Application of Biofire Filmarray Joint Infection Panel for Rapid Identification of Aetiology in a Necrotizing Fasciitis Case
Abstract
1. Introduction
2. Materials and Methods
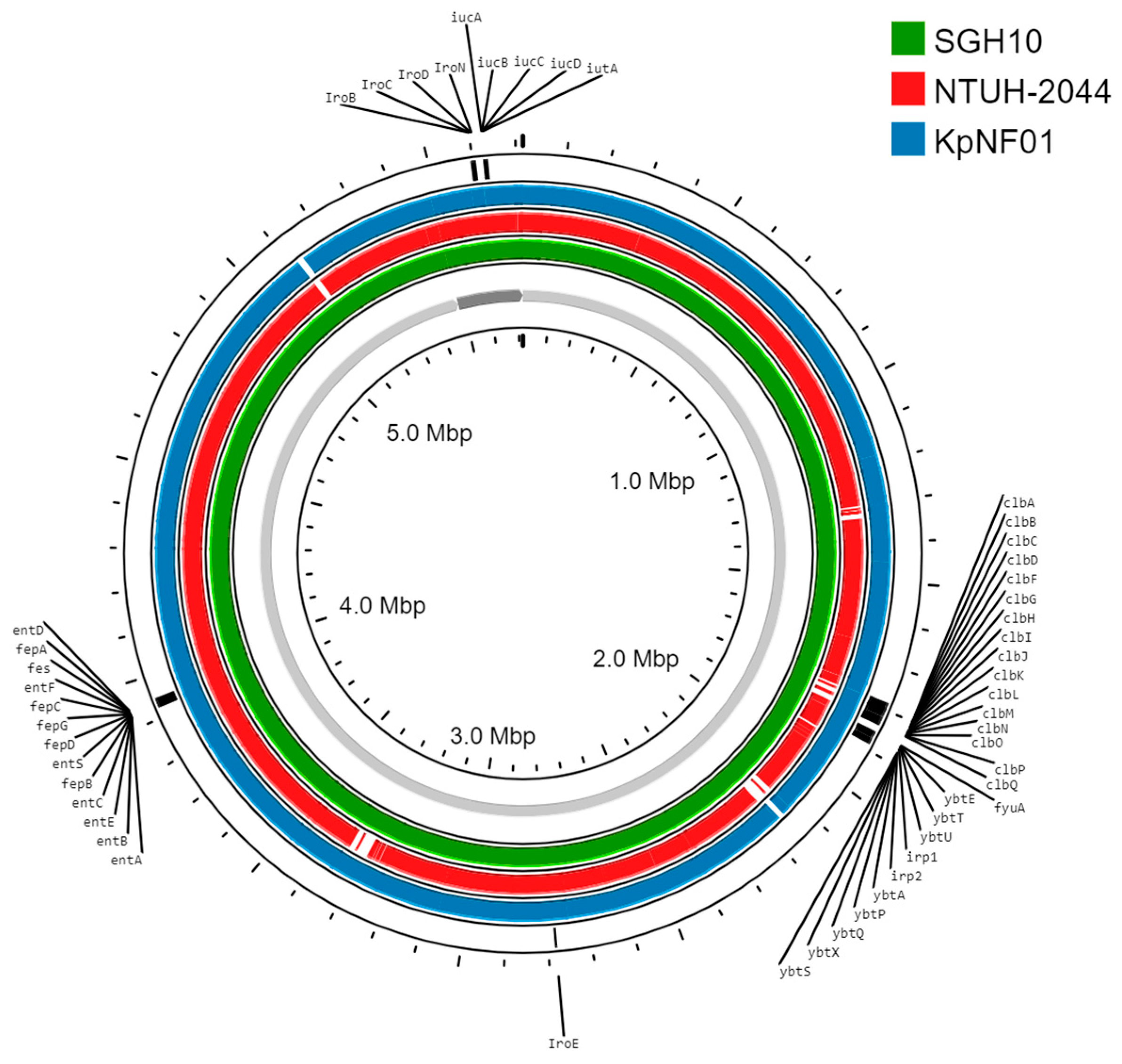
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbina, T.; Razazi, K.; Ourghanlian, C.; Woerther, P.-L.; Chosidow, O.; Lepeule, R.; de Prost, N. Antibiotics in necrotizing soft tissue infections. Antibiotics 2021, 10, 1104. [Google Scholar] [CrossRef]
- Hua, C.; Urbina, T.; Bosc, R.; Parks, T.; Sriskandan, S.; de Prost, N.; Chosidow, O. Necrotising soft-tissue infections. Lancet Infect. Dis. 2023, 23, e81–e94. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft-tissue infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-C.; Tai, H.-C.; Chien, K.-L.; Wang, S.-H.; Chen, Y.-H.; Fang, C.-T.; Hsueh, P.-R. High mortality risk of type III monomicrobial gram-negative necrotizing fasciitis: The role of extraintestinal pathogenic Escherichia coli (ExPEC) and Klebsiella pneumoniae. Int. J. Infect. Dis. 2023, 132, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Tschudin-Sutter, S.; Siegemund, M.; Marsch, S.; Battegay, M.; Wetterauer, C.; Seifert, H.H.; Schaefer, D.J.; Erb, S.; Egli, A. High mortality of non-Fournier necrotizing fasciitis with Enterobacteriales: Time to rethink classification? Clin. Infect. Dis. 2019, 69, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yu, S.N.; Lee, E.J.; Kim, T.; Jeon, M.H.; Choo, E.J.; Park, S.; Chae, J.W.; Bang, H.I.; Kim, T.H. Monomicrobial gram-negative necrotizing fasciitis: An uncommon but fatal syndrome. Diagn. Microbiol. Infect. Dis. 2019, 94, 183–187. [Google Scholar] [CrossRef]
- Burillo, A.; Pulido-Pérez, A.; Bouza, E. Current challenges in acute bacterial skin infection management. Curr. Opin. Infect. Dis. 2024, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.; Kriger, O.; Cohen, S.; Gefen-Halevi, S.; Yahav, D.; Amit, S. Real-life experience and diagnostic utility of the BioFire joint infection PCR panel in bone and joint infections: Analysis of a prospective validation study. Infect. Dis. Ther. 2023, 12, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- BioFire Diagnostics. The FilmArray Panels for Joint Infection. Available online: https://www.biofiredx.com/products/the-filmarray-panels/ji/ (accessed on 30 September 2023).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 22 January 2024).
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef] [PubMed]
