Relationship between Prognostic Nutritional Index and Amputation in Patients with Diabetic Foot Ulcer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Biochemical Analysis
2.3. Clinical Follow-Up
2.4. Statistical Methods
2.5. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Moulik, P.K.; Mtonga, R.; Gill, G.V. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003, 26, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Newton, K.; Blough, D.; Mcculloch, D.K.; Sandhu, N.; Reiber, G.E.; Wagner, E.H. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999, 22, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.S.; Eren, M.A.; Uyar, N.; Kankılıç, N.; Karaaslan, H.; Sabuncu, T.; Çelik, H. Relationship between systemic immune inflammation index and amputation in patients with diabetic foot ulcer. J. Orthop. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Reiber, G.E.; Pecoraro, R.E.; Koepsell, T.D. Risk factors for amputation in patients with diabetes mellitus: A case-control study. Ann. Intern. Med. 1992, 117, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z. Higher systemic immune-inflammation index is associated with higher likelihood of peripheral arterial disease. Ann. Vasc. Surg. 2022, 84, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Demirdal, T.; Sen, P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res. Clin. Pract. 2018, 144, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.; Aguş, H.Z.; Kalkan, A.K.; Uzun, F.; Ertürk, M.; Kalkan, M.E.; Yıldız, M. Prognostic nutritional index predicts mortality in infective endocarditis. Türk Kardiyol. Derneği Arşivi 2020, 48, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, A.N.; Ahrén, B. Relation between malnutrition and development of diabetes mellitus. Int. J. Pancreatol. 1999, 26, 125–130. [Google Scholar] [CrossRef]
- Suliman, M.E.; Stenvinkel, P.; Bárány, P.; Heimbürger, O.; Anderstam, B.; Lindholm, B. Hyperhomocysteinemia and its relationship to cardiovascular disease in ESRD: Influence of hypoalbuminemia, malnutrition, inflammation, and diabetes mellitus. Am. J. Kidney Dis. 2003, 41, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Keskinler, M.V.; Feyİzoglu, G.; Yildiz, K.; Oguz, A. The frequency of malnutrition in patients with type 2 diabetes. Medeni. Med. J. 2021, 36, 117. [Google Scholar]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Xu, S.; Wang, Y.; Hu, Z.; Ma, L.; Zhang, F.; Liu, P. Effects of neutrophil-to-lymphocyte ratio, serum calcium, and serum albumin on prognosis in patients with diabetic foot. Int. Wound J. 2023, 20, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.; Ribeiro, J.; Ribeiro, I.; Dinis-Ribeiro, M. Risk stratification systems for diabetic foot ulcers: A systematic review. Diabetologia 2011, 54, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Margarson, M.; Soni, N. Serum albumin: Touchstone or totem? Anaesthesia 1998, 53, 789–803. