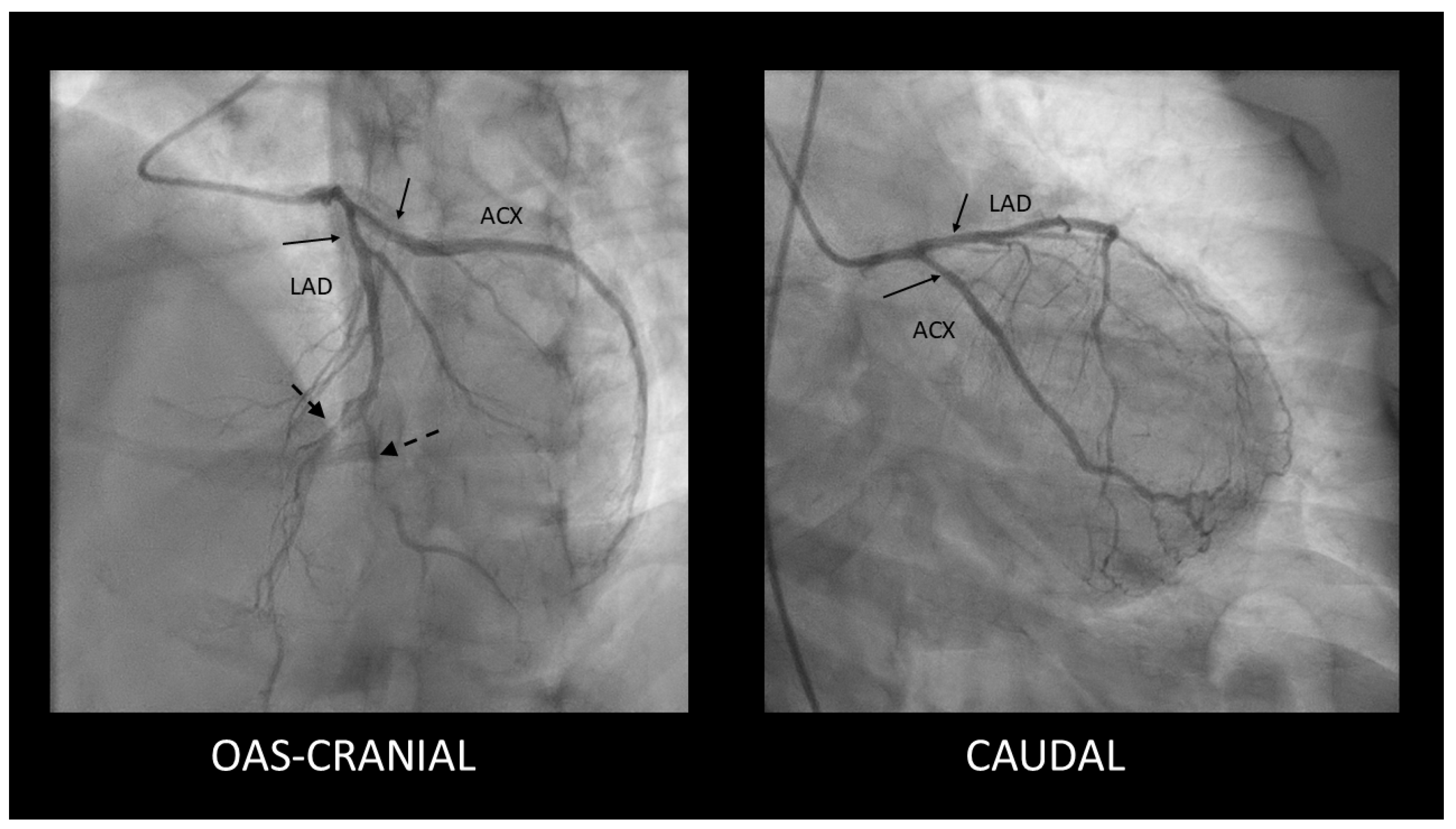
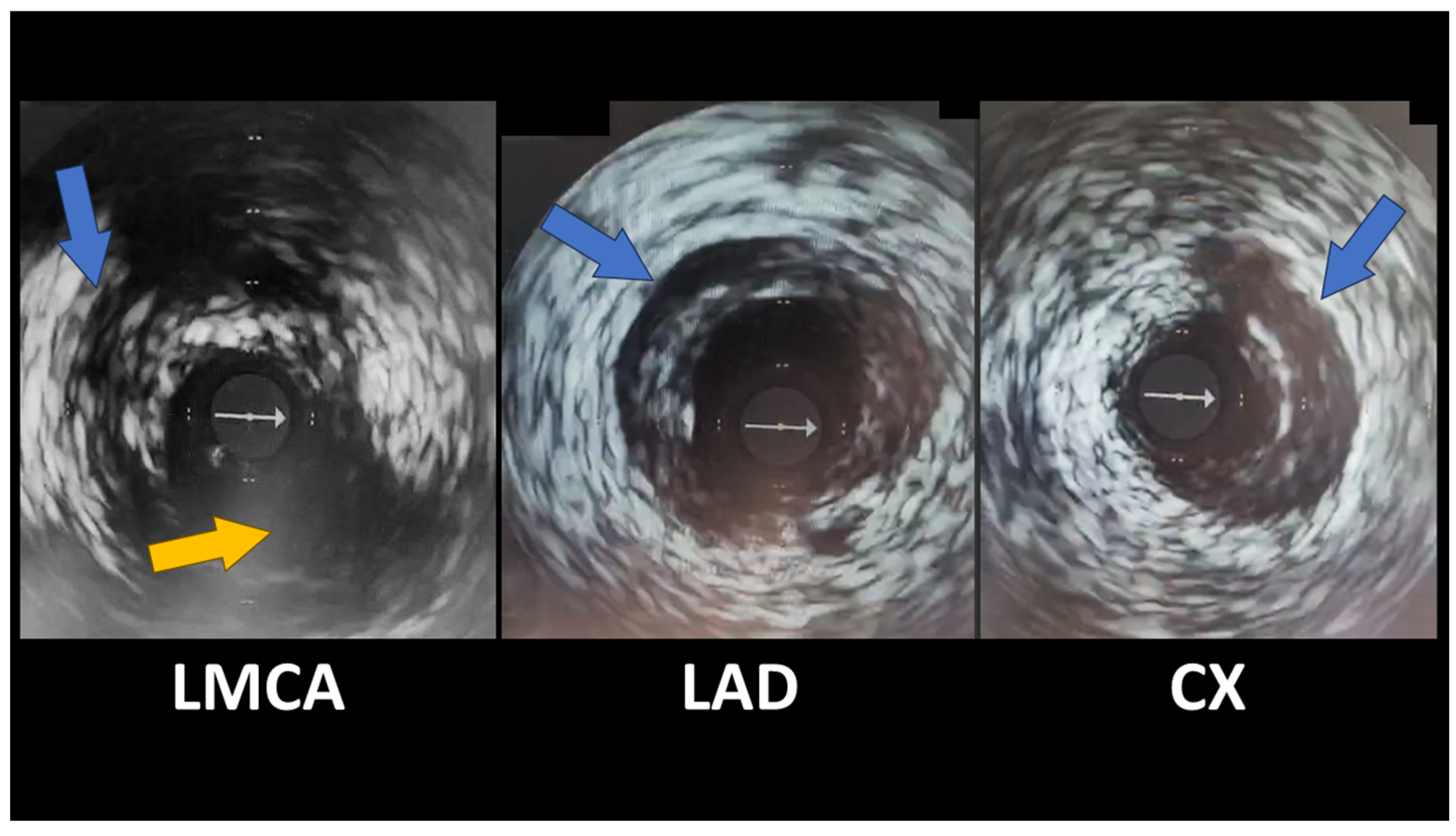
Spontaneous Coronary Artery Dissection Involving the Left Main with Extension to Left Anterior Descending Artery and Left Circumflex Artery: Diagnostic and Management Challenges
Abstract
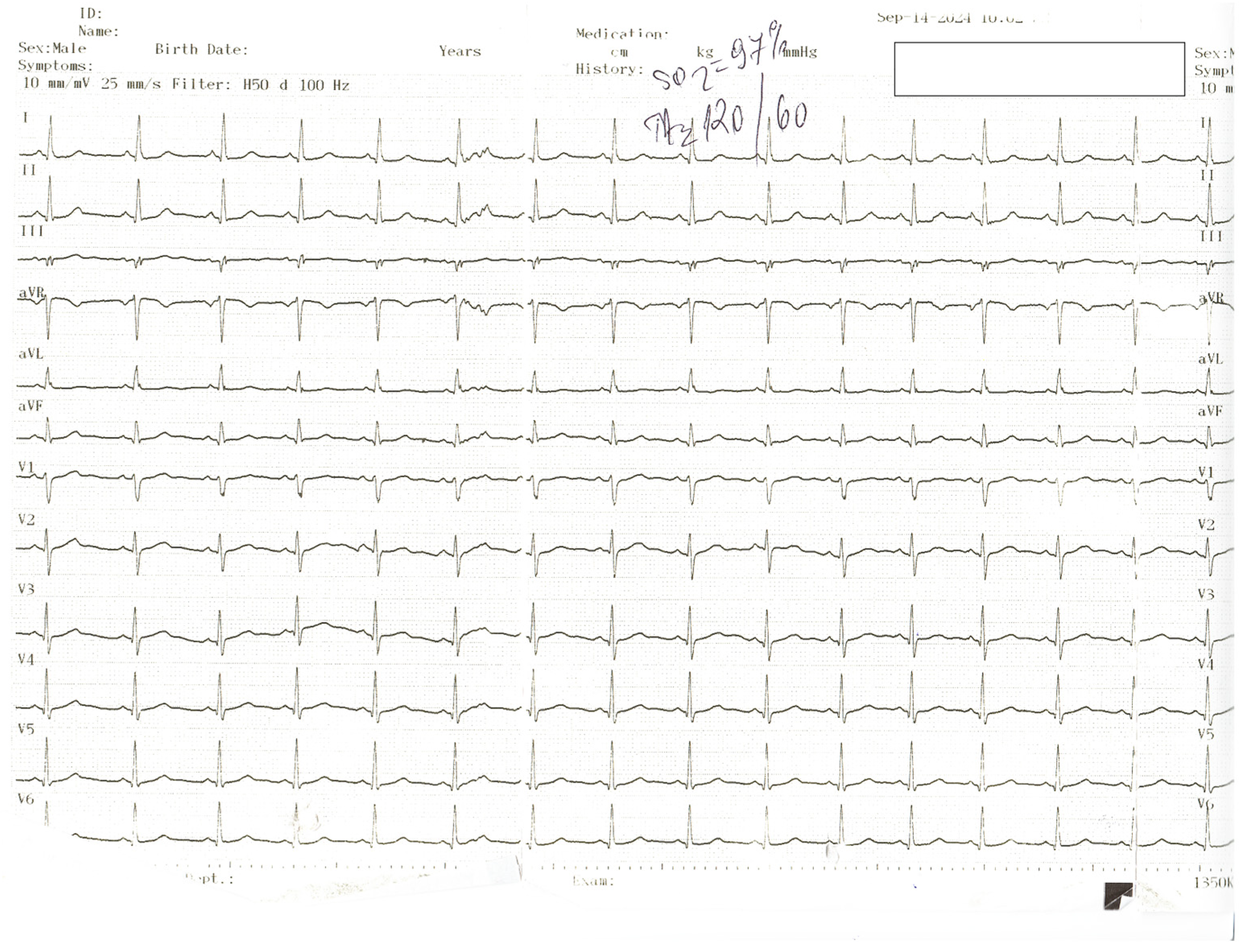
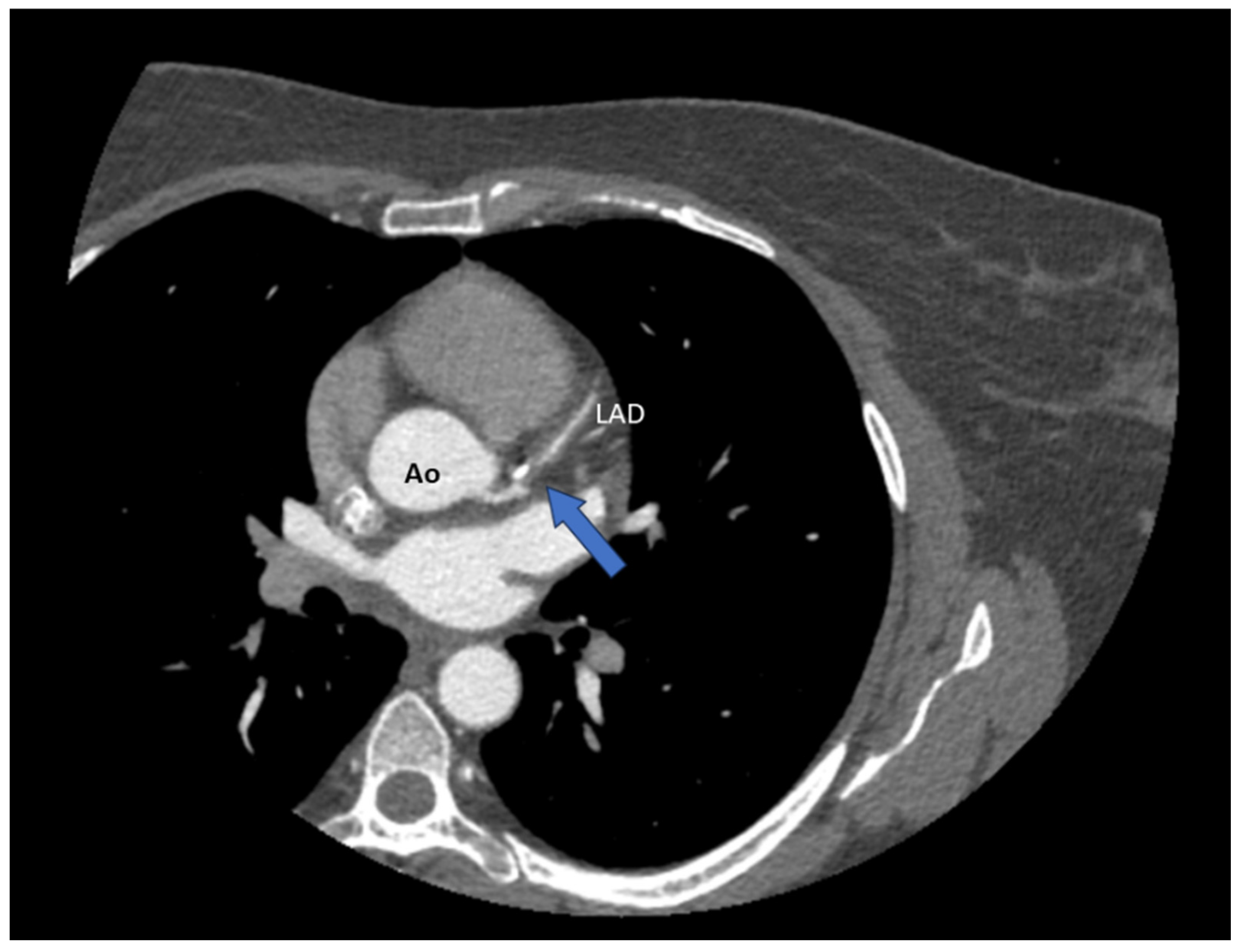
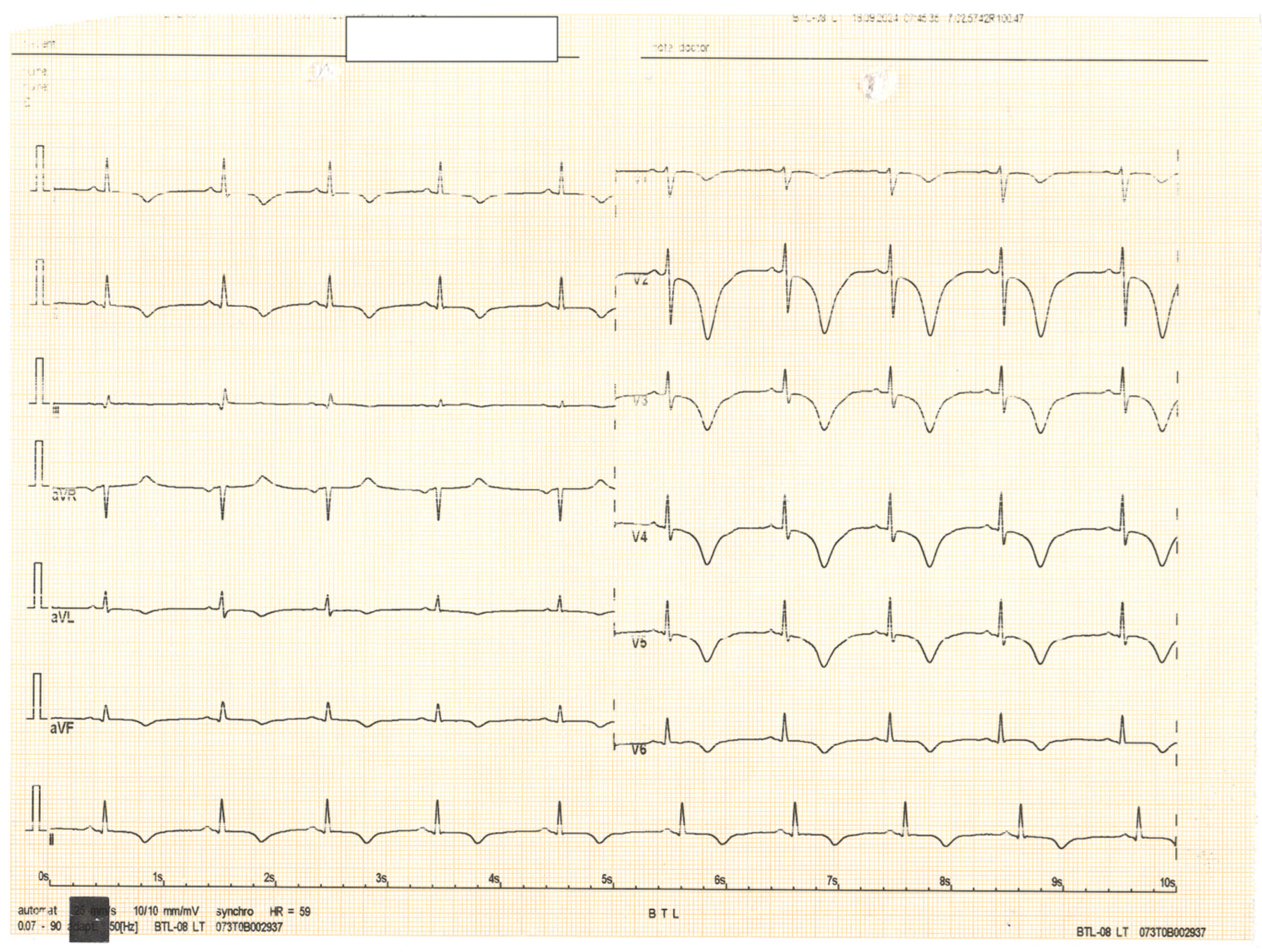
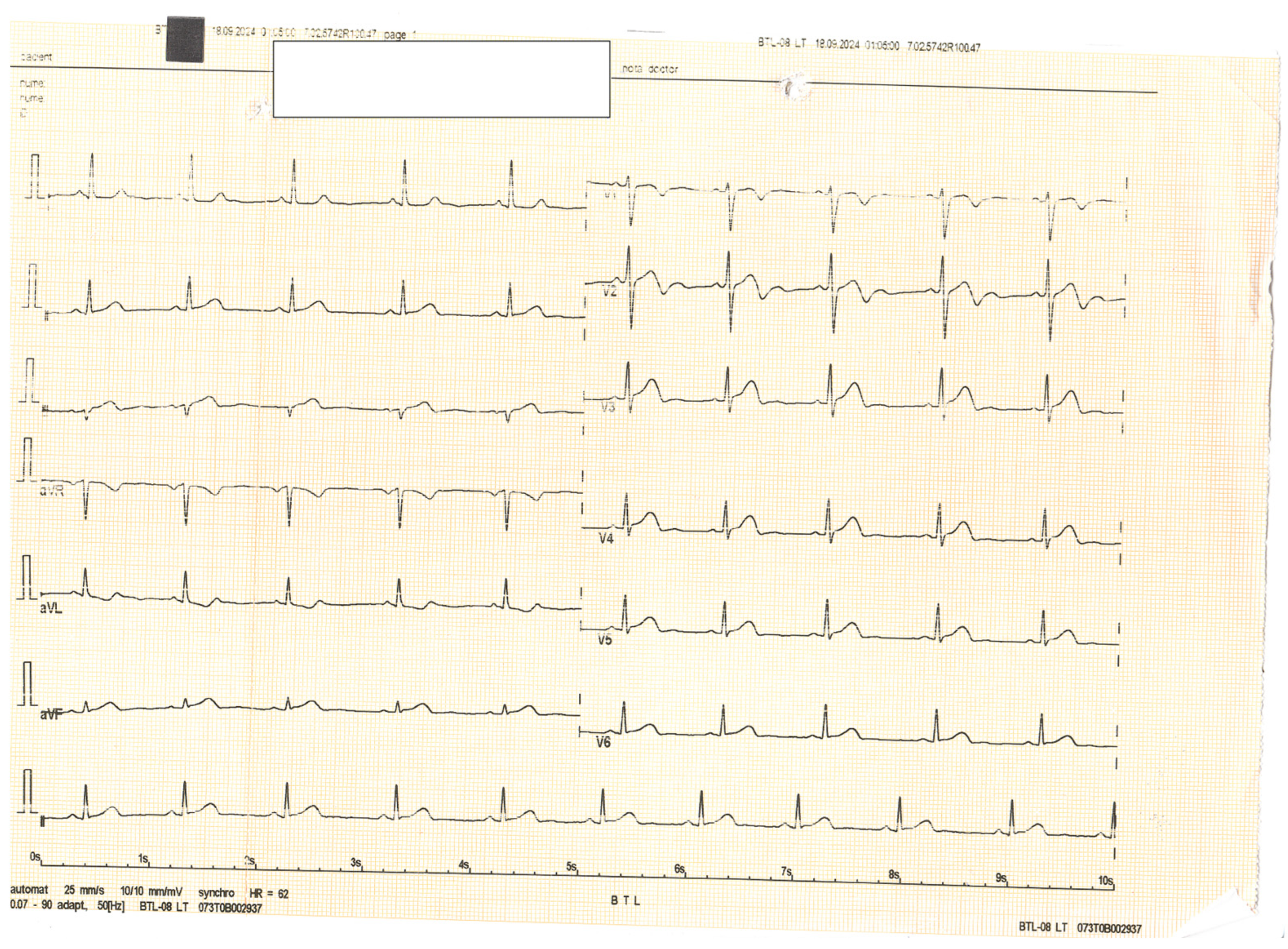
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adlam, D.; Alfonso, F.; Maas, A.; Vrints, C. