Abstract
Background/Objectives: Covert hepatic encephalopathy (CHE) is associated with decreased quality of life. Detection of Child–Pugh class A is necessary for its early diagnosis. This study aimed to establish a simple diagnostic method of CHE in patients with Child–Pugh class A. Methods: One hundred patients with liver cirrhosis without overt hepatic encephalopathy and sixty-eight with liver cirrhosis and Child–Pugh class A who visited our institution were enrolled. CHE was diagnosed using number connection test B in the neuropsychiatric test (NPT). Clinical data were compared. Results: The liver volume/body surface area ratio (LV/BSA) was associated with CHE in patients with all-cause and Child–Pugh class A liver cirrhosis. Multiple logistic regression analysis revealed that low LV/BSA and low serum zinc (Zn) levels were significantly associated with CHE in Child–Pugh class A liver cirrhosis. The best cutoff values in the receiver operating characteristic curve analysis showed that the complication rate of CHE was 54.8% in patients with LV/BSA < 620 mL/m2, which was 2.9 times higher than that in patients with larger liver volume. Referring to the cutoff values for LV/BSA and Zn (<70 µg/dL), in cases with LV/BSA < 620 mL/m2 and Zn < 70 µg/dL, 64.2% had CHE, whereas in cases with LV/BSA ≥ 620 mL/m2 and Zn ≥ 70 µg/dL, 94.5% did not have CHE. Conclusions: Liver volume can be used as a risk assessment tool for CHE. LV/BSA and serum Zn levels are considered effective diagnostic tools for CHE, serving as alternatives to NPT in patients with Child–Pugh class A liver cirrhosis.
1. Introduction
Hepatic encephalopathy (HE) is a complication of liver cirrhosis that can significantly affect a patient’s quality of life (QOL), causing sleep disturbances, depression, driving difficulties, and an increased risk of falls. Covert HE (CHE), a preclinical stage of HE, is associated with these impairments. Psychometric tests are currently used to diagnose CHE; however, establishing standardized criteria is challenging, owing to variations in education, culture, cognitive function, and physical ability, particularly in older adults. Furthermore, these tests are cumbersome and time-consuming, making them difficult to administer in clinical practice. Therefore, identifying clinical markers that predict the onset of CHE is crucial.
To address these issues, the Stroop test has proven effective in reducing diagnostic time and facilitating screening [1,2]. Low albumin (Alb; ≤3.5 g/dL) and high ammonia (≥80 μg/dL) levels have been reported as clinical scores that can be easily determined from hematochemical tests alone [3].
Diagnosis of CHE by blood chemistry is simple and ideal; however, many cases identified as high-risk are Child–Pugh class B. In recent multicenter studies, the onset of CHE has been reported to correlate with a Child–Pugh score [4], and the Child–Pugh score is effective in risk discrimination. Thus, hepatic decompensation is a risk factor for CHE. Therefore, to detect CHE at an early stage, we believe it is necessary to identify cases with a high risk of developing the disease in patients with Child–Pugh class A.
Liver volume has been reported to correlate with the liver reserve capacity. Furthermore, liver volume has been reported to decrease in uncompensated cirrhosis compared with the compensated stage, and the presence or absence of overt HE correlates with liver volume [5,6]. In this study, to subdivide the liver function of patients with Child–Pugh class A, we focused on liver volume. We aimed to investigate the possibility of an association between CHE and liver volume.
2. Materials and Methods
2.1. Study Design
This study enrolled 100 consecutive patients with liver cirrhosis without history of overt HE at the Nagasaki University Hospital (Nagasaki, Japan) between 2019 and 2022. This cohort included patients with all etiologies, including alcoholic liver disease. The number connection test B (NCT-B) was used to diagnose CHE. Clinical data such as liver function, fibrosis markers, presence of esophageal varices, presence of a portosystemic shunt, and liver volume were compared. Blood tests were performed for all patients within the 7-day CHE test. Esophageal varices were defined based on a history of treatment. Computed tomography (CT) images used to measure liver volume and assess portosystemic shunts were obtained within 3 months before and after the CHE test. A portosystemic shunt was defined as having a maximum vessel diameter ≥ 5 mm. All patients with cirrhosis were treated according to the Japanese Clinical Practical Guidelines for Liver Cirrhosis.
