Advancing Evidence Generation for Circulating Tumor DNA: Lessons Learned from A Multi-Assay Study of Baseline Circulating Tumor DNA Levels across Cancer Types and Stages
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Assay Characteristics
3.2. Sample Characteristics
3.3. Baseline ctDNA Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medina, J.E.; Dracopoli, N.C.; Bach, P.B.; Lau, A.; Scharpf, R.B.; Meijer, G.A.; Andersen, C.L.; Velculescu, V.E. Cell-free DNA approaches for cancer early detection and interception. J. Immunother. Cancer 2023, 11, e006013. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, N.P.; Szymanski, J.J.; Earland, N.; Chauhan, P.S.; Pellini, B.; Chaudhuri, A.A. Genomic approaches to cancer and minimal residual disease detection using circulating tumor DNA. J. Immunother. Cancer 2023, 11, e006284. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, P.J.; Ghosh, S.; Pathak, A.; Fusco, M.J.; Bloomquist, E.W.; Tang, S.; Singh, H.; Philip, R.; Pazdur, R.; Beaver, J.A. Regulatory implications of ctDNA in immuno-oncology for solid tumors. J. Immunother. Cancer 2023, 11, e005344. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, L.; Murray, J.C.; Canzoniero, J.V.; Landon, B.; Jackson, J.; Scott, S.; Lam, V.; Levy, B.P.; Sausen, M.; Anagnostou, V. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J. Immunother. Cancer 2023, 11, e005924. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Crucial roles of circulating tumor cells in the metastatic cascade and tumor immune escape: Biology and clinical translation. J. Immunother. Cancer 2022, 10, e005615. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Jones, G.; Severgnini, M.; Alessi, J.V.; Recondo, G.; Lawrence, M.; Forshew, T.; Lydon, C.; Nishino, M.; Cheng, M.; et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2021, 9, e001504. [Google Scholar] [CrossRef] [PubMed]
- Seremet, T.; Jansen, Y.; Planken, S.; Njimi, H.; Delaunoy, M.; El Housni, H.; Awada, G.; Schwarze, J.K.; Keyaerts, M.; Everaert, H.; et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J. Transl. Med. 2019, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Nabet, B.Y.; Esfahani, M.S.; Moding, E.J.; Hamilton, E.G.; Chabon, J.J.; Rizvi, H.; Steen, C.B.; Chaudhuri, A.A.; Liu, C.L.; Hui, A.B.; et al. Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition. Cell 2020, 183, 363–376.e13. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.M.; Nishimura, K.K.; Zariffa, N.; Thompson, J.C.; Hoering, A.; Cilento, V.; Rosenthal, A.; Anagnostou, V.; Baden, J.; Beaver, J.A.; et al. Changes in Circulating Tumor DNA Reflect Clinical Benefit Across Multiple Studies of Patients with Non–Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. JCO Precis. Oncol. 2022, 6, e2100372. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.A.; Howie, L.J.; Pelosof, L.; Kim, T.; Liu, J.; Goldberg, K.B.; Sridhara, R.; Blumenthal, G.M.; Farrell, A.T.; Keegan, P.; et al. A 25-Year Experience of US Food and Drug Administration Accelerated Approval of Malignant Hematology and Oncology Drugs and Biologics. JAMA Oncol. 2018, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Use of Circulating Tumor DNA for Early-Stage Solid Tumor Drug Development Guidance for Industry DRAFT GUIDANCE. Available online: https://www.fda.gov/vaccines-blood-biologics/guidance (accessed on 19 October 2023).
