Current Developments and Role of Intestinal Ultrasound including the Advent of AI
Abstract
1. Introduction
2. Materials and Methods
3. Intestinal Ultrasound Technique
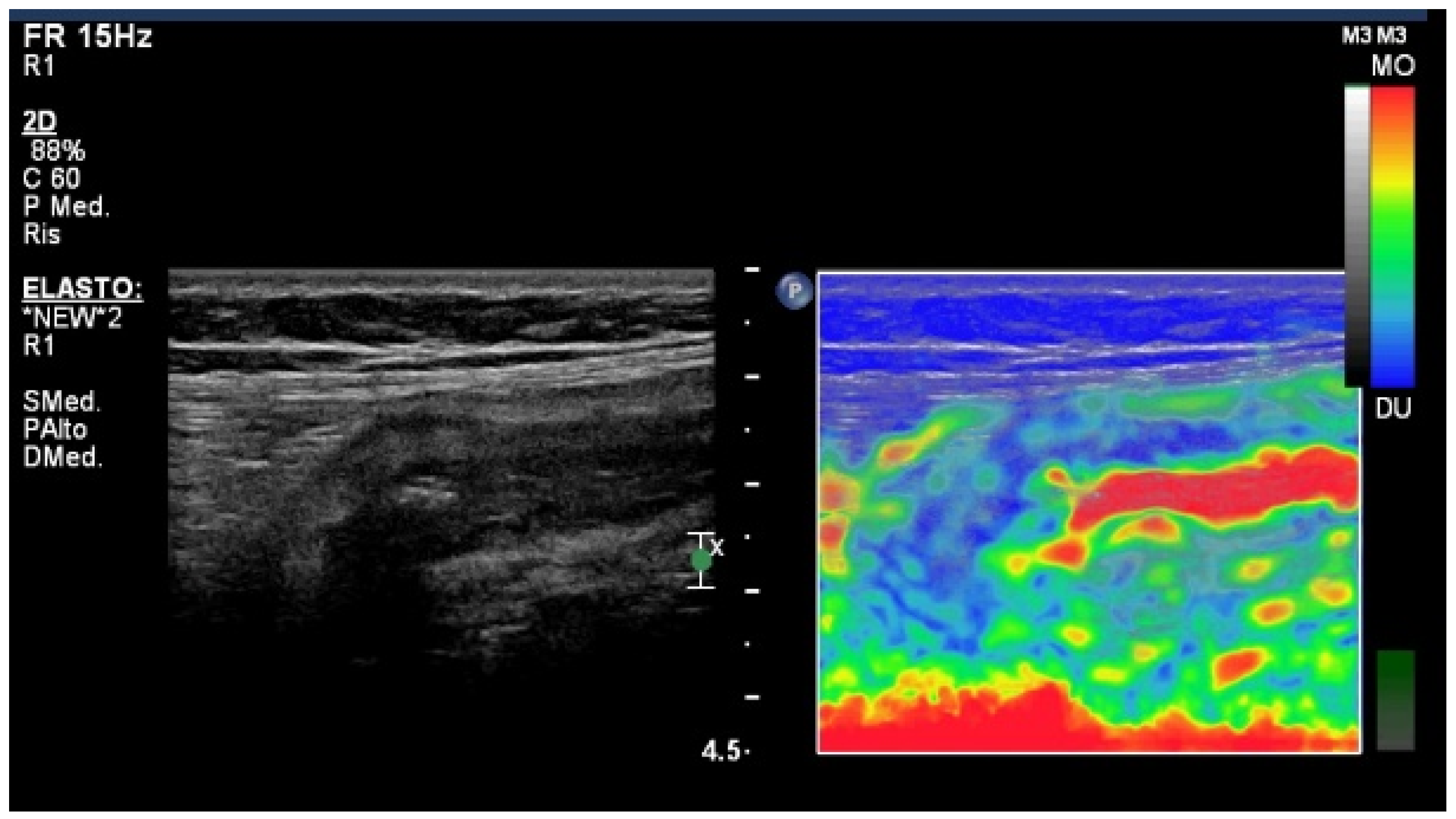
4. Elastography Imaging Techniques
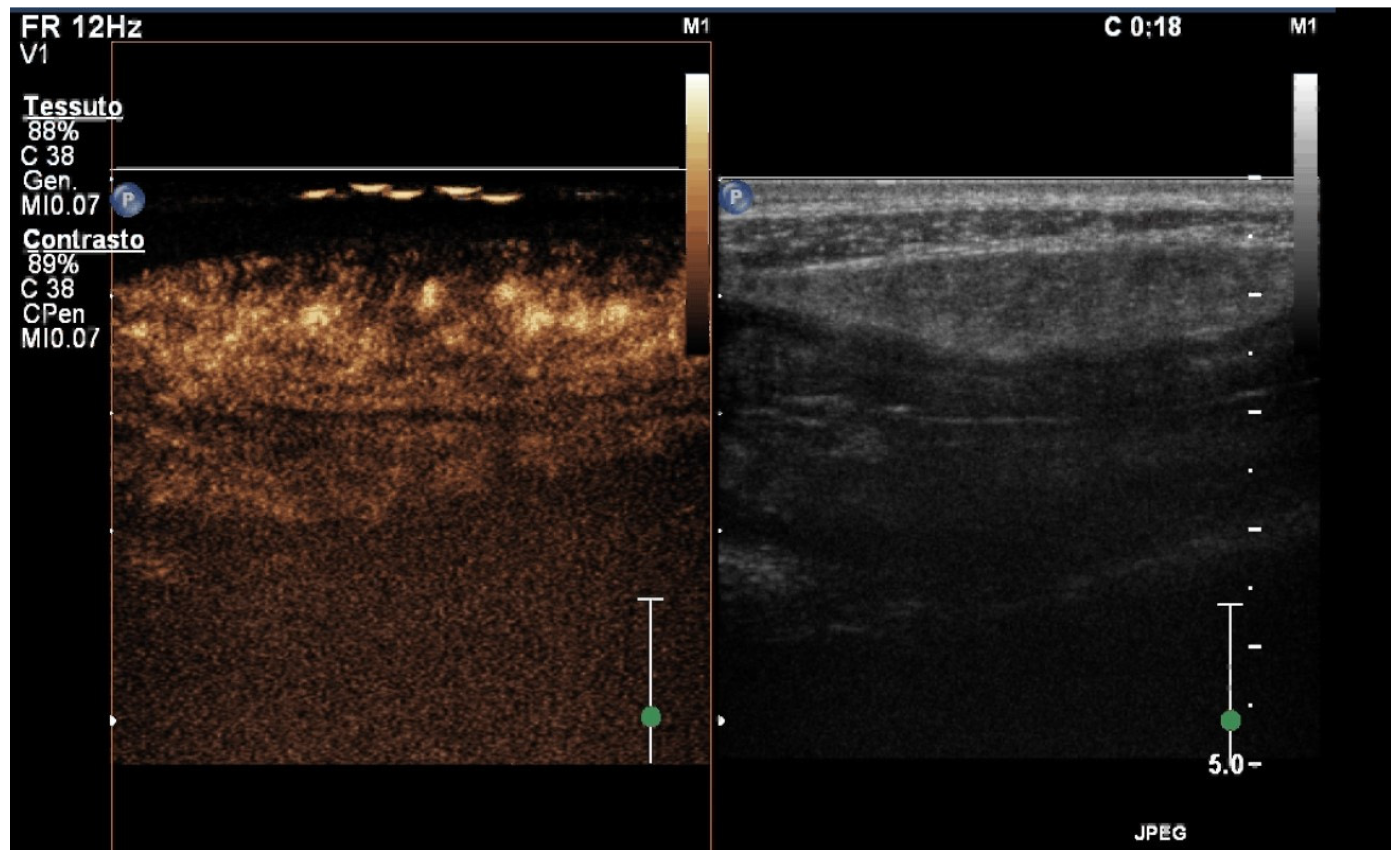
5. Contrast-Enhanced Ultrasound (CEUS)
6. Small Intestine Contrast Ultrasound (SICUS)
7. Artificial Intelligence (AI)
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ARFI | acoustic radiation force impulse |
| AUC | area under the curve |
| BWS | bowel wall stratification |
| BWT | bowel wall thickness |
| CD | Crohn’s disease |
| CDI | color doppler imaging |
| CEUS | contrast-enhanced ultrasound |
| CNNs | convolutional neural networks |
| CPI | color parametric imaging |
| CT | computed tomography |
| CTE | computed tomography enterography |
| DCE | dynamic contrast enhanced |
| DL | deep learning |
| GI | gastrointestinal |
| GvHD | graft-versus-host disease |
| IBD | inflammatory bowel disease |
| IUS | intestinal ultrasound |
| ML | machine learning |
| MRE | magnetic resonance enterography |
| MTT | mean transit time |
| PE | peak enhancement |
| PI | peak intensity |
| Pw | wash-in slope |
| RNNs | recurrent neural networks |
| ROI | region-of-interest |
| SE | strain elastography |
| SICUS | small intestine contrast ultrasound |
| SWD | shear-wave dispersion |
| SWE | shear-wave elastography |
| SWI | shear-wave imaging |
| TE | texture of enhancement |
| TIC | time-intensity curve |
| TTP | time to peak |
| UC | ulcerative colitis |
| US | ultrasound |
| US-E | ultrasound elastography |
References
- Panes, J.; Bouhnik, Y.; Reinisch, W.; Stoker, J.; Taylor, S.; Baumgart, D.; Danese, S.; Halligan, S.; Marincek, B.; Matos, C.; et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohn’s Coli. 2013, 7, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.; Ødegaard, S.; Hausken, T.; Folvik, G.; Lied, G.A.; Viola, I.; Hauser, H.; Gilja, O.-H. Sonography of the small intestine. World J. Gastroenterol. 2009, 15, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Goertz, R.S.; Bernatik, T. Diagnostics in inflammatory bowel disease: Ultrasound. World J. Gastroenterol. 2011, 17, 3192–3197. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, K.; Hata, J.; Haruma, K.; Manabe, N.; Tanaka, S.; Chayama, K. Sonographic detection of longitudinal ulcers in Crohn disease. Scand. J. Gastroenterol. 2004, 39, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Kimmey, M.B.; Wang, K.Y.; Haggitt, R.C.; Mack, L.A.; Silverstein, F.E. Diagnosis of inflammatory bowel disease with ultrasound. An in vitro study. Investig. Radiol. 1990, 25, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Moser, G.; Turetschek, K.; Schober, E.; Moeschl, P.; Oberhuber, G. Transabdominal bowel sonography for the detection of intestinal complications in Crohn’s disease. Gut 1999, 44, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Calavrezos, L.; Bannas, P.; Warncke, M.; Wiegard, C.; Huber, S.; Manthey, C. Transabdominal Ultrasound and Magnetic Resonance Enterography in Inflammatory Bowel Disease: Results of an Observational Retrospective Single-Center Study. Ultrasound Int. Open 2022, 8, E22–E28. [Google Scholar] [CrossRef] [PubMed]