Liver cirrhosis was identified if at least one of the following criteria was met: platelet count < 100,000/µL, presence of esophageal varices, Mac-2 binding protein glycosylation isomer (M2BPGi) > 3, fibrosis 4 (Fib4) index > 2.67, hyaluronan > 130 ng/mL, type 4 collagen 7S > 8 ng/mL, or FibroScan score > 12.5 kPa.
Next, we conducted an analysis, focusing on 68 consecutive cases of liver cirrhosis in patients with Child–Pugh class A without a history of overt HE who visited our hospital between 2019 and 2023. Given the significant effect of alcohol consumption on liver volume, patients with continuous drinking, defined as regularly drinking >20 g/day, were excluded from this study.
2.2. Assessment of Liver Volume
All patients underwent multiphase contrast-enhanced CT of the abdomen. Liver volume data were obtained routinely with a parallel 3D scan of the liver using SYNAPSE VINCENT® image processing software ver7.0 (Fujifilm Medical Co., Tokyo, Japan). Liver volume was corrected for body surface area (LV/BSA) to account for variations in physique. The spleen volume/body surface area ratio (SPV/BSA) and liver volume/spleen volume ratio (LV/SPV) were also measured as findings suggesting portal hypertension [7].
2.3. CHE Diagnosis
CHE was diagnosed using computer-aided neuropsychiatric test (NPT) software ver3.1 on an iPad (Apple Inc., Cupertino, CA, USA). NPT software was developed by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan) and provided by the Japan Society of Hepatology. NPT is composed of four subtests: NCT-A, NCT-B, digit symbol test, and block design test. In Japan, patients with liver cirrhosis are diagnosed with CHE if the results of two or more of the four subtests are abnormal [8].
Scores from the EncephalApp (Stroop-off and Stroop-on tests) and the NCT-B test can identify patients with CHE with approximately 87% accuracy and in a much shorter time than the standard psychometric HE scoring system [1]. In this study, patients who were NCT-B-positive were diagnosed with CHE.
2.4. Statistical Analyses
Based on CHE diagnosis, patients were divided into CHE and non-CHE groups. The significance of differences in these continuous variables across the study groups was calculated using the Mann–Whitney U test. Categorical data were analyzed using Fisher’s exact test. We calculated the odds ratio, 95% confidence interval, and p-values using multiple logistic regression analysis to identify factors associated with CHE. Continuous variables were dichotomized based on median values. CHE was the objective variable and clinical items were explanatory variables. The p values were tested against the null hypothesis of an odds ratio of 1.0 at a two-sided 5% significance level. Statistical significance was set at p < 0.05. Data analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. CHE Correlates with Liver Functional Reserve
Of the 100 patients, 47 had CHE. When comparing the non-CHE group with the background factors, prolonged prothrombin time with international normalized ratio (PT-INR), elevated total bilirubin (TBil), and decreased Alb levels were significantly associated with CHE. These factors were determinant of the Child–Pugh score, and the Child–Pugh score was correlated with CHE. Elevated levels of fibrotic markers, including hyaluronic acid, type 4 collagen, and M2BPGi, were also noted. Additionally, a significant difference was observed in serum zinc (Zn) level, branched-chain amino acid/tyrosine molecular ratio (BTR), LV/BSA, and LV/SPV (Table 1).

Table 1.
Characteristics of the CHE and non-CHE groups in liver cirrhosis.
The incidence rates of CHE according to Child–Pugh classes were 30.9% in class A, 63.2% in class B, and 85.7% in class C (Figure 1). The proportion of CHE complications increased in proportion to the severity of liver function.