- Desai, A.; Lovly, C.M. Challenges in the implementation of ultrasensitive liquid biopsy approaches in precision oncology. J. Immunother. Cancer 2023, 11, e006793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Y.; Xu, Y.; Li, L.; Gong, Y.; Zhang, K.; Zhang, M.; Guan, Y.; Chang, L.; Xia, X.; et al. Pan-cancer circulating tumor DNA detection in over 10,000 Chinese patients. Nat. Commun. 2021, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Zill, O.A.; Banks, K.C.; Fairclough, S.R.; Mortimer, S.A.; Vowles, J.V.; Mokhtari, R.; Gandara, D.R.; Mack, P.C.; Odegaard, J.I.; Nagy, R.J.; et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin. Cancer Res. 2018, 24, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef]
- Febbo, P.G.; Martin, A.; Scher, H.I.; Barrett, J.C.; Beaver, J.A.; Beresford, P.J.; Blumenthal, G.M.; Bramlett, K.; Compton, C.; Dittamore, R.; et al. Minimum Technical Data Elements for Liquid Biopsy Data Submitted to Public Databases. Clin. Pharmacol. Ther. 2020, 107, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.V.; Reinert, T.; Christensen, E.; Sethi, H.; Birkenkamp-Demtröder, K.; Gögenur, M.; Gögenur, I.; Zimmermann, B.G.; Dyrskjøt, L.; Andersen, C.L.; et al. The effect of surgical trauma on circulating free DNA levels in cancer patients—Implications for studies of circulating tumor DNA. Mol. Oncol. 2020, 14, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Lerro, C.C.; Bradley, M.C.; Forshee, R.A.; Rivera, D.R. The Bar Is High: Evaluating Fit-for-Use Oncology Real-World Data for Regulatory Decision Making. JCO Clin. Cancer Inform. 2024, 8, e2300261. [Google Scholar] [CrossRef] [PubMed]
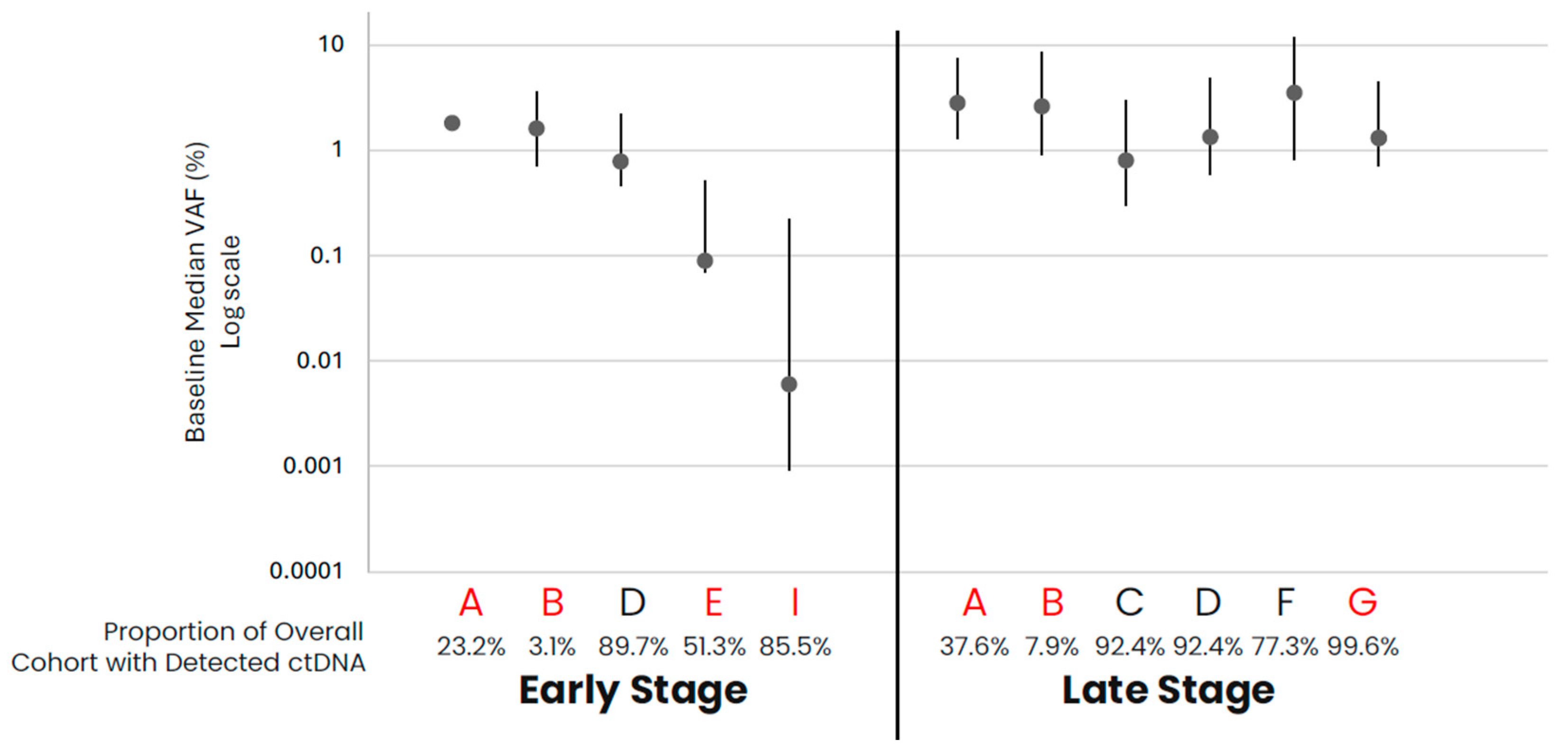
| A: Early- and Late-Stage Non-Small Cell Lung Cancer (NSCLC) | ||||||||||||