- Goodsall, T.M.; Nguyen, T.M.; Parker, C.E.; Ma, C.; Andrews, J.M.; Jairath, V.; Bryant, R.V. Systematic Review: Gastrointestinal Ultrasound Scoring Indices for Inflammatory Bowel Disease. J. Crohn’s Coli. 2021, 15, 125–142. [Google Scholar] [CrossRef]
- Novak, K.L.; Nylund, K.; Maaser, C.; Petersen, F.; Kucharzik, T.; Lu, C.; Allocca, M.; Maconi, G.; de Voogd, F.; Christensen, B.; et al. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn’s Disease. J. Crohn’s Coli. 2021, 15, 609–616. [Google Scholar] [CrossRef]
- Dragoni, G.; Gottin, M.; Innocenti, T.; Lynch, E.N.; Bagnoli, S.; Macrì, G.; Bonanomi, A.G.; Orlandini, B.; Rogai, F.; Milani, S.; et al. Correlation of Ultrasound Scores with Endoscopic Activity in Crohn’s Disease: A Prospective Exploratory Study. J. Crohn’s Coli. 2023, 17, 1387–1394. [Google Scholar] [CrossRef]
- Allocca, M.; Fiorino, G.; Bonovas, S.; Furfaro, F.; Gilardi, D.; Argollo, M.; Magnoni, P.; Peyrin-Biroulet, L.; Danese, S. Accuracy of Humanitas Ultrasound Criteria in Assessing Disease Activity and Severity in Ulcerative Colitis: A Prospective Study. J. Crohn’s Coli. 2018, 12, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Dell’avalle, C.; Craviotto, V.; Furfaro, F.; Zilli, A.; D’amico, F.; Bonovas, S.; Peyrin-Biroulet, L.; Fiorino, G.; Danese, S. Predictive value of Milan ultrasound criteria in ulcerative colitis: A prospective observational cohort study. United Eur. Gastroenterol. J. 2022, 10, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.T.; Florence, M.G.; Johnson, M.G.; Jurkovich, G.J.; Kwon, S.; Schmidt, Z.; Thirlby, R.C.; Flum, D.R. Progress in the diagnosis of appendicitis: A report from Washington State’s Surgical Care and Outcomes Assessment Program. Ann. Surg. 2012, 256, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, S.; Mohebbi, F.; Sodagari, N.; Hedayat, A. Diagnostic values of ultrasound and the Modified Alvarado Scoring System in acute appendicitis. Int. J. Emerg. Med. 2012, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Puylaert, J.B.; van der Zant, F.M.; Rijke, A.M. Sonography and the acute abdomen: Practical considerations. AJR Am. J. Roentgenol. 1997, 168, 179–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fraquelli, M.; Colli, A.; Colucci, A.; Bardella, M.T.; Trovato, C.; Pometta, R.; Pagliarulo, M.; Conte, D. Accuracy of ultrasonography in predicting celiac disease. Arch. Intern. Med. 2004, 164, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kamaya, A.; Machtaler, S.; Sanjani, S.S.; Nikoozadeh, A.; Sommer, F.G.; Khuri-Yakub, B.T.; Willmann, J.K.; Desser, T.S. New technologies in clinical ultrasound. Semin. Roentgenol. 