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018, 39, 3353–3368. [Google Scholar]
- Al-Hussaini, A.; Adlam, D. Spontaneous coronary artery dissection. Heart 2017, 103, 1043–1051. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.M.; Rihal, C.S.; et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Iyasere, C.; Potdar, N. Spontaneous Coronary Artery Dissection Associated with Infertility Treatment. Cureus 2022, 14, e29587. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Macaya, F.; Giacobbe, F.; Moreno, V.; Quadri, G.; Chipayo, D.; Bianco, M.; Salinas, P.; Rolfo, C.; Majia-Renteria, H.; et al. Association between hormone therapy and short-term cardiovascular events in women with spontaneous coronary artery dissection. Rev. Española Cardiol. 2023, 76, 165–172. [Google Scholar] [CrossRef]
- Hirsch, K.; Yogeswaran, V.; Dean, L.S. Spontaneous coronary artery dissection and exogenous estrogen in a transgender female. Catheter. Cardiovasc. Interv. 2022, 100, 96–99. [Google Scholar] [CrossRef]
- Evangelou, D.; Letsas, K.P.; Korantzopoulos, P.; Antonellis, I.; Sioras, E.; Kardaras, F. Spontaneous coronary artery dissection associated with oral contraceptive use: A case report and review of the literature. Int. J. Cardiol. 2006, 112, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Adlam, D.; García-Guimaraes, M.; Maas, A.H.E.M. Spontaneous coronary artery dissection: No longer a rare disease. Eur. Heart J. 2019, 40, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Kim, C.E.S.H.; Saw, J.; Adlam, D.; Arslanian-Engoren, C.; Economy, K.E.; Ganesh, S.; Gulati, R.; Lindsay, M.; Mieres, J.; et