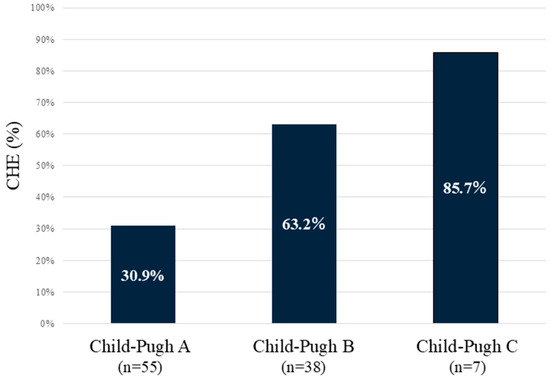
Figure 1.
Incidence of CHE according to Child–Pugh classes. The worse the Child–Pugh class is, the higher the incidence of CHE. CHE—covert hepatic encephalopathy.
3.2. Association Between Alcohol Consumption and Liver Volume
Furthermore, to examine whether alcohol affects liver volume, we compared two groups of alcoholic liver cirrhosis during continuous drinking and during abstinence in 30 patients with alcoholic liver cirrhosis from 100 patients with liver cirrhosis. Continuous drinking was defined as regularly drinking >20 g/day. Among the groups, liver volume was larger in the continuous drinking group (606 vs. 838 mL/m2), with no difference in liver functional reserve among the groups (Table 2). This result indicated that individuals who consumed alcohol were to be excluded when assessing liver volume.

Table 2.
Characteristics of the abstinence and continued drinking groups in alcoholic liver cirrhosis.
3.3. CHE in Child–Pugh Class A Correlates with Liver Volume and Serum Zn Levels
The background factors associated with CHE were compared among cases with Child–Pugh class A, excluding patients with continuous drinking (Table 3). As the liver function severity was aligned, the values for PT-INR, TBil, and Alb were comparable. We performed a multivariate analysis of the factors predicting CHE. The LV/BSA and serum Zn levels were identified as the factor contributing to the deterioration of CHE (Table 4).

Table 3.
Characteristics of the CHE and non-CHE groups in Child–Pugh class A liver cirrhosis.

Table 4.
Factors contributing to CHE in Child–Pugh class A liver cirrhosis (n = 68).
Among these, the LV/BSA showed the most significant difference, with an area under the receiver operating characteristic curve of 0.74. Setting the cutoff value at 620 mL/m2, the incidence of CHE was 54.8%, which was 2.9 times higher in cases exceeding the cutoff value (Figure 2). The cases demonstrated significant differences in liver volume, despite similar liver functional reserves (Figure 3).
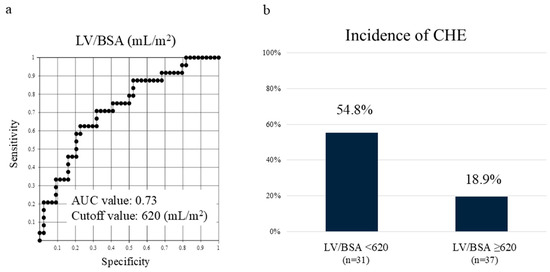
Figure 2.
CHE in Child–Pugh class A correlates with liver volume. (a) Receiver operating characteristic curve of LV/BSA in Child–Pugh class A. (b) Setting the cutoff value at 620 mL/m2, the incidence of CHE was 2.9 times higher in cases below the cutoff value. LV/BSA—liver volume/body surface area; AUC—area under the curve; CHE—covert hepatic encephalopathy.
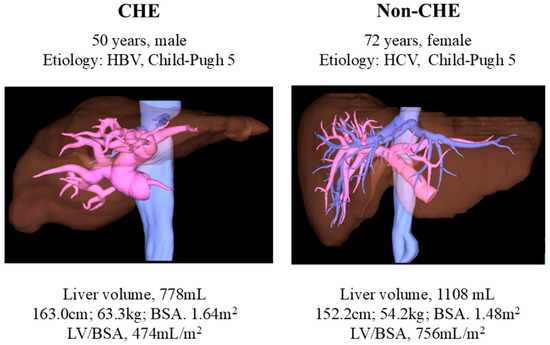
Figure 3.
