Low Rate of Postoperative Pterygium Recurrence in Patients under Treatment with Low-Dose Oral Doxycycline for Chronic Blepharitis: A First Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
Objectives of This Study and Outcome Measures
2.2. Procedures
2.3. Statistical Analysis
3. Results
3.1. Tetra Group: Descriptive Analysis
3.1.1. Primary Pterygium
3.1.2. Recurrent Pterygium
3.2. Control Group: Descriptive Analysis
3.2.1. Primary Pterygium
3.2.2. Recurrent Pterygium
3.3. Comparison between Groups
3.3.1. Primary Pterygium
3.3.2. Recurrent Pterygium
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.J.; Lee, J.; Saw, S.-M.; Gazzard, G.; Koh, D.; Widjaja, D.; Tan, D.T.H. Prevalence and risk factors associated with dry eye symptoms: A population based study in Indonesia. Br. J. Ophthalmol. 2002, 86, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Ariyasu, R.G.; Kaza, V.; LaBree, L.D.; McDonnell, P.J. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am. J. Ophthalmol. 1995, 120, 151–160. [Google Scholar] [CrossRef]
- Ehrlich, D. The Management of Pterygium. Ophthalmic Surg. Lasers Imaging Retin. 1977, 8, 23–30. [Google Scholar] [CrossRef]
- Yu, C.; Liang, W.; Huang, Y.; Guan, W. Comparison of clinical efficacy of three surgical methods in the treatment of pterygium. Eye Sci. 2011, 26, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Oh, J.-H.; Do, J.R.; Chuck, R.S.; Park, C.Y. A Comparison of Anchored Conjunctival Rotation Flap and Conjunctival Autograft Techniques in Pterygium Surgery. Cornea 2013, 32, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.R.; Wagoner, M.D.; Hettinger, M.E. Conjunctival Autograft Transplantation for Advanced and Recurrent Pterygium. Ophthalmology 1985, 92, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Shinozaki, N.; Tsubota, K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br. J. Ophthalmol. 1998, 82, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Poirier, R.H.; Fish, J.R. Lamellar Keratoplasty for Recurrent Pterygium. Ophthalmic Surg. Lasers Imaging Retin. 1976, 7, 38–41. [Google Scholar] [CrossRef]
- Frucht-Pery, J.; Ilsar, M. The Use of Low-Dose Mitomycin C for Prevention of Recurrent Pterygium. Ophthalmology 1994, 101, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, C.; Taner, P.; Ergin, A. 5-Fluorouracil as Chemoadjuvant for Primary Pterygium Surgery: Preliminary Report. Cornea 2003, 22, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Razeghinejad, M.R.; Hosseini, H.; Ahmadi, F.; Rahat, F.; Eghbal, H. Preliminary Results of Subconjunctival Bevacizumab in Primary Pterygium Excision. Ophthalmic Res. 2010, 43, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.M.; Elbendary, A.M.; Elwan, M.M. Intraoperative Subconjunctival Bevacizumab as an Adjunctive Treatment in Primary Pterygium: A Preliminary Report. Ophthalmic Surg. Lasers Imaging Retin. 2012, 43, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kasetsuwan, N.; Reinprayoon, U.; Satitpitakul, V. Prevention of Recurrent Pterygium with Topical Bevacizumab 0.05% Eye Drops: A Randomized Controlled Trial. Clin. Ther. 2015, 37, 2347–2351. [Google Scholar] [CrossRef]
- Leippi, S.; Grehn, F.; Geerling, G. Antiangiogenic therapy for pterygium recurrence. Ophthalmologe 2009, 106, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.C.; Rocha, E.M.; Arruda, G.V. Comparison among adjuvant treatments for primary pterygium: A network meta-analysis. Br. J. Ophthalmol. 2018, 102, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Cai, J.; Jhanji, V.; Chen, H. Comparison of Pterygium Recurrence Rates After Limbal Conjunctival Autograft Transplantation and Other Techniques: Meta-analysis. Cornea 2012, 31, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.C.; Jacobs, D.S.; Lee, W.B.; Deng, S.X.; Rosenblatt, M.I.; Shtein, R.M. Options and adjuvants in surgery for pterygium: A report by the American Academy of Ophthalmology. Ophthalmology 2013, 120, 201–208. [Google Scholar] [CrossRef]
