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2024, 35, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shim, H.S.; Kim, S.; Lee, I.H.; Kim, J.; Yoon, S.; Kim, H.D.; Park, I.; Jeong, J.H.; Yoo, C.; et al. Clinical Practice Recommendations for the Use of Next-Generation Sequencing in Patients with Solid Cancer: A Joint Report from KSMO and KSP. Cancer Res. Treat. 2023, 56, 721–742. [Google Scholar] [CrossRef]
- Tayshetye, P.; Miller, K.; Monga, D.; Brem, C.; Silverman, J.F.; Finley, G.G. Molecular Profiling of Advanced Malignancies: A Community Oncology Network Experience and Review of Literature. Front. Med. 2020, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Colomer, R.; Mondejar, R.; Romero-Laorden, N.; Alfranca, A.; Sanchez-Madrid, F.; Quintela-Fandino, M. When should we order a next generation sequencing test in a patient with cancer? eClinicalMedicine 2020, 25, 100487. [Google Scholar] [CrossRef]
- Kato, S.; Schwaederle, M.C.; Fanta, P.T.; Okamura, R.; Leichman, L.; Lippman, S.M.; Lanman, R.B.; Raymond, V.M.; Talasaz, A.; Kurzrock, R. Genomic Assessment of Blood-Derived Circulating Tumor DNA in Patients with Colorectal Cancers: Correlation with Tissue Sequencing, Therapeutic Response, and Survival. JCO Precis. Oncol. 2019, 3, 1–16. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P.S.; et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Hong, D.S.; Ye, Y.; Cartwright, C.; Wheler, J.J.; Falchook, G.S.; Naing, A.; Fu, S.; Piha-Paul, S.; Janku, F.; et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study. JCO Precis. Oncol. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Haslem, D.S.; Van Norman, S.B.; Fulde, G.; Knighton, A.J.; Belnap, T.; Butler, A.M.; Rhagunath, S.; Newman, D.; Gilbert, H.; Tudor, B.P.; et al. A Retrospective Analysis of Precision Medicine Outcomes in Patients with Advanced Cancer Reveals Improved Progression-Free Survival Without Increased Health Care Costs. J. Oncol. Pract. 2017, 13, e108–e119. [Google Scholar] [CrossRef]