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Doehner, W.; Anker, S.D. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc. Res. 2007, 73, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Sanz París, A.; García, J.M.; Gómez-Candela, C.; Burgos, R.; Martín, Á.; Matía, P. Prevalencia de desnutrición en ancianos hospitalizados con diabetes mellitus. Nutr. Hosp. 2013, 28, 592–599. [Google Scholar]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system: An introduction. Am. J. Clin. Nutr. 1997, 66, 460S–463S. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Toft, A.D. Effects of exercise on lymphocytes and cytokines. Br. J. Sports Med. 2000, 34, 246–251. [Google Scholar] [CrossRef]
- Ünver Ulusoy, T.; Hekimoğlu, C.H.; Kayhan, S.; Altın, N.; Şencan, İ. Prognostic nutritional index: Is it associated with the prognosis of Crimean Congo hemorrhagic fever. J. Med. Virol. 2022, 94, 4910–4917. [Google Scholar] [CrossRef] [PubMed]
- Kozan, F.B.; Ertuğrul, M.B.; Başak, O.; Utlu, Y. The strongest predictor of major amputation in diabetic foot ulcers: Peripheral arterial disease; frequency and related factors. J. Turk. Fam. Physician 2020, 11, 2–8. [Google Scholar]
- Prompers, L.; Huijberts, M.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; Mauricio, D. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007, 50, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, R.; Brownrigg, J.; Hinchliffe, R. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes. Metab. 2015, 17, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Huijberts, M.S.; Schaper, N.C.; Schalkwijk, C.G. Advanced glycation end products and diabetic foot disease. Diabetes/Metab. Res. Rev. 2008, 24, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Gariani, K.; Pataky, Z.; Lipsky, B.A. Diabetic foot infections: State-of-the-art. Diabetes Obes. Metab. 2014, 16, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A.; Board, I.E. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3266. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Sánchez, J.; Víquez-Molina, G.; López-Valverde, M.E.; Rojas-Bonilla, J.M. Systemic Immune-Inflammation Index in Diabetic Foot Infections and Osteomyelitis. Int. J. Low. Extrem. Wounds 2023. [Google Scholar] [CrossRef] [PubMed]