Representative image showing differences in liver volume. CHE—covert hepatic encephalopathy; HCV—hepatitis C virus; HBV—hepatitis B virus; LV/BSA—liver volume/body surface area.
Although both groups had similar liver functional reserves, the liver volumes were significantly different. Liver volume was small in patients with CHE.
3.4. CHE Prediction Using Liver Volume and Serum Zn Levels
The cutoff value for serum Zn levels was set at 70 µg/dL. The CHE prevalence was 64.2% (9/14) in patients with low LV/BSA (<620 mL/m2) and low Zn level (<70 µg/dL), 38.8% (14/36) in patients with low LV/BSA or low Zn level, and 5.5% (1/18) in patients with high LV/BSA and high Zn level (Figure 4). This suggests that, in cases of LV/BSA ≥ 620 mL/m2 and Zn ≥ 70 µg/dL, the possibility of CHE is extremely low.
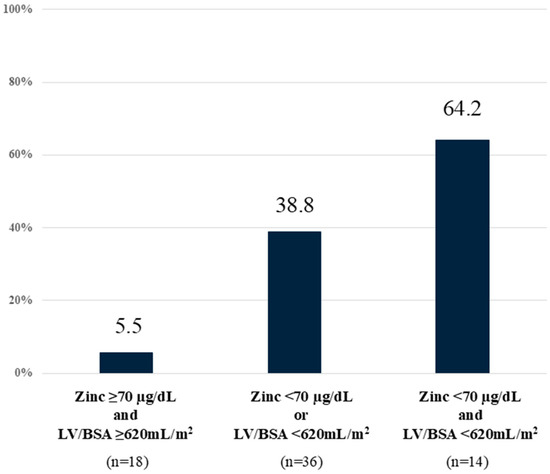
Figure 4.
CHE prediction using liver volume and serum zinc levels. Low LV/BSA (<620 mL/m2) and low zinc level (<70 µg/dL) were associated with high incidence of CHE. CHE—covert hepatic encephalopathy; LV/BSA—liver volume/body surface area.
4. Discussion
This study is one of the first attempts to assess the liver volume to stratify the risk of CHE development in patients with liver cirrhosis. We found that in patients with Child–Pugh class A and no difference in liver functional reserve, CHE was more commonly observed in those with smaller liver volumes. Liver volume, measurable readily through imaging modalities such as CT, provides an objective measure unaffected by patient background compared with NPT. In addition, the addition of serum Zn levels may enable the diagnosis of CHE with higher accuracy. We believe that this method has clinical significance and can serve as a valuable tool for risk assessment in patients with liver cirrhosis.
The prevalence of CHE in patients with cirrhosis has been reported to be 30–50% [9,10]. The gold standard in the diagnosis of CHE is the psychometric hepatic encephalopathy score test, which includes five tests: digit symbol test; NCT A (letters) and B (letters and numbers); serial dotting test; line tracing test [11]. However, the line tracing and serial dotting tests are not common in Japan, and applying them is difficult. Therefore, in Japan, the NPT was developed to perform eight tests: figure design test; digit symbol test; block design test; reaction time A, B, and C tests [12]. Performing all these tests originally required 30–40 min. However, Kawaguchi et al. reported that reducing the number of NPTs from eight to four—the NCT-A, NCT-B, digit symbol test, and block design test—did not affect the diagnosis of CHE, enabling CHE diagnosis within 15–20 min. Therefore, this approach is currently used to diagnose CHE [8].