2013, 48, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imag. 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.-M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Coli. 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Rieder, F.; Latella, G.; Magro, F.; Yuksel, E.S.; Higgins, P.D.R.; Di Sabatino, A.; de Bruyn, J.R.; Rimola, J.; Brito, J.; Bettenworth, D.; et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn’s Coli. 2016, 10, 873–885. [Google Scholar] [CrossRef]
- Gordon, I.O.; Bettenworth, D.; Bokemeyer, A.; Srivastava, A.; Rosty, C.; de Hertogh, G.; Robert, M.E.; Valasek, M.A.; Mao, R.; Kurada, S.; et al. Histopathology Scoring Systems of Stenosis Associated With Small Bowel Crohn’s Disease: A Systematic Review. Gastroenterology 2020, 158, 137–150.e1. [Google Scholar] [CrossRef]
- Choi, D.; Lee, S.J.; Cho, Y.A.; Lim, H.K.; Kim, S.H.; Lee, W.J.; Lim, J.H.; Park, H.; Lee, Y.R. Bowel wall thickening in patients with Crohn’s disease: CT patterns and correlation with inflammatory activity. Clin. Radiol. 2003, 58, 68–74. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Fraquelli, M.; Branchi, F.; Cribiù, F.M.; Orlando, S.; Casazza, G.; Magarotto, A.; Massironi, S.; Botti, F.; Contessini-Avesani, E.; Conte, D.; et al. The Role of Ultrasound Elasticity Imaging in Predicting Ileal Fibrosis in Crohn’s Disease Patients. Inflamm. Bowel Dis. 2015, 21, 2605–2612. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Müller, H.P.; Grittner, U.; Metzke, D.; Fischer, A.; Guckelberger, O.; Pascher, A.; Sack, I.; Vieth, M.; Rudolph, B. US-based Real-time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology 2015, 275, 889–899. [Google Scholar] [CrossRef]
- Re, G.L.; Picone, D.; Vernuccio, F.; Scopelliti, L.; Di Piazza, A.; Tudisca, C.; Serraino, S.; Privitera, G.; Midiri, F.; Salerno, S.; et al. Comparison of US Strain Elastography and Entero-MRI to Typify the Mesenteric and Bowel Wall Changes during Crohn’s Disease: A Pilot Study. Biomed. Res. Int. 2017, 2017, 4257987. [Google Scholar] [CrossRef]
- Serra, C.; Rizzello, F.; Pratico’, C.; Felicani, C.; Fiorini, E.; Brugnera, R.; Mazzotta, E.; Giunchi, F.; Fiorentino, M.; D’errico, A.; et al. Real-time elastography for the detection of fibrotic and inflammatory tissue in patients with stricturing Crohn’s disease. J. Ultrasou. 2017, 20, 273–284. [Google Scholar] [CrossRef]
- Ding, S.; Fang, Y.; Wan, J.; Zhao, C.; Xiang, L.; Liu, H.; Pu, H.; Xu, G.; Zhang, K.; Xu, X.; et al. Usefulness of Strain Elastography, ARFI Imaging, and Point Shear Wave Elastography for the Assessment of Crohn Disease Strictures. J. Ultrasound Med. 2019, 38, 2861–2870. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, E.; Liu, M.; Mei, X.; Dai, Y. Retrospective Cohort Study of Shear-Wave Elastography and Computed Tomography Enterography in Crohn’s Disease. Diagnostics 2023, 13, 1980. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, R.; Li, X.; Cao, Q.; Chen, Z.; Liu, B.; Chen, S.; Chen, B.; He, Y.; Zeng, Z.; et al. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 2183–2190. [Google Scholar] [CrossRef]
