Whole Blood as a Sample Matrix in Homogeneous Time-Resolved Assay—Förster Resonance Energy Transfer-Based Antibody Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum and Whole Blood Samples: Collection and Handling
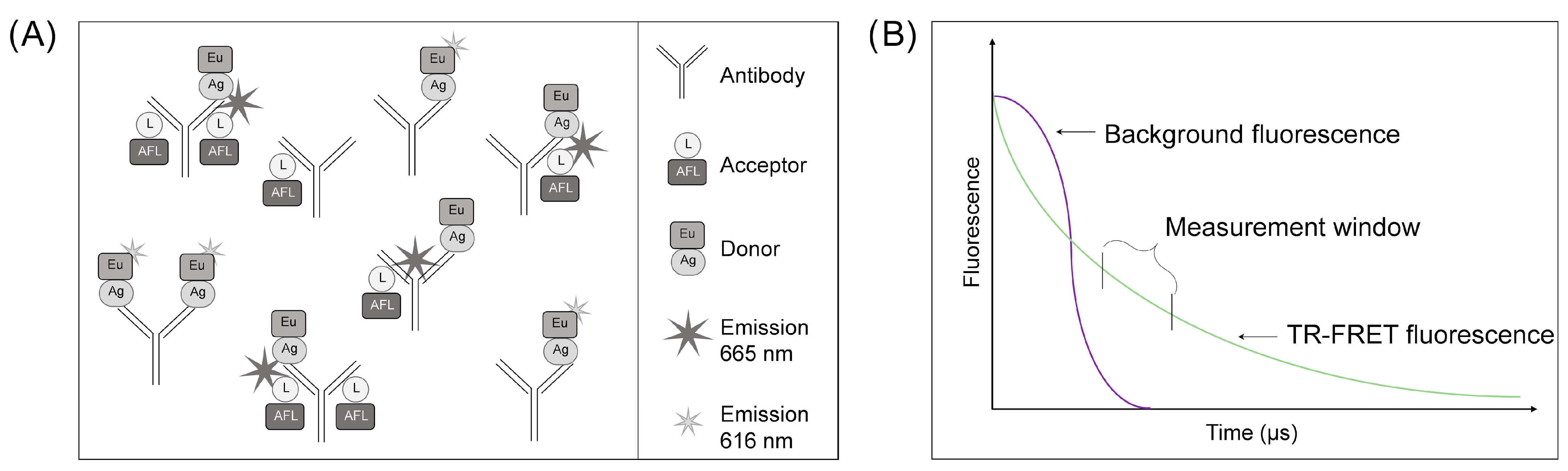
2.2. LFRET Assay Optimization and Set-Up
2.3. Statistical Analyses
3. Results
3.1. LFRET Assay Protocol Optimization for WB Samples
3.2. Optimization of the Incubation Time and Normalization Method for the WB LFRET Assay
3.3. Serum and WB Produce Similar LFRET Signal Levels
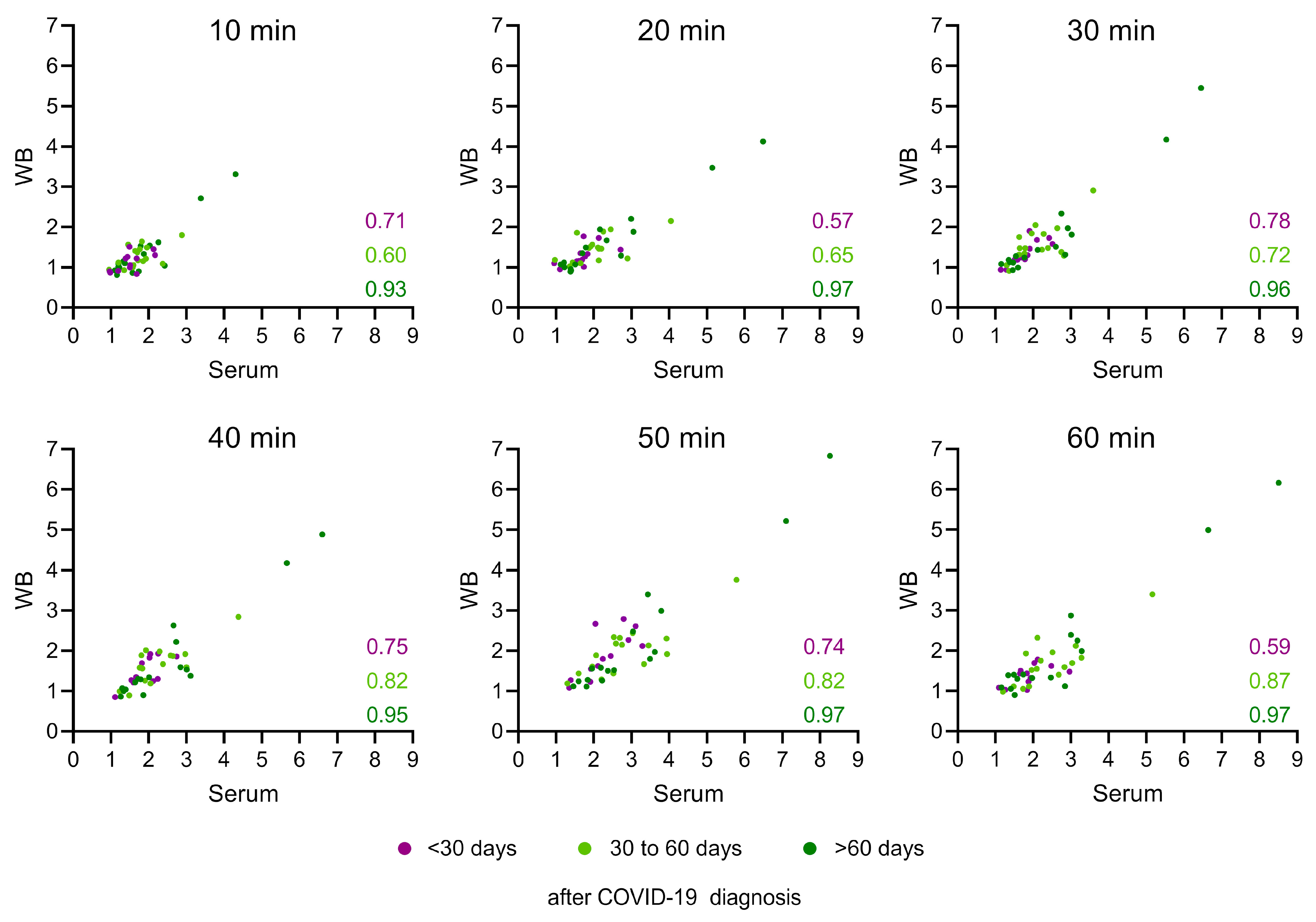
3.4. LFRET Results Show a Strong Correlation with Neutralizing Antibodies but Correlate Only Weakly with Anti-Spike IgG ELISA Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepojoki, S.; Nurmi, V.; Vaheri, A.; Hedman, K.; Vapalahti, O.; Hepojoki, J. A protein L-based immunodiagnostic approach utilizing time-resolved Forster resonance energy transfer. PLoS ONE 2014, 9, e106432. [Google Scholar] [CrossRef]
- Saraheimo, S.; Hepojoki, J.; Nurmi, V.; Lahtinen, A.; Hemmila, I.; Vaheri, A.; Vapalahti, O.; Hedman, K. Time-resolved FRET -based approach for antibody detection—A new serodiagnostic concept. PLoS ONE 2013, 8, e62739. [Google Scholar] [CrossRef]
- Kareinen, L.; Hepojoki, S.; Huhtamo, E.; Korhonen, E.M.; Schmidt-Chanasit, J.; Hedman, K.; Hepojoki, J.; Vapalahti, O. Immunoassay for serodiagnosis of Zika virus infection based on time-resolved Forster resonance energy transfer. PLoS ONE 2019, 14, e0219474. [Google Scholar] [CrossRef]