- Argimón, S.; David, S.; Underwood, A.; Abrudan, M.; Wheeler, N.E.; Kekre, M.; Abudahab, K.; Yeats, C.A.; Goater, R.; Taylor, B.; et al. Rapid genomic characterization and global surveillance of Klebsiella using Pathogenwatch. Clin. Infect. Dis. 2021, 73, S325–S335. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.-Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wyres, K.L.; Duchêne, S.; Wick, R.R.; Judd, L.M.; Gan, Y.-H.; Hoh, C.-H.; Archuleta, S.; Molton, J.S.; Kalimuddin, S.; et al. Population genomics of hypervirulent Klebsiella pneumoniae clonal-group 23 reveals early emergence and rapid global dissemination. Nat. Commun. 2018, 9, 2703. [Google Scholar] [CrossRef]
- Rahim, G.R.; Gupta, N.; Maheshwari, P.; Singh, M.P. Monomicrobial Klebsiella pneumoniae necrotizing fasciitis: An emerging life-threatening entity. Clin. Microbiol. Infect. 2019, 25, 316–323. [Google Scholar] [CrossRef]
- Shankar, C.; Jacob, J.J.; Vasudevan, K.; Biswas, R.; Manesh, A.; Sethuvel, D.P.M.; Varughese, S.; Biswas, I.; Veeraraghavan, B. Emergence of multidrug resistant hypervirulent ST23 Klebsiella pneumoniae: Multidrug resistant plasmid acquisition drives evolution. Front. Cell Infect. Microbiol. 2020, 10, 575289. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Howard-Anderson, J.; Weiss, D.S. Hypervirulent Klebsiella pneumoniae—Clinical and molecular perspectives. J. Intern. Med. 2020, 287, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Chen, Y.-T.; Chiang, M.-K.; Wang, Y.-C.; Hsiao, P.-Y.; Huang, Y.-J.; Lin, C.-T.; Cheng, C.-C.; Liang, C.-L.; Lai, Y.-C. Colibactin contributes to the hypervirulence of pks+ K1 CC23 Klebsiella pneumoniae in mouse meningitis infections. Front. Cell Infect. Microbiol. 2017, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Al-Busaidi, B.; Al-Muzahmi, M.; Al-Shabibi, Z.; Rizvi, M.; Al-Rashdi, A.; Al-Jardani, A.; Farzand, R.; Al-Jabri, Z. Hypervirulent capsular serotypes K1 and K2 Klebsiella pneumoniae strains demonstrate resistance to serum bactericidal activity and Galleria mellonella lethality. Int. J. Mol. Sci. 2024, 25, 1944. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, G.L.; Brandt, P.B.; Gad, D.; Struve, C.; Justesen, U.S. Monomicrobial necrotizing fasciitis in a white male caused by hypermucoviscous Klebsiella pneumoniae. J. Med. Microbiol. 2009, 58, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Meziere, A.; Monie, M.; Drieux-Rouzet, L.; Nzili, B.; Dicko, M.; Greffard, S.; Decre, D.; Goursot, C. Klebsiella pneumoniae necrotizing fasciitis of the leg in an elderly French woman. Clin. Interv. Aging 2014, 9, 1171–1174. [Google Scholar] [CrossRef]