The liver volume is usually smaller in patients with cirrhosis than in healthy individuals. Liver volume assessment is utilized in preoperative planning for liver resection or transplantation, and the postoperative remnant liver volume is correlated with postoperative outcomes [13]. Liver volume has been identified as an independent predictor of prognosis in patients with cirrhosis, separate from the model for end-stage liver disease (MELD) score [14]. Additionally, liver volume also correlates with the Child–Pugh score [15,16], suggesting its potential as an independent indicator of liver functional reserve, akin to MELD and Child–Pugh scores. Furthermore, recent multicenter studies have reported an association between CHE onset and Child–Pugh score [4]. Although the Child–Pugh score serves as a useful score for assessing CHE risk, a one-point difference in the Child–Pugh score indicates a significant difference in liver function. Therefore, we focused on the liver volume as an item that can express the difference between Child–Pugh scores in more detail.
Liver volume was correlated with body surface area, and a method for calculating the standard liver volume using BSA was applied [17,18]. In the Urata formula, the standard liver volume is expressed as 706.2 × BSA + 2.4 [18]. Therefore, in this study, the LV/BSA ratio was used to correct for differences in liver volume due to differences in body size. Liver volume increases during alcohol consumption [19]. In our study, compared with the abstinence group, liver volume was larger in the continuous drinking group with no difference in liver functional reserve; therefore, when liver volume is used as an indicator of liver functional reserve, ensuring that the patient is not drinking alcohol is crucial.
Ammonia metabolism, which contributes to HE, requires the liver’s ability to process ammonia. If the liver cannot process it, glutamate is used to process ammonia as a compensatory measure, resulting in increased protein catabolism in skeletal muscle and a deficiency associated with branched-chain amino acid consumption, which causes a decrease in the BTR [20]. The mechanism of ammonia metabolism is initially a decrease in BTR, followed by a decrease in Alb, and finally an increase in ammonia. Patients with Child–Pugh grade A had no decrease in the BTR or Alb levels, making risk assessment with ammonia difficult. Therefore, LV/BSA, which was found to be a predictor of CHE development in this study, can predict CHE risk earlier than Alb and ammonia, which have been previously reported.
In this study, low liver volume and serum Zn level were risk factors of CHE in patients with Child–Pugh grade A. Zn deficiency frequently occurs in patients with liver cirrhosis [21]. Zn deficiency is attributed to impaired absorption in the gastrointestinal tract associated with cirrhosis and increased urinary excretion due to the enhanced relative binding of amino acids and Zn as a consequence of low albumin levels. Zn is essential for the urea cycle that metabolizes ammonia, and elevated ammonia resulting from Zn deficiency is postulated to contribute to HE [22,23]. Consequently, Zn levels decrease prior to the elevation of ammonia levels, rendering Zn a potential biomarker for the early detection of CHE. In a cohort with 73.8% Child–Pugh class A, Soma et al. reported that Zn < 60 μg/dL was an independent risk factor for CHE. This finding corroborates the observation that in the Child–Pugh class A-only cohort of this study, Zn levels < 70 μg/dL constituted a risk factor [24].
In living donor liver transplantation, at least 35% of the donor’s remaining liver is required [25], and at least 40% of the standard liver volume is required for the recipient’s graft [18,26]. If 40% of the standard liver volume is required, the LV/BSA would be approximately 300 mL/m2. The cutoff value for the risk of CHE in our study was 620 mL/m2, which is a considerably small liver volume. However, we believe that liver transplant grafts and donor liver remnants function without complications because the liver is composed of healthy hepatocytes. Therefore, we believe that the function of a cirrhotic liver may be reduced to half of that of a normal liver. Serum Zn level and liver volume may be useful in determining liver function in patients with cirrhosis before abnormalities in Alb, PT, and TBil are observed, as expressed by the Child–Pugh classification. In this study, hyaluronic acid levels also showed a significant difference in univariate analysis, suggesting an association with CHE among fibrosis markers. The fibrosis marker hyaluronic acid differs from other fibrosis markers in that it is produced by astrocytes and is characterized by degradation by sinusoidal endothelial cells [27,28]. High levels of hyaluronic acid in the CHE group may reflect functional defects in terms of reduced liver degradation.