| NSCLC | ||||||||||||
| Early Stage | Late Stage | |||||||||||
| Cohort | A | B | D | E | I | A | B | C | D | F | G | |
| N (samples) | 245 | 1873 | 679 | 78 | 131 | 1232 | 31,889 | 23,157 | 2452 | 264 | 4000 | |
| Age (median, years) | 70 | 70 | 70 | Unkn | 63 | 69 | 67 | 68 | Unkn | 70 | 73 | |
| Gender (% female) | 48 | 49 | 49 | 49 | 35 | 49 | 50 | 52 | 49 | 52 | 53 | |
| Clinical Stage | ||||||||||||
| I | 5 | 19 | 15 | 53 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | |
| II | 2 | 15 | 17 | 28 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | |
| III | 11 | 29 | 68 | 19 | 35 | 5 | 1 | 0 | 0 | 15 | 0 | |
| IV | 0 | 0 | 0 | 0 | 0 | 13 | 7 | 0 | 100 | 82 | 0 | |
| Unknown | 82 | 37 | 0 | 0 | 0 | 82 | 92 | 100 | 0 | 3 | 100 | |
| Prior Anti-Cancer Treatments | ||||||||||||
| Known Tx | 18 | 21 | 0 | 0 | 0 | 18 | 3 | 0 | 0 | 0 | 0 | |
| None | 1 | 42 | 0 | 0 | 100 | 11 | 6 | 0 | 0 | 0 | 0 | |
| Unknown | 81 | 37 | 100 | 100 | 0 | 71 | 91 | 100 | 100 | 100 | 100 | |
| Recurrence/Progression Status | ||||||||||||
| No prior cancer | 2 | 11 | 0 | 0 | 100 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Unknown | 95 | 86 | 100 | 100 | 0 | 97 | 98 | 100 | 100 | 100 | 100 | |
| Timing of Sampling, days from diagnosis to sampling, (median (IQR)) | 461 (145.5, 891.5) | 40 (16, 134.3) | 22 (13, 36) | <84 | 1 (1, 1) | 602.5 (334, 850.8) | 29 (13, 31.8) | 18 (7, 52) | 17 (9, 36) | <84 | Unkn | |
| Frequency of ctDNA Detected in Samples (%) | 23.2 | 3.1 | 89.7 | 51.3 | 85.5 | 37.6 | 7.9 | 92.4 | 92.4 | 77.3 | 99.6 | |
| Median VAF (IQR) | 1.8 (0.7, 2.3) | 1.6 (0.9, 2) | 0.78 (0.32, 1.44) | 0.09 (0.02, 0.42) | 0.001 (0.001, 0.2) | 2.8 (1.5, 4.6) | 2.6 (1.7, 5.8) | 0.8 (0.5, 2.2) | 1.33 (0.75, 3.47) | 3.49 (2.67, 8.21) | 1.3 (0.6, 3.2) | |