- Dumbrava, E.E.I.; Balaji, K.; Raghav, K.; Hess, K.; Javle, M.; Blum-Murphy, M.; Ajani, J.; Kopetz, S.; Broaddus, R.; Routbort, M.; et al. Targeting ERBB2 (HER2) Amplification Identified by Next-Generation Sequencing in Patients with Advanced or Metastatic Solid Tumors Beyond Conventional Indications. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Kato, S.; Kurasaki, K.; Ikeda, S.; Kurzrock, R. Rare Tumor Clinic: The University of California San Diego Moores Cancer Center Experience with a Precision Therapy Approach. Oncologist 2018, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.F.; et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Janku, F.; Naing, A.; Fu, S.; Piha-Paul, S.; Cartwright, C.; Broaddus, R.R.; et al. Long-term overall survival and prognostic score predicting survival: The IMPACT study in precision medicine. J. Hematol. Oncol. 2019, 12, 145. [Google Scholar] [CrossRef]
- Carter, P.; Alifrangis, C.; Cereser, B.; Chandrasinghe, P.; Del Bel Belluz, L.; Fotopoulou, C.; Frilling, A.; Herzog, T.; Moderau, N.; Tabassum, N.; et al. Assessing tumor molecular profiling to guide treatments for patients with advanced female genital tract malignancy. Oncotarget 2018, 9, 6007–6014. [Google Scholar] [CrossRef]
- Presley, C.J.; Tang, D.; Soulos, P.R.; Chiang, A.C.; Longtine, J.A.; Adelson, K.B.; Herbst, R.S.; Zhu, W.; Nussbaum, N.C.; Sorg, R.A.; et al. Association of Broad-Based Genomic Sequencing with Survival Among Patients with Advanced Non-Small Cell Lung Cancer in the Community Oncology Setting. JAMA 2018, 320, 469–477. [Google Scholar] [CrossRef]
- Kopetz, S.; Mills Shaw, K.R.; Lee, J.J.; Zhang, J.; Litzenburger, B.; Holla, V.; Kinyua, W.; Broaddus, E.; Daniels, M.S.; Meric-Bernstam, F.; et al. Use of a Targeted Exome Next-Generation Sequencing Panel Offers Therapeutic Opportunity and Clinical Benefit in a Subset of Patients with Advanced Cancers. JCO Precis. Oncol. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Charo, L.M.; Eskander, R.N.; Okamura, R.; Patel, S.P.; Nikanjam, M.; Lanman, R.B.; Piccioni, D.E.; Kato, S.; McHale, M.T.; Kurzrock, R. Clinical implications of plasma circulating tumor DNA in gynecologic cancer patients. Mol. Oncol. 2021, 15, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Okamura, R.; Kurzrock, R.; Mallory, R.J.; Fanta, P.T.; Burgoyne, A.M.; Clary, B.M.; Kato, S.; Sicklick, J.K. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int. J. Cancer 2021, 148, 702–712. [Google Scholar] [CrossRef]