- Lu, C.; Gui, X.; Chen, W.; Fung, T.; Novak, K.; Wilson, S.R. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn’s Disease Strictures. Inflamm. Bowel Dis. 2017, 23, 421–430. [Google Scholar] [CrossRef]
- Kapoor, A.; Singh, A.; Kapur, A.; Mahajan, G.; Sharma, S. Use of shear wave imaging with intestinal ultrasonography in patients with chronic diarrhea. J. Clin. Ultrasound 2023, 52, 163–175. [Google Scholar] [CrossRef]
- Garcovich, M.; Paratore, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Shear Wave Dispersion in Chronic Liver Disease: From Physical Principles to Clinical Usefulness. J. Pers. Med. 2023, 13, 945. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, B.-L.; Liang, M.-J.; Chen, S.-L.; Li, X.-H.; Qiu, Y.; Pang, L.-L.; Xia, Q.-Q.; He, Y.; Zeng, Z.-R.; et al. Longitudinal Bowel Behavior Assessed by Bowel Ultrasound to Predict Early Response to Anti-TNF Therapy in Patients With Crohn’s Disease: A Pilot Study. Inflamm. Bowel Dis. 2022, 28 (Suppl. S2), S67–S75. [Google Scholar] [CrossRef]
- Orlando, S.; Fraquelli, M.; Coletta, M.; Branchi, F.; Magarotto, A.; Conti, C.B.; Mazza, S.; Conte, D.; Basilisco, G.; Caprioli, F. Ultrasound Elasticity Imaging Predicts Therapeutic Outcomes of Patients with Crohn’s Disease Treated with Anti-Tumour Necrosis Factor Antibodies. J. Crohn’s Coli. 2018, 12, 63–70. [Google Scholar] [CrossRef]
- Yamada, K.; Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Ishikawa, E.; Mizutani, Y.; Sawada, T.; Maeda, K.; Yamamura, T.; et al. Evaluation of ulcerative colitis activity using transabdominal ultrasound shear wave elastography. Quant. Imaging Med. Surg. 2022, 12, 618–626. [Google Scholar] [CrossRef]
- Goertz, R.S.; Lueke, C.; Schellhaas, B.; Pfeifer, L.; Wildner, D.; Neurath, M.F.; Strobel, D. Acoustic radiation force impulse (ARFI) shear wave elastography of the bowel wall in healthy volunteers and in ulcerative colitis. Acta Radiol. Open 2019, 8, 205846011984096. [Google Scholar] [CrossRef]
- Kapoor, A.; Kapoor, A.; Mahajan, G. Real-time elastography in acute appendicitis. J. Ultrasound Med. 2010, 29, 871–877. [Google Scholar] [CrossRef]
- Goya, C.; Hamidi, C.; Okur, M.H.; Icer, M.; Oguz, A.; Hattapoglu, S.; Cetincakmak, M.G.; Teke, M. The utility of acoustic radiation force impulse imaging in diagnosing acute appendicitis and staging its severity. Diagn. Interv. Radiol. 2014, 20, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Keven, A.; Tekin, A.F.; Arslan, F.Z.; Özer, H.; Durmaz, M.S. Two-dimensional shear wave elastography can improve the diagnostic accuracy of ultrasonography in acute appendicitis. J. Ultrasound 2022, 26, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.W.; Kim, I.Y.; Kim, Y.W. Quantitative Measurement of Elasticity of the Appendix Using Shear Wave Elastography in Patients with Suspected Acute Appendicitis. PLoS ONE 2014, 9, e101292. [Google Scholar] [CrossRef] [PubMed]