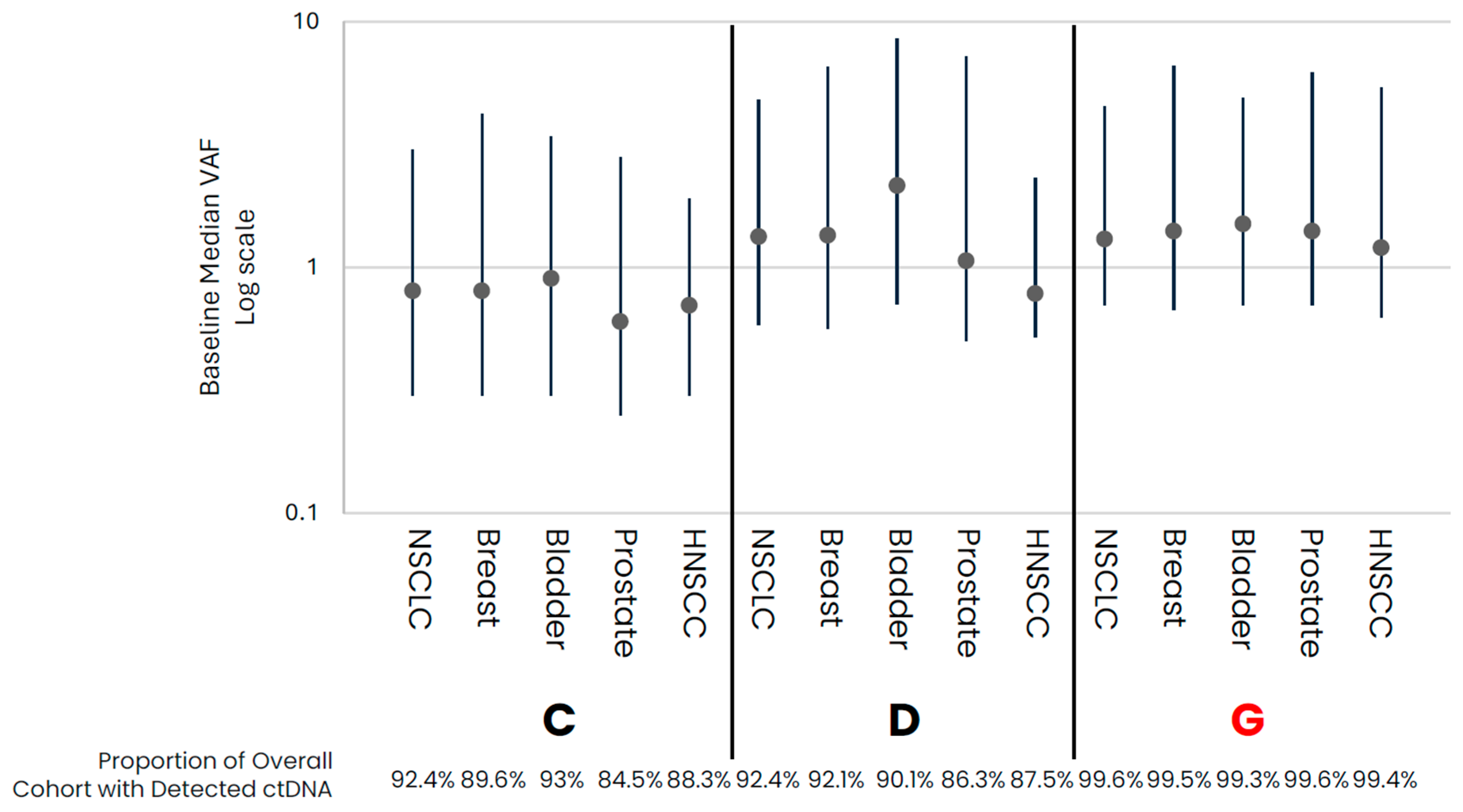
| B: Late-Stage Breast, Bladder, Prostate, and HNSCC Cancers | ||||||||||||
| Late Stage | ||||||||||||
| Breast | Bladder | Prostate | HNSCC | |||||||||
| Cohort | C | D | G | C | D | G | C | D | G | C | D | G |
| N (samples) | 2572 | 1020 | 6940 | 500 | 282 | 577 | 1100 | 633 | 9502 | 274 | 136 | 546 |
| Age (median, years) | 62 | 61 | 64 | 72 | 71 | 73 | 70 | 68 | 74 | 64 | 62 | 64 |
| Gender (% female) | 98 | 100 | 99 | 29 | 26 | 25 | 0 | 0 | 0 | 22 | 24 | 23 |
| Clinical Stage | ||||||||||||
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| III | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IV | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| Unknown | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 0 | 100 |
| Prior Anti-Cancer Treatments | ||||||||||||
| Known Tx | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Recurrence/Progression Status | ||||||||||||
| No prior cancer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Timing of Sampling, days from diagnosis to Ssmpling, (median (IQR)) | 262 (16, 1220) | 35 (20, 75) | Unkn | 70 (11, 557) | 241 (35, 741) | Unkn | 126.5 (9, 1130.5) | 42 (21, 1255) | Unkn | 97.5 (9, 728.5) | 34 (24, 135) | Unkn |
| Frequency of ctDNA Detected in Samples (%) | 89.6 | 92.1 | 99.5 | 93 | 90.1 | 99.3 | 84.5 | 86.3 | 99.6 | 88.3 | 87.5 | 99.4 |
| Median VAF (IQR) | 0.8 (0.5, 3.4) | 1.35 (0.79, 5.19) | 1.4 (0.73, 5.22) | 0.9 (0.6, 2.5) | 2.15 (1.44, 6.36) | 1.5 (0.8, 3.4) | 0.6 (0.35, 2.2) | 1.06 (0.56, 6.14) | 1.4 (0.7, 4.8) | 0.7 (0.4, 1.2) | 0.78 (0.26, 1.53) | 1.2 (0.58, 