- Kato, S.; Okamura, R.; Adashek, J.J.; Khalid, N.; Lee, S.; Nguyen, V.; Sicklick, J.K.; Kurzrock, R. Targeting G1/S phase cell-cycle genomic alterations and accompanying co-alterations with individualized CDK4/6 inhibitor-based regimens. JCI Insight 2021, 6, e142547. [Google Scholar] [CrossRef]
- Jones, T.E.; Zou, J.; Tseng, G.C.; Roy, S.; Bhargava, R. The Utility of Next-Generation Sequencing in Advanced Breast and Gynecologic Cancers. Am. J. Clin. Pathol. 2021, 156, 455–460. [Google Scholar] [CrossRef]
- Groisberg, R.; Hong, D.S.; Holla, V.; Janku, F.; Piha-Paul, S.; Ravi, V.; Benjamin, R.; Kumar Patel, S.; Somaiah, N.; Conley, A.; et al. Clinical genomic profiling to identify actionable alterations for investigational therapies in patients with diverse sarcomas. Oncotarget 2017, 8, 39254–39267. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Fuller, G.N.; Powell, S.Z.; Sulman, E.P.; Patel, K.P.; Luthra, R.; Routbort, M.J. Retrospective Analysis of Molecular and Immunohistochemical Characterization of 381 Primary Brain Tumors. J. Neuropathol. Exp. Neurol. 2017, 76, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Coquerelle, S.; Darlington, M.; Michel, M.; Durand, M.; Borget, I.; Baffert, S.; Marino, P.; Perrier, L.; Durand-Zaleski, I.; Group, N.G. Impact of Next Generation Sequencing on Clinical Practice in Oncology in France: Better Genetic Profiles for Patients Improve Access to Experimental Treatments. Value Health 2020, 23, 898–906. [Google Scholar] [CrossRef]
- Bertucci, F.; Goncalves, A.; Guille, A.; Adelaide, J.; Garnier, S.; Carbuccia, N.; Billon, E.; Finetti, P.; Sfumato, P.; Monneur, A.; et al. Prospective high-throughput genome profiling of advanced cancers: Results of the PERMED-01 clinical trial. Genome Med. 2021, 13, 87. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Chen, S.; Lin, S.; Ding, S.; Zhou, X.; Liu, T.; Wang, R.; Wang, W. Characteristics and response to next-generation sequencing-guided therapy in locally advanced or metastatic esophageal cancer. Int. J. Cancer 2023, 152, 436–446. [Google Scholar] [CrossRef]