- Lam, D.S.; Wong, A.K.; Fan, D.S.; Chew, S.; Kwok, P.S.; Tso, M.O. Intraoperative mitomycin C to prevent recurrence of pterygium after excision: A 30-month follow-up study. Ophthalmology 1998, 105, 901–904; discussion 904–905. [Google Scholar] [CrossRef]
- Clearfield, E.; Hawkins, B.S.; Kuo, I.C. Conjunctival Autograft Versus Amniotic Membrane Transplantation for Treatment of Pterygium: Findings From a Cochrane Systematic Review. Am. J. Ophthalmol. 2017, 182, 8–17. [Google Scholar] [CrossRef]
- Macarie, S.S.; Macarie, D.M. Conjunctival autograft in pterygium treatment. Rom. J. Ophthalmol. 2016, 60, 170–173. [Google Scholar] [PubMed]
- Röck, T.; Bramkamp, M.; Bartz-Schmidt, K.U.; Röck, D. A Retrospective Study to Compare the Recurrence Rate After Treatment of Pterygium by Conjunctival Autograft, Primary Closure, and Amniotic Membrane Transplantation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7976–7981. [Google Scholar] [CrossRef] [PubMed]
- Dursun, D.; Kim, M.C.; Solomon, A.; Pflugfelder, S.C. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am. J. Ophthalmol. 2001, 132, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Luo, L.; Pflugfelder, S.C.; Li, D.-Q. Doxycycline Inhibits TGF-β1–Induced MMP-9 via Smad and MAPK Pathways in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 840–848. [Google Scholar] [CrossRef] [PubMed]
- García-López, C.; Rodríguez-Calvo-de-Mora, M.; Borroni, D.; Sánchez-González, J.-M.; Romano, V.; Rocha-de-Lossada, C. The role of matrix metalloproteinases in infectious corneal ulcers. Surv. Ophthalmol. 2023, 68, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Arun, M.Z.; Guzeloglu, M.; Onursal, C.; Gokce, G.; Korkmaz, C.G.; Reel, B. Low-dose doxycycline inhibits hydrogen peroxide-induced oxidative stress, MMP-2 up-regulation and contractile dysfunction in human saphenous vein grafts. Drug Des. Devel. Ther. 2019, 13, 1791–1801. [Google Scholar] [CrossRef]
- Palomino-Morales, R.; Torres, C.; Perales, S.; Linares, A.; Alejandre, M.J. Inhibition of extracellular matrix production and remodeling by doxycycline in smooth muscle cells. J. Pharmacol. Sci. 2016, 132, 218–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, V.A.; Cook, S.D. Doxycycline-a role in ocular surface repair. Br. J. Ophthalmol. 2004, 88, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-M.; Park, J.-H.; Kang, B.; Lee, S.-A.; Park, I.-H.; Lee, H.-M. Effect of doxycycline on transforming growth factor-beta-1-induced matrix metalloproteinase 2 expression, migration, and collagen contraction in nasal polyp-derived fibroblasts. Am. J. Rhinol. Allergy 2016, 30, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, Z.; Li, Y.; Lin, M.; Yao, L.; Liu, Y.; He, Z.; Wu, C.; Liang, D. Doxycycline enhances the inhibitory effects of bevacizumab on corneal neovascularization and prevents its side effects. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9108–9115. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Lokeshwar, B.L.; Solomon, A.; Monroy, D.; Ji, Z.; Pflugfelder, S.C. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye Res. 2001, 73, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-E.; Lee, D.-C.; Chang, M.-H. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J. Ophthalmol. 2005, 19, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Iovieno, A.; Lambiase, A.; Micera, A.; Stampachiacchiere, B.; Sgrulletta, R.; Bonini, S. In Vivo Characterization of Doxycycline Effects on Tear Metalloproteinases in Patients with Chronic Blepharitis. Eur. J. Ophthalmol. 2009, 19, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, B.; Doycheva, D.; Deuter, C.; Pfeffer, I.; Schaller, M.; Zierhut, M. Treatment of Ocular Rosacea with Once-Daily Low-Dose Doxycycline. Cornea 2014, 33, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Nemet, A.Y.; Vinker, S.; Kaiserman, I. Associated Morbidity of Blepharitis. Ophthalmology 2011, 118, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Blepharitis PPP-2018. American Academy of Ophthalmology. Published 13 November 2018. Available online: https://www.aao.org/preferred-practice-pattern/blepharitis-ppp-2018 (accessed on 3 February 2022).