In recent years, there have been reports on the utility of novel serum biomarkers such as interleukin 6 and glial fibrillary acidic protein that have been utilized for the diagnosis of CHE [29,30]. The strength of liver volume evaluation in the diagnosis of CHE lies in its utilization of CT, eliminating the need for additional tests and enabling assessment independent of factors such as the patient’s mental state or educational background.
One limitation of this study is that it was a single-center retrospective analysis with a small number of cases. Due to the small number of cases, evaluation by etiology was not possible in this study. Differences may exist in the appropriate cutoff value for each etiology in liver volume; therefore, further accumulation of cases and additional analysis are required in the future. Additionally, verification of long-term outcomes, such as progression to overt HE, in patients diagnosed with CHE is required.
5. Conclusions
Despite the limitation of this study, this is the first to describe the relationship between liver volume and CHE. In this study, liver volume was used as an index reflecting the liver functional reserve capacity and as a risk assessment tool for the development of CHE. Our study indicates that identifying cases with small liver volumes is beneficial in efficiently detecting the risk of CHE in patients with Child–Pugh class A liver cirrhosis. Furthermore, the integration of serum Zn level measurements enhances the diagnostic accuracy for CHE. LV/BSA and serum Zn levels can be considered as effective diagnostic tools for CHE and can act as alternatives to the current diagnostic tools.
Author Contributions
Conceptualization, M.F. and H.M.; data curation, M.F., R.S., Y.N. and M.H.; formal analysis, M.F.; funding acquisition, M.F.; investigation, M.F., H.M., R.S., Y.N., M.H., K.T., E.O., S.M. and K.N.; methodology, M.F.; project administration, M.F.; resources, M.F.; software, M.F.; supervision, M.F.; validation, M.F. and R.S.; visualization, M.F.; writing—original draft, M.F.; writing—review and editing, M.F. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Nagasaki University Hospital (protocol code 23022004-2; 21 March 2023).
Informed Consent Statement
Informed consent was obtained from all individual participants included in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, as they contain sensitive medical information.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Alb | Albumin |
| AL | Alcoholic liver |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| BCAA | Branched-chain amino acid |
| BMI | Body mass index |
| BTR | Branched-chain amino acid-to-tyrosine molecular ratio |
| CHE | Covert hepatic encephalopathy |
| CI | Confidence interval |
| CT | Computed tomography |
| eGFR | Estimated glomerular filtration rate |
| Fib4 | Fibrosis-4 index |
| γ-GTP | γ-Glutamyl transpeptidase |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HE | Hepatic encephalopathy |
| LV/BSA | Liver volume-to-body surface area ratio |
| LV/SPV | Liver volume-to-spleen volume ratio |
| M2BPGi | Mac-2 binding protein glycosylation isomer |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MELD | Model for end-stage liver disease score |
| Na | Sodium |
| NCT-B | Number connection test B |
| NH3 | Ammonia |
| NPT | Neuropsychiatric test |
| OR | Odds ratio |
| Plt | Platelets |
| PT-INR | Prothrombin time/international normalized ratio |
| QOL | Quality of life |
| SPV/BSA | Spleen volume-to-body surface area ratio |
| TBil | Total bilirubin |
| TLA | Three-letter acronym |
| Zn | Zinc |
References