- Rusanen, J.; Kareinen, L.; Levanov, L.; Mero, S.; Pakkanen, S.H.; Kantele, A.; Amanat, F.; Krammer, F.; Hedman, K.; Vapalahti, O.; et al. A 10-Minute “Mix and Read” Antibody Assay for SARS-CoV-2. Viruses 2021, 13, 143. [Google Scholar] [CrossRef]
- Hepojoki, S.; Rusanen, J.; Hepojoki, J.; Nurmi, V.; Vaheri, A.; Lundkvist, A.; Hedman, K.; Vapalahti, O. Competitive Homogeneous Immunoassay for Rapid Serodiagnosis of Hantavirus Disease. J. Clin. Microbiol. 2015, 53, 2292–2297. [Google Scholar] [CrossRef][Green Version]
- Rusanen, J.; Toivonen, A.; Hepojoki, J.; Hepojoki, S.; Arikoski, P.; Heikkinen, M.; Vaarala, O.; Ilonen, J.; Hedman, K. LFRET, a novel rapid assay for anti-tissue transglutaminase antibody detection. PLoS ONE 2019, 14, e0225851. [Google Scholar] [CrossRef]
- Åkerstrom, B.; Bjorck, L. Protein L: An immunoglobulin light chain-binding bacterial protein. Characterization of binding and physicochemical properties. J. Biol. Chem. 1989, 264, 19740–19746. [Google Scholar] [CrossRef]
- Hepojoki, S.; Hepojoki, J.; Hedman, K.; Vapalahti, O.; Vaheri, A. Rapid homogeneous immunoassay based on time-resolved Forster resonance energy transfer for serodiagnosis of acute hantavirus infection. J. Clin. Microbiol. 2015, 53, 636–640. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef]
- WHO. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody. Available online: https://www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed on 24 October 2023).