- Tak, B.T.; Cay, S.; Pamukcu, H.E.; Ekizler, F.A.; Kafes, H.; Cetin, E.H.O.; Ulvan, N.; Ozeke, O.; Ozcan, F.; Topaloglu, S. Prognostic nutritional index as a novel marker for prediction of prognosis in patients with peripartum cardiomyopathy. Medicine 2020, 99, e19524. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, K.; Xu, Z.; Hu, Y.; Liu, Y.; Liu, W.; Hu, X.; Ye, T.; Hong, J.; Zhu, H. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict mortality in patients with diabetic foot ulcers undergoing amputations. Diabetes Metab. Syndr. Obes. 2021, 14, 821–829. [Google Scholar] [CrossRef]
| Non-Amputees (n = 276) | Amputees (n = 110) | p Values | ||
|---|---|---|---|---|
| Age, mean ± standard deviation (SD) | 60.36 ± 14.26 | 57.77 ± 13.08 | 0.101 c | |
| Gender | Male | 177 (64.1%) | 64 (58.2%) | 0.276 a |
| Female | 99 (35.9%) | 46 (41.8%) | ||
| Smoking | No | 239 (86.6%) | 99 (90%) | 0.360 a |
| Yes | 37 (13.4%) | 11 (10%) | ||
| At least one comorbidity | No | 107 (38.8%) | 41 (37.3%) | 0.785 a |
| Yes | 169 (61.2%) | 69 (62.7%) | ||
| Rheumatological disease | No | 271 (98.2%) | 104 (94.5%) | 0.083 b |
| Yes | 5 (1.8%) | 6 (5.5%) | ||
| Peripheral neuropathy | No | 260 (94.2%) | 105 (95.5%) | 0.625 a |
| Yes | 16 (5.8%) | 5 (4.5%) | ||
| Venous insufficiency | No | 274 (99.3%) | 106 (96.4%) | 0.058 b |
| Yes | 2 (0.7%) | 4 (3.6%) | ||
| CAD | No | 211 (76.4%) | 87 (79.1%) | 0.577 a |
| Yes | 65 (23.6%) | 23 (20.9%) | ||
| CHF | No | 257 (93.1%) | 101 (91.8%) | 0.657 a |
| Yes | 19 (6.9%) | 9 (8.2%) | ||
| CRD | No | 256 (92.8%) | 101 (91.8%) | 0.753 a |
| Yes | 20 (7.2%) | 9 (8.2%) | ||
| Arterial thrombosis | No | 235 (85.1%) | 84 (76.4%) | 0.040 a |
| Yes | 41 (14.9%) | 26 (23.6%) | ||
| Venous thrombosis | No | 268 (97.1%) | 100 (90.9%) | 0.009 a |
| Yes | 8 (2.9%) | 10 (9.1%) | ||
| Osteomyelitis | No | 190 (68.8%) | 65 (59.1%) | 0.068 a |
| Yes | 86 (31.2%) | 45 (40.9%) |
| Non-Amputees (n = 276) | Amputees (n = 110) | p Values | |
|---|---|---|---|
| WBC (103/mm3) | 8.72 (7.21–11.18) | 10.78 (7.63–13.27) | 0.002 b |
| Neutrophil (103/mm3) | 5.49 (4.27–7.73) | 6.66 (4.99–9.41) | 0.003 b |
| Lymphocyte (103/mm3) | 1.71 (1.23–2.12) | 1.68 (1.28–2.46) | 0.536 b |
| PLT (103/mm3) | 300 (218–393) | 328 (240–423) | 0.135 b |
| NLR | 3.1 (2.27–4.57) | 3.22 (2.42–5.86) | 0.349 b |
| PLR | 17.51 (12.55–23.54) | 17.81 (12.45–27.12) | 0.867 b |
| Albumin (g/L) | 43 (41–44) | 36 (35–38) | <0.001 b |