- Han, Y.-L.; Wen, X.-H.; Zhao, W.; Cao, X.-S.; Wen, J.-X.; Wang, J.-R.; Hu, Z.-D.; Zheng, W.-Q. Epidemiological characteristics and molecular evolution mechanisms of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 1003783. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, D.; Chan, E.W.-C.; Gu, D.; Chen, G.-X.; Chen, S. Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob. Agents Chemother. 2015, 60, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, N.; Chan, E.W.-C.; Zhang, R.; Chen, S. Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in Klebsiella pneumoniae. Trends Microbiol. 2021, 29, 65–83. [Google Scholar] [CrossRef]
- Bento, M.L.; de Matos, L.V.; Ribeiro, L.A.; Gomes, O.; Nogueira, F.; Esteves, G.; Valle, S.; Martins, H.; Raposo, J. Necrotizing fasciitis of the vulva due to carbapenem-resistant Enterobacteriaceae as a complication of acute myeloid leukemia treatment: A case report. J. Med. Case Rep. 2022, 16, 148. [Google Scholar] [CrossRef]
- Hasman, H.; Saputra, D.; Sicheritz-Ponten, T.; Lund, O.; Svendsen, C.A.; Frimodt-Møller, N.; Aarestrup, F.M. Rapid Whole-Genome Sequencing for Detection and Characterization of Microorganisms Directly from Clinical Samples. J. Clin. Microbiol. 2014, 52, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.S. Whole Genome Sequencing: Applications in Clinical Bacteriology. Med. Princ. Pract. 2024, 33, 185–197. [Google Scholar] [CrossRef]
- Rossen, J.W.A.; Friedrich, A.W.; Moran-Gilad, J. ESCMID Study Group for Genomic and Molecular Diagnostics (ESGMD). Practical Issues in Implementing Whole-Genome Sequencing in Routine Diagnostic Microbiology. Clin. Microbiol. Infect. 2018, 24, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Realegeno, S.; Mirasol, R.; Tsan, A.; Chandrasekaran, S.; Garner, O.B.; Yang, S. Validation, Implementation, and Clinical Utility of Whole Genome Sequence-Based Bacterial Identification in the Clinical Microbiology Laboratory. J. Mol. Diagn. 2021, 23, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Michos, A.; Palili, A.; Koutouzis, E.I.; Sandu, A.; Lykopoulou, L.; Syriopoulou, V.P. Detection of bacterial pathogens in synovial and pleural fluid with the FilmArray Blood Culture Identification System. IDCases 2016, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Hirai, J.; Mori, N.; Sakanashi, D.; Morishita, Y.; Kuge, Y.; Kishino, T.; Asai, N.; Hagihara, M.; Takahashi, N.; Mikamo, H. Usefulness of the FilmArray blood culture identification panel for identifying causative pathogens of bone and joint infections. J. Infect. Chemother. 2023, 29, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, N.; Di Bella, S.; Principe, L.; Luppino, D.; Conti, J.; Costantino, V.; Di Santolo, M.; Busetti, M.; Luzzati, R.; Zerbato, V. BioFire® Joint Infection Panel for Samples Other than Synovial Fluid. Antibiotics 2024, 13, 1198. [Google Scholar] [CrossRef]