- Kondo, Y.; Iwasa, M.; Kawaratani, H.; Miyaaki, H.; Hanai, T.; Kon, K.; Hirano, H.; Shimizu, M.; Yoshiji, H.; Okita, K.; et al. Proposal of Stroop test cut-off values as screening for neuropsychological impairments in cirrhosis: A Japanese multicenter study. Hepatol. Res. 2021, 51, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Miyaaki, H.; Hiraoka, A.; Haraguchi, M.; Uojima, H.; Kawaratani, H.; Hiramatsu, A.; Hanai, T.; Hiasa, Y.; Yoshiji, H.; Okita, K.; et al. Proposal for new sleep disorder criteria in patients with chronic liver disease: Influence of liver-related complications. Hepatol. Res. 2022, 52, 364–370. [Google Scholar] [CrossRef]
- Miwa, T.; Hanai, T.; Nishimura, K.; Maeda, T.; Tajirika, S.; Imai, K.; Suetsugu, A.; Takai, K.; Yamamoto, M.; Shimizu, M. A simple covert hepatic encephalopathy screening model based on blood biochemical parameters in patients with cirrhosis. PLoS ONE 2022, 17, e0277829. [Google Scholar] [CrossRef] [PubMed]
- Gairing, S.J.; Mangini, C.; Zarantonello, L.; Gioia, S.; Nielsen, E.J.; Danneberg, S.; Gabriel, M.; Ehrenbauer, A.F.; Bloom, P.P.; Ripoll, C.; et al. Prevalence of minimal hepatic encephalopathy in patients with liver cirrhosis: A multicenter study. Am. J. Gastroenterol. 2023, 118, 2191–2200. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Leng, X.S.; Dong, N.; Qi, G.Y.; Du, R.Y. Measurement of liver volume and its clinical significance in cirrhotic portal hypertensive patients. World J. Gastroenterol. 1999, 5, 525–526. [Google Scholar] [CrossRef]
- Patel, M.; Puangsricharoen, P.; Arshad, H.M.S.; Garrison, S.; Techasatian, W.; Ghabril, M.; Sandrasegaran, K.; Liangpunsakul, S.; Tann, M. Does providing routine liver volume assessment add value when performing CT surveillance in cirrhotic patients? Abdom. Radiol. 2019, 44, 3263–3272. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Vazquez, O.; Terraz, S.; Majno, P.; Spahr, L.; Poncet, A.; Morel, P.; Mentha, G.; Toso, C. Accurate computed tomography-based portal pressure assessment in patients with hepatocellular carcinoma. J. Hepatol. 2014, 60, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Konishi, M.; Kato, A.; Kato, M.; Kooka, Y.; Sawara, K.; Endo, R.; Torimura, T.; Suzuki, K.; Takikawa, Y. Updating the neuropsychological test system in Japan for the elderly and in a modern touch screen tablet society by resetting the cut-off values. Hepatol. Res. 2017, 47, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, M.M.; Jepsen, P.; Vilstrup, H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: A comparative study of 154 patients with liver disease. Metab. Brain Dis. 2011, 26, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Tanaka, H.; Kawaguchi, T.; Kanazawa, H.; Iwasa, M.; Sakaida, I.; Moriwaki, H.; Murawaki, Y.; Suzuki, K.; Okita, K. Nutritional management contributes to improvement in minimal hepatic encephalopathy and quality of life in patients with liver cirrhosis: A preliminary, prospective, open-label study. Hepatol. Res. 2013, 43, 452–458. [Google Scholar] [CrossRef]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Kato, A.; Watanabe, Y.; Sawara, K.; Suzuki, K. Diagnosis of sub-clinical hepatic encephalopathy by Neuropsychological Tests (NP-tests). Hepatol. Res. 2008, 38 (Suppl. S1), S122–S127. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Tan, C.H.; Cai, J.; Zheng, J.; Kow, A.W. CT volumetry of the liver: Where does it stand in clinical practice? Clin. Radiol. 2014, 69, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.T.; Sayuk, G.S.; Lisker-Melman, M.; Korenblat, K.M.; Kerr, T.A.; Chapman, W.C.; Crippin, J.S. Liver volume in the cirrhotic patient: Does size matter? Dig. Dis. Sci. 2014, 59, 886–891. [Google Scholar] [CrossRef]