| CRP (mg/L) | 19.5 (10–65) | 30 (8.75–114) | 0.035 b |
| Glucose (mg/dL) | 133.5 (95–195) | 152 (103–238.5) | 0.021 b |
| HgbA1c | 7 (6–8.2) | 7.4 (6.4–9) | 0.015 b |
| Urea (mg/dL) | 42 (32–54) | 44 (32–55.25) | 0.257 b |
| Creatinine (mg/dL) | 0.9 (0.75–1.1) | 0.9 (0.78–1.3) | 0.300 b |
| ESR (mm/h) | 44.63 ± 28.34 | 50.67 ± 30.48 | 0.065 a |
| SII | 871 (574–1569) | 1039 (607–1878) | 0.184 b |
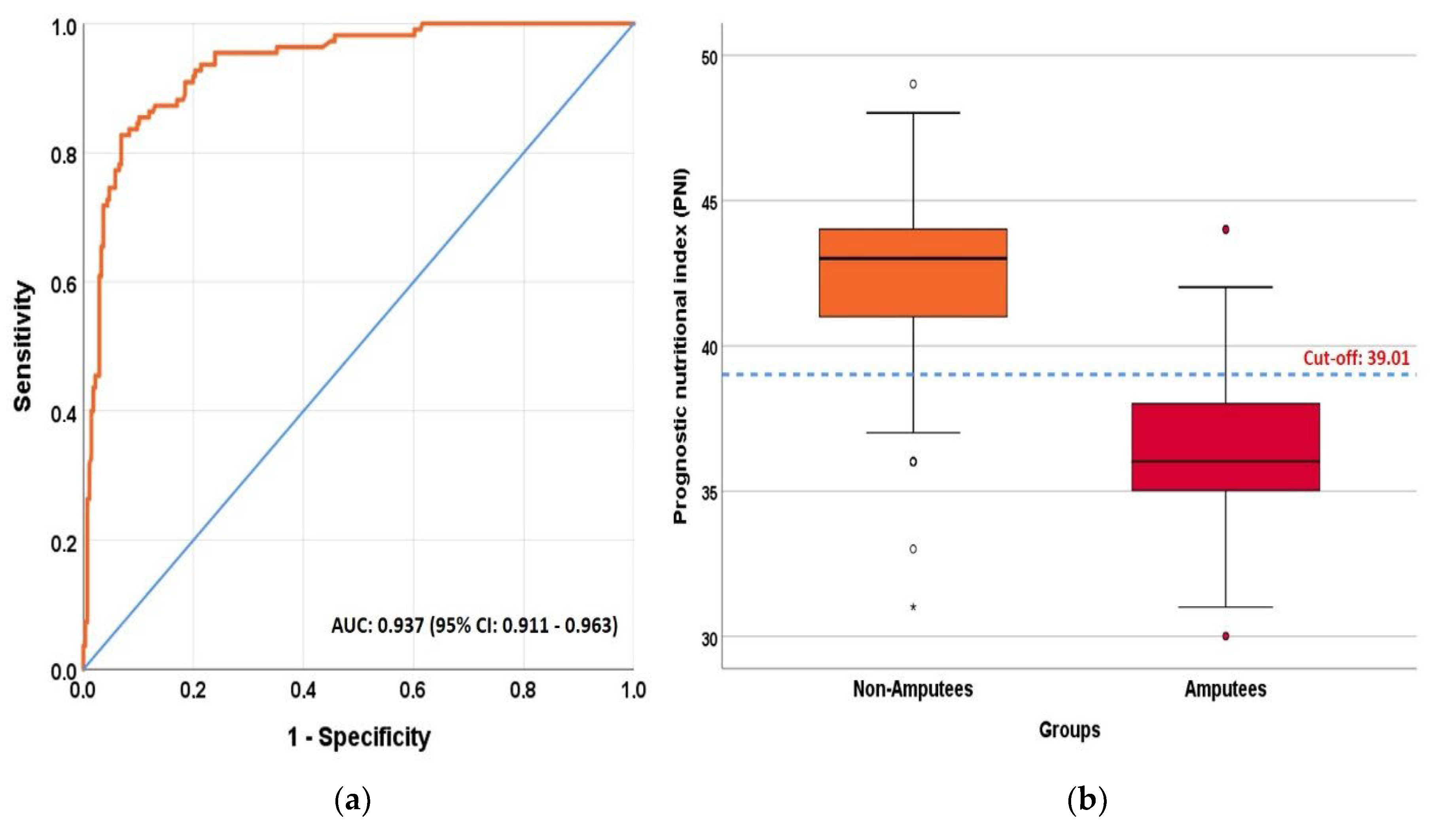
| PNI | 43.01 (41–44.02) | 36.01 (35.01–38.01) | <0.001 b |
| Prognostic Nutritional Index (PNI) | Albumin | |
|---|---|---|
| AUC (95% CI) | 0.937 (0.911–0.963) | 0.938 (0.912–0.964) |
| p values | <0.001 | <0.001 |
| Cut off | ≤39.005 | ≤38.5 |
| Sensitivity (95% CI) | 82.7% (74.1–89) | 81.8% (73.1–88.3) |
| Specificity (95% CI) | 93.1% (89.3–95.7) | 93.1% (89.3–95.7) |
| PPV (95% CI) | 82.7% (74.1–89) | 82.6% (73.9–88.9) |
| NPV (95% CI) | 93.1% (89.3–95.7) | 92.8% (88.9–95.4) |
| LR+ (95% CI) | 12 (7.7–18.7) | 11.9 (7.6–18.5) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p Values | OR (CI 95%) | p Values | OR (CI 95%) | |
| Venous thrombosis (Yes vs. no) | 0.013 | 3.35 (1.28–8.72) | ns | - |
| WBC | 0.006 | 1.00 (1.00–1.00) | ns | - |
| Neutrophil | 0.036 | 1.00 (1.00–1.00) | ns | - |
| CRP | 0.006 | 1.01 (1.00–1.01) | ns | - |
| Arterial thrombosis (Yes vs. no) | 0.041 | 1.77 (1.02–3.08) | 0.014 | 3.04 (1.25–7.37) |
| HbA1c | 0.006 | 1.17 (1.04–1.31) | 0.022 | 1.24 (1.03–1.48) |
| PNI (≤39 vs. >39) | <0.001 | 64.7 (32.8–127.7) | <0.001 | 81.8 (38.5–173.7) |
| Multivariate model: Nagelkerke R Square = 0.653, Classification accuracy: 90.2% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coşkun, B.; Ayhan, M.; Ulusoy, S. Relationship between Prognostic Nutritional Index and Amputation in Patients with Diabetic Foot Ulcer. Diagnostics 2024, 14, 738. https://doi.org/10.3390/diagnostics14070738
Coşkun B, Ayhan M, Ulusoy S. Relationship between Prognostic Nutritional Index and Amputation in Patients with Diabetic Foot Ulcer. Diagnostics. 2024; 14(7):738. https://doi.org/10.3390/diagnostics14070738
Chicago/Turabian StyleCoşkun, Belgin, Müge Ayhan, and Serap Ulusoy. 2024. "Relationship between Prognostic Nutritional Index and Amputation in Patients with Diabetic Foot Ulcer" Diagnostics 14, no. 7: 738. https://doi.org/10.3390/diagnostics14070738
APA StyleCoşkun, B., Ayhan, M., & Ulusoy, S. (2024). Relationship between Prognostic Nutritional Index and Amputation in Patients with Diabetic Foot Ulcer. Diagnostics, 14(7), 738. https://doi.org/10.3390/diagnostics14070738