- Toth, Z.; Balázs, B.; Majoros, L.; Kovács, R. A case of necrotizing fasciitis and severe sepsis due to ST23 hypervirulent Klebsiella pneumoniae. Acta Microbiol. Immunol. Hung. 2023, 70 (Suppl. S1), 85–86. [Google Scholar] [CrossRef]
| Antibiotic | Interpretation by Disk Diffusion | MIC mg/L (MTS) |
|---|---|---|
| Ampicillin | Resistant | >64 |
| Amoxicillin/Clavulanic acid | Susceptible | 2 |
| Piperacillin/Tazobactam | Susceptible | 1 |
| Cefotaxime | Susceptible | 0.125 |
| Ceftazidime | Susceptible | 0.125 |
| Cefepime | Susceptible | 0.125 |
| Meropenem | Susceptible | 0.06 |
| Imipenem | Susceptible | 0.125 |
| Ertapenem | Susceptible | 0.06 |
| Trimethoprim/Sulfamethoxazole | Susceptible | 0.25 |
| Ciprofloxacin | Susceptible | 0.06 |
| Gentamicin | Susceptible | 1 |
| Tobramycin | Susceptible | 1 |
| Amikacin | Susceptible | 4 |
| Ceftazidime/Avibactam | Susceptible | 0.125 |
| Ceftolozane/Tazobactam | Susceptible | 0.125 |
| Tigecycline 1 | NA 2 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, Z.; Balázs, B.; Pfliegler, W.P.; Csoma, E.; Majoros, L.; Szűcs, D.; Kovács, R. Application of Biofire Filmarray Joint Infection Panel for Rapid Identification of Aetiology in a Necrotizing Fasciitis Case. Diagnostics 2025, 15, 58. https://doi.org/10.3390/diagnostics15010058
Tóth Z, Balázs B, Pfliegler WP, Csoma E, Majoros L, Szűcs D, Kovács R. Application of Biofire Filmarray Joint Infection Panel for Rapid Identification of Aetiology in a Necrotizing Fasciitis Case. Diagnostics. 2025; 15(1):58. https://doi.org/10.3390/diagnostics15010058
Chicago/Turabian StyleTóth, Zoltán, Bence Balázs, Walter P. Pfliegler, Eszter Csoma, László Majoros, Dorka Szűcs, and Renátó Kovács. 2025. "Application of Biofire Filmarray Joint Infection Panel for Rapid Identification of Aetiology in a Necrotizing Fasciitis Case" Diagnostics 15, no. 1: 58. https://doi.org/10.3390/diagnostics15010058
APA StyleTóth, Z., Balázs, B., Pfliegler, W. P., Csoma, E., Majoros, L., Szűcs, D., & Kovács, R. (2025). Application of Biofire Filmarray Joint Infection Panel for Rapid Identification of Aetiology in a Necrotizing Fasciitis Case. Diagnostics, 15(1), 58. https://doi.org/10.3390/diagnostics15010058