al. Spontaneous coronary artery dissection: Current state of the science: A scientific statement from the American Heart Association. Circulation 2018, 137, e523–e557. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusali, C.A.; Cojocaru, L.; Lupu, I.C.; Uzea, C.-D.; Rusali, M.L. Spontaneous Coronary Artery Dissection Involving the Left Main with Extension to Left Anterior Descending Artery and Left Circumflex Artery: Diagnostic and Management Challenges. Diagnostics 2025, 15, 61. https://doi.org/10.3390/diagnostics15010061
Rusali CA, Cojocaru L, Lupu IC, Uzea C-D, Rusali ML. Spontaneous Coronary Artery Dissection Involving the Left Main with Extension to Left Anterior Descending Artery and Left Circumflex Artery: Diagnostic and Management Challenges. Diagnostics. 2025; 15(1):61. https://doi.org/10.3390/diagnostics15010061
Chicago/Turabian StyleRusali, Constantin Andrei, Lucia Cojocaru, Ioana Caterina Lupu, Cezar-Dan Uzea, and Maria Lavinia Rusali. 2025. "Spontaneous Coronary Artery Dissection Involving the Left Main with Extension to Left Anterior Descending Artery and Left Circumflex Artery: Diagnostic and Management Challenges" Diagnostics 15, no. 1: 61. https://doi.org/10.3390/diagnostics15010061
APA StyleRusali, C. A., Cojocaru, L., Lupu, I. C., Uzea, C.-D., & Rusali, M. L. (2025). Spontaneous Coronary Artery Dissection Involving the Left Main with Extension to Left Anterior Descending Artery and Left Circumflex Artery: Diagnostic and Management Challenges. Diagnostics, 15(1), 61. https://doi.org/10.3390/diagnostics15010061