4.19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKelvey, B.A.; Andrews, H.S.; Baehner, F.L.; Chen, J.; Espenschied, C.R.; Fabrizio, D.; Gorton, V.; Gould, C.; Guinney, J.; Jones, G.; et al. Advancing Evidence Generation for Circulating Tumor DNA: Lessons Learned from A Multi-Assay Study of Baseline Circulating Tumor DNA Levels across Cancer Types and Stages. Diagnostics 2024, 14, 912. https://doi.org/10.3390/diagnostics14090912
McKelvey BA, Andrews HS, Baehner FL, Chen J, Espenschied CR, Fabrizio D, Gorton V, Gould C, Guinney J, Jones G, et al. Advancing Evidence Generation for Circulating Tumor DNA: Lessons Learned from A Multi-Assay Study of Baseline Circulating Tumor DNA Levels across Cancer Types and Stages. Diagnostics. 2024; 14(9):912. https://doi.org/10.3390/diagnostics14090912
Chicago/Turabian StyleMcKelvey, Brittany A., Hillary S. Andrews, Frederick L. Baehner, James Chen, Carin R. Espenschied, David Fabrizio, Vanessa Gorton, Claire Gould, Justin Guinney, Greg Jones, and et al. 2024. "Advancing Evidence Generation for Circulating Tumor DNA: Lessons Learned from A Multi-Assay Study of Baseline Circulating Tumor DNA Levels across Cancer Types and Stages" Diagnostics 14, no. 9: 912. https://doi.org/10.3390/diagnostics14090912
APA StyleMcKelvey, B. A., Andrews, H. S., Baehner, F. L., Chen, J., Espenschied, C. R., Fabrizio, D., Gorton, V., Gould, C., Guinney, J., Jones, G., Lv, X., Nahorski, M. S., Palomares, M. R., Pestano, G. A., Sausen, M., Silk, A., Zhang, N., Zhang, Z., Stewart, M. D., & Allen, J. D. (2024). Advancing Evidence Generation for Circulating Tumor DNA: Lessons Learned from A Multi-Assay Study of Baseline Circulating Tumor DNA Levels across Cancer Types and Stages. Diagnostics, 14(9), 912. https://doi.org/10.3390/diagnostics14090912