- Hayashi, H.; Takiguchi, Y.; Minami, H.; Akiyoshi, K.; Segawa, Y.; Ueda, H.; Iwamoto, Y.; Kondoh, C.; Matsumoto, K.; Takahashi, S.; et al. Site-Specific and Targeted Therapy Based on Molecular Profiling by Next-Generation Sequencing for Cancer of Unknown Primary Site: A Nonrandomized Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.P.; Goncalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Tredan, O.; Massiani, M.A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Weiss, G.J. Precision medicine: Lessons learned from the SHIVA trial. Lancet Oncol. 2015, 16, e580. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Kurzrock, R. Precision medicine: Lessons learned from the SHIVA trial. Lancet Oncol. 2015, 16, e579–e580. [Google Scholar] [CrossRef]
- Weiss, M.C.; Blank, A.; Gitelis, S.; Fidler, M.J.; Batus, M. Clinical benefit of next generation sequencing in soft tissue and bone sarcoma: Rush University Medical Center’s experience. J. Clin. Oncol. 2019, 37, e22552. [Google Scholar] [CrossRef]
- Victor, A.I.; Alvarez, O.; Baumgart, M.A.; Goyal, G.; Sahasrabudhe, D.M. Next generation sequencing of sarcomas: Response to crizotinib in two cases with MET amplification. J. Clin. Oncol. 2021, 39, 11538. [Google Scholar] [CrossRef]
- Johnson, D.B.; Frampton, G.M.; Rioth, M.J.; Yusko, E.; Xu, Y.; Guo, X.; Ennis, R.C.; Fabrizio, D.; Chalmers, Z.R.; Greenbowe, J.; et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol. Res. 2016, 4, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Ricagno, G.; Angerilli, V.; Maddalena, G.; Toni, C.D.; Gasparello, J.; Barsotti, G.; Mattiuzzo, E.; Ceccon, C.; Montagna, A.; Sabbadin, M.; et al. Real-world performance and clinical outcome of comprehensive genomic profiling of gastrointestinal tumors: High-volume, single-center experience. J. Clin. Oncol. 2024, 42, 727. [Google Scholar] [CrossRef]
- Lebedeva, A.; Kuznetsova, O.A.; Fedyanin, M.; Ivanov, M.V.; Tryakin, A. 164P Clinical utility of comprehensive molecular profiling tests for advanced gastrointestinal tumors. ESMO Open 2023, 8, 102013. [Google Scholar] [CrossRef]
- Tsimberidou, A.M. Initiative for Molecular Profiling and Advanced Cancer Therapy and challenges in the implementation of precision medicine. Curr. Probl. Cancer 2017, 41, 176–181. [Google Scholar] [CrossRef]
- Yohe, S.; Thyagarajan, B. Review of Clinical Next-Generation Sequencing. Arch. Pathol. Lab. Med. 2017, 141, 1544–1557. [Google Scholar] [CrossRef]
- Veeramah, K.R.; Hammer, M.F. The impact of whole-genome sequencing on the reconstruction of human population history. Nat. Rev. Genet. 2014, 15, 149–162. [Google Scholar] [CrossRef]