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Pietiäinen, V.; Polso, M.; Migh, E.; Guckelsberger, C.; Harmati, M.; Diosdi, A.; Turunen, L.; Hassinen, A.; Potdar, S.; Koponen, A.; et al. Image-based and machine learning-guided multiplexed serology test for SARS-CoV-2. Cell Rep. Methods 2023, 3, 100565. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Cheng, M.P.; Papenburg, J.; Desjardins, M.; Kanjilal, S.; Quach, C.; Libman, M.; Dittrich, S.; Yansouni, C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 172, 726–734. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef]
- Rose, R.; Neumann, F.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022, 20, 31. [Google Scholar] [CrossRef]
- Bewley, K.R.; Coombes, N.S.; Gagnon, L.; McInroy, L.; Baker, N.; Shaik, I.; St-Jean, J.R.; St-Amant, N.; Buttigieg, K.R.; Humphries, H.E.; et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat. Protoc. 2021, 16, 3114–3140. [Google Scholar] [CrossRef]
- Samper, I.C.; Sánchez-Cano, A.; Khamcharoen, W.; Jang, I.; Siangproh, W.; Baldrich, E.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Electrochemical Capillary-Flow Immunoassay for Detecting Anti-SARS-CoV-2 Nucleocapsid Protein Antibodies at the Point of Care. ACS Sens. 2021, 6, 4067–4075. [Google Scholar] [CrossRef]
- Martinez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Resendiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Bellamkonda, N.; Lambe, U.P.; Sawant, S.; Nandi, S.S.; Chakraborty, C.; Shukla, D. Immune Response to SARS-CoV-2 Vaccines. Biomedicines 2022, 10, 1464. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Zhang, F.; Jahan, S.; Yuan, B.; Saleh, M.S.; Gao, S.J.; Panat, R. N protein-based ultrasensitive SARS-CoV-2 antibody detection in seconds via 3D nanoprinted, microarchitected array electrodes. J. Med. Virol. 2022, 94, 2067–2078. [Google Scholar] [CrossRef]
- Song, W.; Fang, Z.; Ma, F.; Li, J.; Huang, Z.; Zhang, Y.; Li, J.; Chen, K. The role of SARS-CoV-2 N protein in diagnosis and vaccination in the context of emerging variants: Present status and prospects. Front. Microbiol. 2023, 14, 1217567. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Ekström, N.; Haveri, A.; Solastie, A.; Virta, C.; Österlund, P.; Nohynek, H.; Nieminen, T.; Ivaska, L.; Tähtinen, P.A.; Lempainen, J.; et al. Strong Neutralizing Antibody Responses to SARS-CoV-2 Variants Following a Single Vaccine Dose in Subjects with Previous SARS-CoV-2 Infection. Open Forum Infect. Dis. 2022, 9, ofac625. [Google Scholar] [CrossRef]
- Kolehmainen, P.; Huttunen, M.; Iakubovskaia, A.; Maljanen, S.; Tauriainen, S.; Yatkin, E.; Pasternack, A.; Naves, R.; Toivonen, L.; Tähtinen, P.A.; et al. Coronavirus spike protein-specific antibodies indicate frequent infections and reinfections in infancy and among BNT162b2-vaccinated healthcare workers. Sci. Rep. 2023, 13, 8416. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Quattrocchi, F.; Karnes, H.T.; Robinson, J.D.; Hendeles, L. Effect of serum separator blood collection tubes on drug concentrations. Ther. Drug Monit. 1983, 5, 359–362. [Google Scholar] [CrossRef]
- Jokiranta, S.T.; Miettinen, S.; Salonen, S.; Kareinen, L.; Uusitalo, R.; Korhonen, E.M.; Virtanen, J.; Kivistö, I.; Aaltonen, K.; Mosselhy, D.A.; et al. Stable levels of antibodies against unrelated toxoid vaccines after COVID-19. Pathog. Immun. 2023, 8, 74. [Google Scholar] [CrossRef]