- Medellin, A.; Merrill, C.; Wilson, S.R. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom. Radiol. 2018, 43, 918–933. [Google Scholar] [CrossRef]
- Versluis, M.; Stride, E.; Lajoinie, G.; Dollet, B.; Segers, T. Ultrasound Contrast Agent Modeling: A Review. Ultrasound Med. Biol. 2020, 46, 2117–2144. [Google Scholar] [CrossRef] [PubMed]
- Paratore, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Gasbarrini, A.; Zocco, M.A. Dynamic contrast enhanced ultrasound in gastrointestinal diseases: A current trend or an indispensable tool? World J. Gastroenterol. 2023, 29, 4021–4035. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Migaleddu, V.; Scanu, A.M.; Quaia, E.; Rocca, P.C.; Dore, M.P.; Scanu, D.; Azzali, L.; Virgilio, G. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology 2009, 137, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Serafin, Z.; Białecki, M.; Białecka, A.; Sconfienza, L.M.; Kłopocka, M. Contrast-enhanced Ultrasound for Detection of Crohn’s Disease Activity: Systematic Review and Meta-analysis. J. Crohn’s Coli. 2016, 10, 354–362. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Takayama, R.; Kudo, T.; Maruyama, K.; Sumino, Y. Hepatic parenchymal hemodynamics of cholangitis with portal trunk thrombus using contrast-enhanced ultrasonography with Sonazoid: Delineation of so-called central and peripheral zonal differentiation by arrival-time parametric imaging. J. Med. Ultrason. 2013, 40, 73–76. [Google Scholar] [CrossRef]
- Wu, X.-F.; Bai, X.-M.; Yang, W.; Sun, Y.; Wang, H.; Wu, W.; Chen, M.-H.; Yan, K. Differentiation of atypical hepatic hemangioma from liver metastases: Diagnostic performance of a novel type of color contrast enhanced ultrasound. World J. Gastroenterol. 2020, 26, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; de Castro, F.D.; Silva, V.M.; Arieira, C.; Gonçalves, T.C.; Leite, S.; Moreira, M.J.; Cotter, J. Ultrasonographic scores for ileal Crohn’s disease assessment: Better, worse or the same as contrast-enhanced ultrasound? BMC Gastroenterol. 2022, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Huang, P.-L.; Kang, N.; Zhang, J.; Xiao, M.; Zhang, J.-Y.; Cao, X.-C.; Dai, X.-C. The clinical value of multimodal ultrasound for the evaluation of disease activity and complications in inflammatory bowel disease. Ann. Palliat. Med. 2020, 9, 4146–4155. [Google Scholar] [CrossRef]