- Pinxten, W.; Howard, H.C. Ethical issues raised by whole genome sequencing. Best. Pract. Res. Clin. Gastroenterol. 2014, 28, 269–279. [Google Scholar] [CrossRef]
- Martinez-Martin, N.; Magnus, D. Privacy and ethical challenges in next-generation sequencing. Expert. Rev. Precis. Med. Drug Dev. 2019, 4, 95–104. [Google Scholar] [CrossRef]
- Abou Tayoun, A.N.; Fakhro, K.A.; Alsheikh-Ali, A.; Alkuraya, F.S. Genomic medicine in the Middle East. Genome Med. 2021, 13, 184. [Google Scholar] [CrossRef]
- Bilani, N.; Dagher, M.; Zgheib, N.K. Precision Genetic and Genomic Medicine in the Middle East and North Africa Region: Are We There Yet? Public Health Genom. 2017, 20, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ashbury, F.D.; Thompson, K.; Williams, C.; Williams, K. Challenges adopting next-generation sequencing in community oncology practice. Curr. Opin. Oncol. 2021, 33, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for Validation of Next-Generation Sequencing-Based Oncology Panels: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2017, 19, 341–365. [Google Scholar] [CrossRef]
- Pointer, K.B.; Pitroda, S.P.; Weichselbaum, R.R. Radiotherapy and immunotherapy: Open questions and future strategies. Trends Cancer 2022, 8, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Ford, J.M.; O’Dwyer, P.J.; Shapiro, G.I.; McShane, L.M.; Freidlin, B.; O’Cearbhaill, R.E.; George, S.; Glade-Bender, J.; Lyman, G.H.; et al. National Cancer Institute Combination Therapy Platform Trial with Molecular Analysis for Therapy Choice (ComboMATCH). Clin. Cancer Res. 2023, 29, 1412–1422. [Google Scholar] [CrossRef]
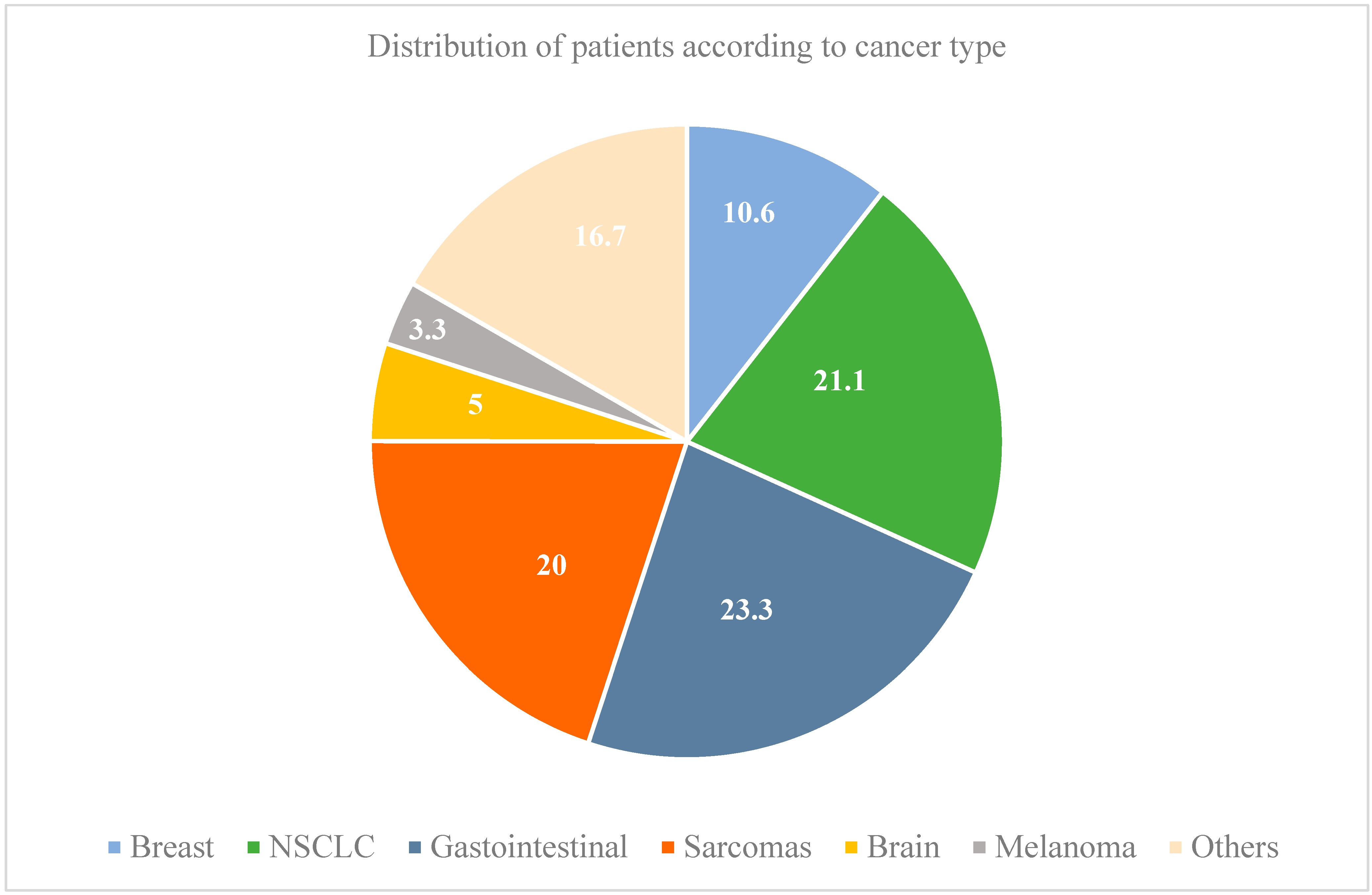

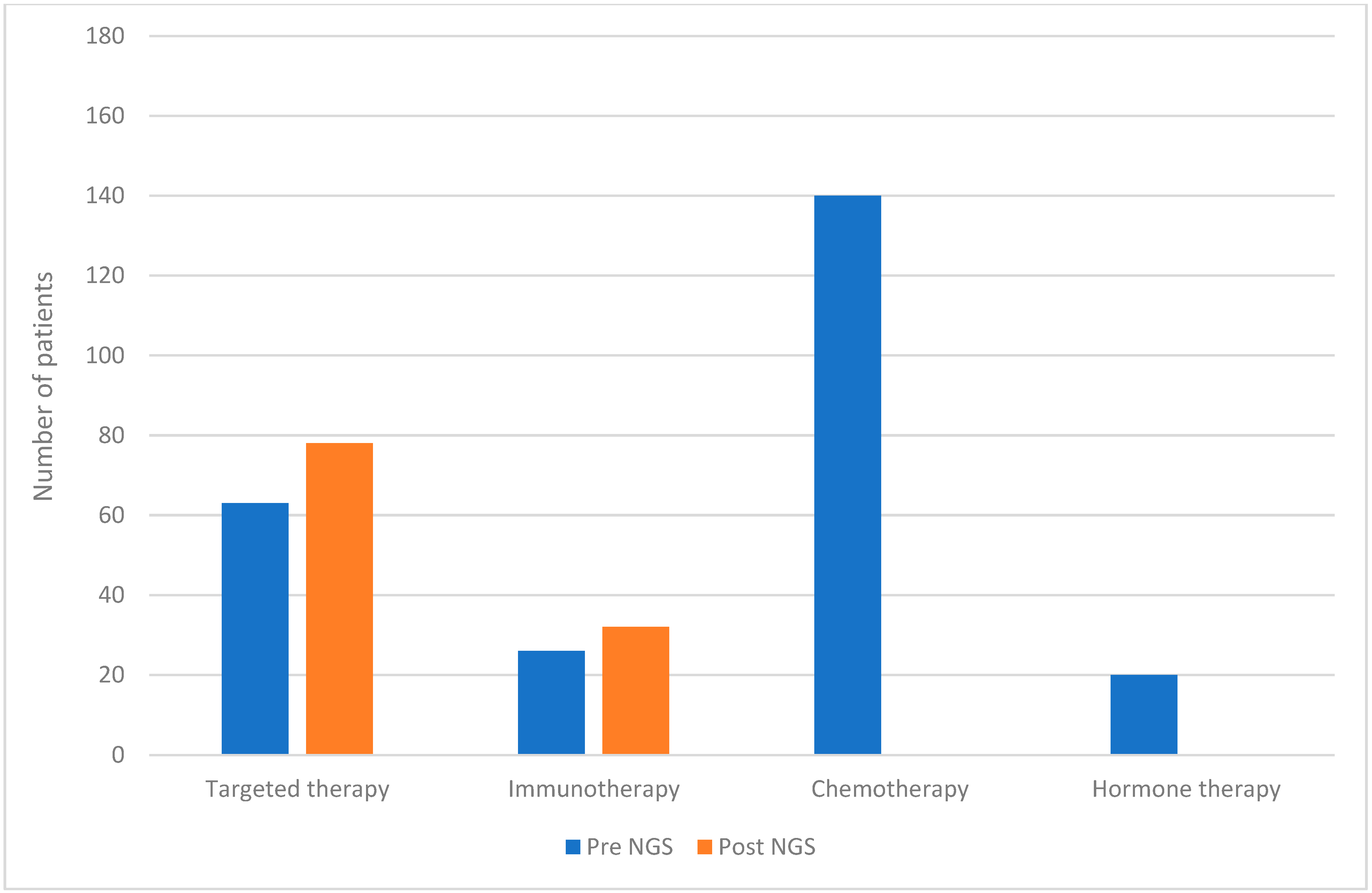


| Patients N = 180 | |
|---|---|
| Median age | 57 (19–92) |
| Gender | |
| Female | 80 (44.4) |
| Male | 100 (55.6) |
| Cancer stage at diagnosis | |
| I | 18 (10) |
| II | 16 (8.9) |
| III | 30 (16.7) |
| IV | 105 (58.3) |
| Not reported | 11 (6.1) |
| Cancer status prior to molecular profiling | |
| Stable disease | 37 (20.6) |
| Partial response | 39 (21.7) |
| Progressive disease | 104 (57.8) |
| Molecular profiling in disease course | |
| Early | 38 (21.1) |
| Late * | 142 (78.9) |