- Klingler, J.; Weiss, S.; Itri, V.; Liu, X.; Oguntuyo, K.Y.; Stevens, C.; Ikegame, S.; Hung, C.T.; Enyindah-Asonye, G.; Amanat, F.; et al. Role of Immunoglobulin M and A Antibodies in the Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2. J. Infect. Dis. 2021, 223, 957–970. [Google Scholar] [CrossRef]
- Noval, M.G.; Kaczmarek, M.E.; Koide, A.; Rodriguez-Rodriguez, B.A.; Louie, P.; Tada, T.; Hattori, T.; Panchenko, T.; Romero, L.A.; Teng, K.W.; et al. Antibody isotype diversity against SARS-CoV-2 is associated with differential serum neutralization capacities. Sci. Rep. 2021, 11, 5538. [Google Scholar] [CrossRef]
- Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Viant, C.; Gaebler, C.; Cipolla, M.; Hoffmann, H.-H.; Oliveira, T.Y.; Oren, D.A.; et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021, 13, eabf1555. [Google Scholar] [CrossRef]
- Underwood, A.P.; Solund, C.; Fernandez-Antunez, C.; Villadsen, S.L.; Winckelmann, A.A.; Bollerup, S.; Mikkelsen, L.S.; Sorensen, A.L.; Feng, S.; Fahnoe, U.; et al. Neutralisation titres against SARS-CoV-2 are sustained 6 months after onset of symptoms in individuals with mild COVID-19. EBioMedicine 2021, 71, 103519. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.H.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef]
- Yadav, A.K.; Ghosh, S.; Kotwal, A.; Kaushik, S.K.; Bobdey, S.; Sahu, R.; Kapoor, S.; Faujdar, D.S.; Teli, P.T.; Anand, V. Seroconversion among COVID-19 patients admitted in a dedicated COVID hospital: A longitudinal prospective study of 1000 patients. Med. J. Armed Forces India 2021, 77, S379–S384. [Google Scholar] [CrossRef]
- Toh, Z.Q.; Anderson, J.; Mazarakis, N.; Neeland, M.; Higgins, R.A.; Rautenbacher, K.; Dohle, K.; Nguyen, J.; Overmars, I.; Donato, C.; et al. Comparison of Seroconversion in Children and Adults with Mild COVID-19. JAMA Netw. Open 2022, 5, e221313. [Google Scholar] [CrossRef]
- Zhou, F.; Vahokoski, J.; Bergen, C.-R.G.; Langeland, N.; Cox, R.J. Impact of ageing on homologous and human-coronavirus-reactive antibodies after SARS-CoV-2 vaccination or infection. NPJ Vaccines 2024, 9, 37. [Google Scholar] [CrossRef]
- Howard, W.A.; Gibson, K.L.; Dunn-Walters, D.K. Antibody quality in old age. Rejuven. Res. 2006, 9, 117–125. [Google Scholar] [CrossRef]


| Dilution of WB | 1st Thaw | 2nd Thaw | |
|---|---|---|---|
| 20 min | 1:300 | 1.2 | 1.7 |
| 1:400 | 1.2 | 1.1 | |
| 1:600 | 1.1 | 1.1 | |
| 60 min | 1:300 | 2.3 | 2.3 |
| 1:400 | 1.7 | 2.0 | |
| 1:600 | 1.6 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lintala, A.; Vapalahti, O.; Nousiainen, A.; Kantele, A.; Hepojoki, J. Whole Blood as a Sample Matrix in Homogeneous Time-Resolved Assay—Förster Resonance Energy Transfer-Based Antibody Detection. Diagnostics 2024, 14, 720. https://doi.org/10.3390/diagnostics14070720
Lintala A, Vapalahti O, Nousiainen A, Kantele A, Hepojoki J. Whole Blood as a Sample Matrix in Homogeneous Time-Resolved Assay—Förster Resonance Energy Transfer-Based Antibody Detection. Diagnostics. 2024; 14(7):720. https://doi.org/10.3390/diagnostics14070720
Chicago/Turabian StyleLintala, Annika, Olli Vapalahti, Arttu Nousiainen, Anu Kantele, and Jussi Hepojoki. 2024. "Whole Blood as a Sample Matrix in Homogeneous Time-Resolved Assay—Förster Resonance Energy Transfer-Based Antibody Detection" Diagnostics 14, no. 7: 720. https://doi.org/10.3390/diagnostics14070720
APA StyleLintala, A., Vapalahti, O., Nousiainen, A., Kantele, A., & Hepojoki, J. (2024). Whole Blood as a Sample Matrix in Homogeneous Time-Resolved Assay—Förster Resonance Energy Transfer-Based Antibody Detection. Diagnostics, 14(7), 720. https://doi.org/10.3390/diagnostics14070720