- Jing, J.; Wu, Y.; Zhang, H.; Zhang, Y.; Mu, J.; Luo, Y.; Zhuang, H. The establishment of a regression model from four modes of ultrasound to predict the activity of Crohn’s disease. Sci. Rep. 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Statie, R.-C.; Iordache, S.; Florescu, L.M.; Gheonea, I.-A.; Sacerdoțianu, V.-M.; Ungureanu, B.S.; Rogoveanu, I.; Gheonea, D.-I.; Ciurea, T.; Florescu, D.N. Assessment of Ileal Crohn’s Disease Activity by Gastrointestinal Ultrasound and MR Enterography: A Pilot Study. Life 2023, 13, 1754. [Google Scholar] [CrossRef]
- Wilkens, R.; Peters, D.A.; Nielsen, A.H.; Hovgaard, V.P.; Glerup, H.; Krogh, K. Dynamic Contrast-Enhanced Magnetic Reso-nance Enterography and Dynamic Contrast-Enhanced Ultrasonography in Crohn’s Disease: An Observational Compari-son Study. Ultrasound Int. Open 2017, 03, E13–E24. [Google Scholar] [CrossRef][Green Version]
- Wilkens, R.; Liao, D.-H.; Gregersen, H.; Glerup, H.; Peters, D.A.; Buchard, C.; Tøttrup, A.; Krogh, K. Biomechanical Properties of Strictures in Crohn’s Disease: Can Dynamic Contrast-Enhanced Ultraso-nography and Magnetic Resonance Enterography Predict Stiffness? Diagnostics 2022, 12, 1370. [Google Scholar] [CrossRef]
- Ripollés, T.; Rausell, N.; Paredes, J.M.; Grau, E.; Martínez, M.J.; Vizuete, J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: A comparison with surgical histopathology analysis. J. Crohn’s Coli. 2013, 7, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ripollés, T.; Paredes, J.M.; Martínez-Pérez, M.J.; Rimola, J.; Jauregui-Amezaga, A.; Bouzas, R.; Martin, G.; Moreno-Osset, E. Ultrasonographic Changes at 12 Weeks of Anti-TNF Drugs Predict 1-year Sonographic Response and Clinical Outcome in Crohn’s Disease: A Multicenter Study. Inflamm. Bowel Dis. 2016, 22, 2465–2473. [Google Scholar] [CrossRef]
- Quaia, E.; Sozzi, M.; Angileri, R.; Gennari, A.G.; Cova, M.A. Time-Intensity Curves Obtained after Microbubble Injection Can Be Used to Differentiate Responders from Nonresponders among Patients with Clinically Active Crohn Disease after 6 Weeks of Pharmacologic Treatment. Radiology 2016, 281, 606–616. [Google Scholar] [CrossRef]
- Quaia, E.; Gennari, A.G.; Cova, M.A. Early Predictors of the Long-term Response to Therapy in Patients With Crohn Disease Derived From a Time-Intensity Curve Analysis After Microbubble Contrast Agent Injection. J. Ultrasound Med. 2019, 38, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Laterza, L.; Ainora, M.E.; Garcovich, M.; Galasso, L.; Poscia, A.; Di Stasio, E.; Lupascu, A.; Riccardi, L.; Scaldaferri, F.; Armuzzi, A.; et al. Bowel contrast-enhanced ultrasound perfusion imaging in the evaluation of Crohn’s disease patients undergoing anti-TNFα therapy. Dig. Liver Dis. 2021, 53, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Saevik, F.; Nylund, K.; Hausken, T.; Ødegaard, S.; Gilja, O.H. Bowel Perfusion Measured with Dynamic Contrast-enhanced Ultrasound Predicts Treatment Outcome in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Gummadi, S.; Koenig, G.; Wessner, C.E.; Machado, P.; Stem, J.; Forsberg, F.; Liu, J.; Lyshchik, A.; O’Kane, P.; Eisenbrey, J.R. Contrast-Enhanced Ultrasound in Small Intestinal Ischemia: Proof of Concept. J. Ultrasound Med. 2022, 41, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Pausch, A.