- Zhou, X.P.; Lu, T.; Wei, Y.G.; Chen, X.Z. Liver volume variation in patients with virus-induced cirrhosis: Findings on MDCT. AJR Am. J. Roentgenol. 2007, 189, W153–W159. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Matsui, O.; Kobayashi, S.; Sanada, J.; Koda, W.; Minami, T.; Kawai, K.; Gabata, T. Selective atrophy of the middle hepatic venous drainage area in hepatitis C-related cirrhotic liver: Morphometric study by using multidetector CT. Radiology 2010, 257, 705–714. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Abdalla, E.K.; Doherty, D.A.; Gertsch, P.; Fenstermacher, M.J.; Loyer, E.M.; Lerut, J.; Materne, R.; Wang, X.; Encarnacion, A.; et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002, 8, 233–240. [Google Scholar] [CrossRef]
- Urata, K.; Kawasaki, S.; Matsunami, H.; Hashikura, Y.; Ikegami, T.; Ishizone, S.; Momose, Y.; Komiyama, A.; Makuuchi, M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995, 21, 1317–1321. [Google Scholar] [CrossRef]
- Leung, N.W.; Farrant, P.; Peters, T.J. Liver volume measurement by ultrasonography in normal subjects and alcoholic patients. J. Hepatol. 1986, 2, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Kakita, N. Possible pathogenetic role of ammonia in liver cirrhosis without hyperammonemia of venous blood: The so-called latency period of abnormal ammonia metabolism. Hepatol. Res. 2024, 54, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Iritani, S.; Kawamura, Y.; Muraishi, N.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Akuta, N.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; et al. The useful predictors of zinc deficiency for the management of chronic liver disease. J. Gastroenterol. 2022, 57, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Fabbri, A.; Bianchi, G.; Brizi, M.; Zoli, M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology 1996, 23, 1084–1092. [Google Scholar] [CrossRef]
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9. [Google Scholar] [CrossRef]
- Soma, N.; Uchida, Y.; Kouyama, J.I.; Naiki, K.; Usui, N.; Sato, A.; Yamada, S.; Tsuji, S.; Ando, S.; Sugawara, K.; et al. Serum zinc levels as predictors of covert hepatic encephalopathy in patients with liver cirrhosis. J. Gastroenterol. 2024. [Google Scholar] [CrossRef]
- Kiuchi, T.; Kasahara, M.; Uryuhara, K.; Inomata, Y.; Uemoto, S.; Asonuma, K.; Egawa, H.; Fujita, S.; Hayashi, M.; Tanaka, K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 1999, 67, 321–327. [Google Scholar] [CrossRef]
- Kokudo, T.; Hasegawa, K.; Uldry, E.; Matsuyama, Y.; Kaneko, J.; Akamatsu, N.; Aoki, T.; Sakamoto, Y.; Demartines, N.; Sugawara, Y.; et al. A new formula for calculating standard liver volume for living donor liver transplantation without using body weight. J. Hepatol. 2015, 63, 848–854. [Google Scholar] [CrossRef]
- Guéchot, J.; Laudat, A.; Loria, A.; Serfaty, L.; Poupon, R.; Giboudeau, J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin. Chem. 1996, 42, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Cohen, L.B.; Nanau, R.M. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin. Biochem. 2016, 49, 302–315. [Google Scholar] [CrossRef]
- Gairing, S.J.; Anders, J.; Kaps, L.; Nagel, M.; Michel, M.; Kremer, W.M.; Hilscher, M.; Galle, P.R.; Schattenberg, J.M.; Wörns, M.A.; et al. Evaluation of IL-6 for stepwise diagnosis of minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol. Commun. 2022, 6, 1113–1122. [Google Scholar] [CrossRef]
- Gairing, S.J.; Danneberg, S.; Kaps, L.; Nagel, M.; Schleicher, E.M.; Quack, C.; Engel, S.; Bittner, S.; Galle, P.R.; Schattenberg, J.M.; et al. Elevated serum levels of glial fibrillary acidic protein are associated with covert hepatic encephalopathy in patients with cirrhosis. JHEP Rep. 2023, 5, 100671. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).