| Malignancy | FoundationOne CDx | FoundationOne Heme | FoundationOne Liquid | FoundationOne Liquid CDx | Guardant 360 |
|---|---|---|---|---|---|
| Sarcomas | 3 | 31 | 1 | 0 | 1 |
| Brain | 9 | 0 | 0 | 0 | 0 |
| Breast | 17 | 0 | 1 | 0 | 1 |
| Gastrointestinal | 34 | 0 | 1 | 4 | 3 |
| Melanoma | 5 | 0 | 0 | 1 | 0 |
| NSCLC | 29 | 1 | 1 | 5 | 2 |
| Others | 25 | 0 | 0 | 5 | 0 |
| Breast | NSCLC | GI | Brain | Sarcomas | Others | |
|---|---|---|---|---|---|---|
| Genes detected by NGS | n = 19 | n = 38 | n = 42 | n = 9 | n = 36 | n = 30 |
| Most commonly detected genes | TP53 | TP53 | KRAS | TERT | TP53 | TP53 |
| PIK3CA | NF1/2 | APC | TP53 | CDK4 | CDKN2A/B | |
| ZNF | KRAS | TP53 | PTEN | MDM2 | CCNE1 | |
| AKT | KEAP1 | NRAS | CDKN2A/B | FRS2 | KRAS | |
| NSD3 | STK11 | PIK3CA | NF1 | ATRX | FGF | |
| MDM2 | ERBB2 | FGF | ATRX | CDKN2A/B | TERT | |
| RAD21 | CDKN2A/B | CDKN2A/B | EGFR | RB1 | MYC | |
| GATA3 | MET | FGF | MTAP | TET2 | BRAF | |
| MYC | EGFR | SMAD4 | IDH1 | EWSR1 | PIK3R1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfouz, R.; Abou Zeidane, R.; Diab, T.; Tarhini, A.; Sbaity, E.; Kazarian, H.; El Zibaoui, Y.; Naji, N.S.; Barake, M.; Assi, H.I. Real-World Data: Implementation and Outcomes of Next-Generation Sequencing in the MENA Region. Diagnostics 2025, 15, 1183. https://doi.org/10.3390/diagnostics15101183
Mahfouz R, Abou Zeidane R, Diab T, Tarhini A, Sbaity E, Kazarian H, El Zibaoui Y, Naji NS, Barake M, Assi HI. Real-World Data: Implementation and Outcomes of Next-Generation Sequencing in the MENA Region. Diagnostics. 2025; 15(10):1183. https://doi.org/10.3390/diagnostics15101183
Chicago/Turabian StyleMahfouz, Rami, Reine Abou Zeidane, Tasnim Diab, Ali Tarhini, Eman Sbaity, Houry Kazarian, Yomna El Zibaoui, Nour Sabiha Naji, Mounir Barake, and Hazem I. Assi. 2025. "Real-World Data: Implementation and Outcomes of Next-Generation Sequencing in the MENA Region" Diagnostics 15, no. 10: 1183. https://doi.org/10.3390/diagnostics15101183
APA StyleMahfouz, R., Abou Zeidane, R., Diab, T., Tarhini, A., Sbaity, E., Kazarian, H., El Zibaoui, Y., Naji, N. S., Barake, M., & Assi, H. I. (2025). Real-World Data: Implementation and Outcomes of Next-Generation Sequencing in the MENA Region. Diagnostics, 15(10), 1183. https://doi.org/10.3390/diagnostics15101183