-M.; Kammerer, S.; Weber, F.; Herr, W.; Stroszczynski, C.; Holler, E.; Edinger, M.; Wolff, D.; Weber, D.; Jung, E.-M.; et al. Parametric Imaging of Contrast-Enhanced Ultrasound (CEUS) for the Evaluation of Acute Gastrointestinal Graft-Versus-Host Disease. Cells 2021, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Tews, H.; Kandulski, A.; Schmid, S.; Schlosser, S.; Schirner, S.; Putz, F.; Cosma, L.; Gülow, K.; Müller, M.; Jung, E. Multimodal ultrasound imaging with conventional B-mode, elastography, and parametric analysis of contrast-enhanced ultrasound (CEUS): A novel approach to assess small bowel manifestation in severe COVID-19 disease. Clin. Hemorheol. Microcirc. 2022, 82, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; La Seta, F.; Buccellato, A.; Virdone, R.; Pallotta, N.; Corazziari, E.; Cottone, M. Crohn’s Disease: A Comparative Prospective Study of Transabdominal Ultrasonography, Small Intestine Contrast Ultrasonography, and Small Bowel Enema. Inflamm. Bowel Dis. 2005, 11, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Parente, F. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease. A prospective comparison with conventional ultrasound, X ray studies, and ileocolonoscopy. Gut 2004, 53, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, P.; Pietrini, L.; Barone, M.; Cela, E.M.; Valle, N.D.; Amoruso, A.; Minenna, M.F.; Penna, A.; De Francesco, V.; Panella, C.; et al. Small intestinal contrast ultrasonography-based scoring system: A promising approach for the diagnosis and follow-up of celiac disease. J. Clin. Gastroenterol. 2005, 39, 591–595. [Google Scholar] [CrossRef]
- Onali, S.; Calabrese, E.; Petruzziello, C.; Zorzi, F.; Sica, G.; Fiori, R.; Ascolani, M.; Lolli, E.; Condino, G.; Palmieri, G.; et al. Small intestine contrast ultrasonography vs computed tomography enteroclysis for assessing ileal Crohn’s disease. World J. Gastroenterol. 2012, 18, 6088–6095. [Google Scholar] [CrossRef][Green Version]
- Zhu, C.; Ma, X.; Xue, L.; Xu, J.; Li, Q.; Wang, Y.; Zhang, J. Small intestine contrast ultrasonography for the detection and assessment of Crohn disease: A meta-analysis. Medicine 2016, 95, e4235. [Google Scholar] [CrossRef] [PubMed]
- Kröner, P.T.; Engels, M.M.; Glicksberg, B.S.; Johnson, K.W.; Mzaik, O.; van Hooft, J.E.; Wallace, M.B.; El-Serag, H.B.; Krittanawong, C. Artificial intelligence in gastroenterology: A state-of-the-art review. World J. Gastroenterol. 2021, 27, 6794–6824. [Google Scholar] [CrossRef] [PubMed]
- Parasa, S.; Wallace, M.; Bagci, U.; Antonino, M.; Berzin, T.; Byrne, M.; Celik, H.; Farahani, K.; Golding, M.; Gross, S.; et al. Proceedings from the First Global Artificial Intelligence in Gastroenterology and Endoscopy Summit. Gastrointest. Endosc. 2020, 92, 938–945.e1. [Google Scholar] [CrossRef] [PubMed]
- Stidham, R.W.; Stidham, R.W.; Enchakalody, B.; Enchakalody, B.; Waljee, A.K.; Waljee, A.K.; Higgins, P.D.R.; Higgins, P.D.R.; Wang, S.C.; Wang, S.C.; et al. Assessing Small Bowel Stricturing and Morphology in Crohn’s Disease Using Semi-automated Image Analysis. Inflamm. Bowel Dis. 2020, 26, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.; Albshesh, A.; Shimon, C.; Segal, B.; Yershov, A.; Kopylov, U.; Meyers, A.; Brzezinski, R.Y.; Ben Horin, S.; Hoffer, O. Automatized Detection of Crohn’s Disease in Intestinal Ultrasound Using Convolutional Neural Network. Inflamm. Bowel Dis. 2023, 29, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Park, H.J.; Song, D.H.; Han, S.-S. Developing an Intelligent Automatic Appendix Extraction Method from Ultrasonography Based on Fuzzy ART and Image Processing. Comput. Math. Methods Med. 2015, 2015, 389057. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Song, D.H.; Park, H.J. Automatic Extraction of Appendix from Ultrasonography with Self-Organizing Map and Shape-Brightness Pattern Learning. Biomed. Res. Int. 2016, 2016, 5206268. [Google Scholar] [CrossRef]
- Kim, K.B.; Park, H.J.; Song, D.H. Semi-dynamic Control of FCM Initialization for Automatic Extraction of Inflamed Appendix from Ultrasonography. Curr. Med. Imaging Rev. 2019, 15, 810–816. [Google Scholar] [CrossRef]
- Ghareeb, W.M.; Emile, S.H.; Elshobaky, A. Artificial Intelligence Compared to Alvarado Scoring System Alone or Combined with Ultrasound Criteria in the Diagnosis of Acute Appendicitis. J. Gastrointest. Surg. 2022, 26, 655–658. [Google Scholar] [CrossRef]
- Rispo, A.; Calabrese, G.; Testa, A.; Imperatore, N.; Patturelli, M.; Allocca, M.; Guarino, A.D.; Cantisani, N.M.; Toro, B.; Castiglione, F. Hocus Pocus: The Role of Hand-held Ultrasonography in Predicting Disease Extension and Endoscopic Activity in Ulcerative Colitis. J. Crohn’s Coli. 2023, 17, 1089–1096. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliamonte, G.; Santagata, F.; Fraquelli, M. Current Developments and Role of Intestinal Ultrasound including the Advent of AI. Diagnostics 2024, 14, 759. https://doi.org/10.3390/diagnostics14070759
Tagliamonte G, Santagata F, Fraquelli M. Current Developments and Role of Intestinal Ultrasound including the Advent of AI. Diagnostics. 2024; 14(7):759. https://doi.org/10.3390/diagnostics14070759
Chicago/Turabian StyleTagliamonte, Gennaro, Fabrizio Santagata, and Mirella Fraquelli. 2024. "Current Developments and Role of Intestinal Ultrasound including the Advent of AI" Diagnostics 14, no. 7: 759. https://doi.org/10.3390/diagnostics14070759
APA StyleTagliamonte, G., Santagata, F., & Fraquelli, M. (2024). Current Developments and Role of Intestinal Ultrasound including the Advent of AI. Diagnostics, 14(7), 759. https://doi.org/10.3390/diagnostics14070759