- Ben Ephraim Noyman, D.; Chan, C.C.; Mimouni, M.; Safir, M. Systemic antibiotic treatment for meibomian gland dysfunction—A systematic review and meta-analysis. Acta Ophthalmol. 2024, 102, e1–e10. [Google Scholar] [CrossRef]
- Onghanseng, N.; Ng, S.M.; Halim, M.S.; Nguyen, Q.D. Oral antibiotics for chronic blepharitis. Cochrane Database Syst. Rev. 2021, 2021, CD013697. [Google Scholar] [CrossRef]
- Oguz, H.; Kilitcioglu, A.; Yasar, M. Limbal Conjunctival Mini-Autografting for Preventing Recurrence after Pterygium Surgery. Eur. J. Ophthalmol. 2006, 16, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Cavas-Martínez, F.; De la Cruz Sánchez, E.; Nieto Martínez, J.; Fernández Cañavate, F.J.; Fernández-Pacheco, D.G. Corneal topography in keratoconus: State of the art. Eye Vis. 2016, 3, 5. [Google Scholar] [CrossRef]
- Calossi, A. Corneal asphericity and spherical aberration. J. Refract. Surg. 2007, 23, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Doctor, K.; Vunnava, K.P.; Shroff, R.; Kaweri, L.; Lalgudi, V.G.; Gupta, K.; Kundu, G. Simplifying and understanding various topographic indices for keratoconus using Scheimpflug based topographers. Indian J. Ophthalmol. 2020, 68, 2732–2743. [Google Scholar] [CrossRef] [PubMed]
- Fuest, M.; Mehta, J.S.; Coroneo, M.T. New treatment options for pterygium. Expert Rev. Ophthalmol. 2017, 12, 193–196. [Google Scholar] [CrossRef][Green Version]
- Rezvan, F.; Khabazkhoob, M.; Hooshmand, E.; Yekta, A.; Saatchi, M.; Hashemi, H. Prevalence and risk factors of pterygium: A systematic review and meta-analysis. Surv. Ophthalmol. 2018, 63, 719–735. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Geng, J.; Yuan, Z.; Huang, D. Geographical prevalence and risk factors for pterygium: A systematic review and meta-analysis. BMJ Open 2013, 3, e003787. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.; Di Girolamo, N.; Wakefield, D.; Coroneo, M.T. The pathogenesis of pterygium: Current concepts and their therapeutic implications. Ocul. Surf. 2008, 6, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Di Girolamo, N.; McCluskey, P.; Lloyd, A.; Coroneo, M.T.; Wakefield, D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 671–679. [Google Scholar]
- Kato, N.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Ogawa, Y.; Yoshida, S.; Higa, K.; Okano, H.; Tsubota, K. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1511–1517. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Wakefield, D.; Coroneo, M.T. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4142–4149. [Google Scholar] [PubMed]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar]
- Dushku, N.; John, M.K.; Schultz, G.S.; Reid, T.W. Pterygia pathogenesis: Corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch. Ophthalmol. 2001, 119, 695–706. [Google Scholar] [CrossRef]
- Yang, S.-F.; Lin, C.-Y.; Yang, P.-Y.; Chao, S.-C.; Ye, Y.-Z.; Hu, D.-N. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4588–4596. [Google Scholar] [CrossRef]
- Solomon, A.; Rosenblatt, M.; Li, D.-Q.; Liu, Z.; Monroy, D.; Ji, Z.; Lokeshwar, B.L.; Pflugfelder, S.C. Doxycycline Inhibition of Interleukin-1 in the Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef] [PubMed]
- de Meneghim, R.L.F.S.; Satto, L.H.; Natsuaki, K.L.; de Oliveira, A.C.; Padovani, C.R.; Viveiros, M.M.H.; Schellini, S.A. Topical cyclosporine A 0.05% before and after surgery to prevent pterygium recurrence. Arq. Bras. Oftalmol. 2019, 82, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Yalcin Tok, O.; Burcu Nurozler, A.; Ergun, G.; Akbas Kocaoglu, F.; Duman, S. Topical cyclosporine A in the prevention of pterygium recurrence. Ophthalmologica 2008, 222, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Yaisawang, S.; Piyapattanakorn, P. Role of post-operative topical corticosteroids in recurrence rate after pterygium excision with conjunctival autograft. J. Med. Assoc. Thail. 2003, 86 (Suppl. S2), S215–S223. [Google Scholar]
- Sul, S.; Korkmaz, S.; Alacamli, G.; Ozyol, P.; Ozyol, E. Application of autologous serum eye drops after pterygium surgery: A prospective study. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Kampitak, K.; Leelawongtawun, W.; Leeamornsiri, S.; Suphachearaphan, W. Role of artificial tears in reducing the recurrence of pterygium after surgery: A prospective randomized controlled trial. Acta Ophthalmol. 2017, 95, e227–e229. [Google Scholar] [CrossRef]
- Chu, W.K.; Choi, H.L.; Bhat, A.K.; Jhanji, V. Pterygium: New insights. Eye 2020, 34, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Karalezli, A.; Kucukerdonmez, C.; Akova, Y.A.; Koktekir, B.E. Does topical bevacizumab prevent postoperative recurrence after pterygium surgery with conjunctival autografting? Int. J. Ophthalmol. 2014, 7, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Courville, C.B.; Smolek, M.K.; Klyce, S.D. Contribution of the ocular surface to visual optics. Exp. Eye Res. 2004, 78, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Yuksel, T.; Maden, A. Corneal topographic changes after four types of pterygium surgery. J. Refract. Surg. 2008, 24, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef]
- Eljaaly, K.; Alghamdi, H.; Almehmadi, H.; Aljawi, F.; Hassan, A.; Thabit, A.K. Long-term gastrointestinal adverse effects of doxycycline. J. Infect. Dev. Ctries. 2023, 17, 281–285. [Google Scholar] [CrossRef] [PubMed]
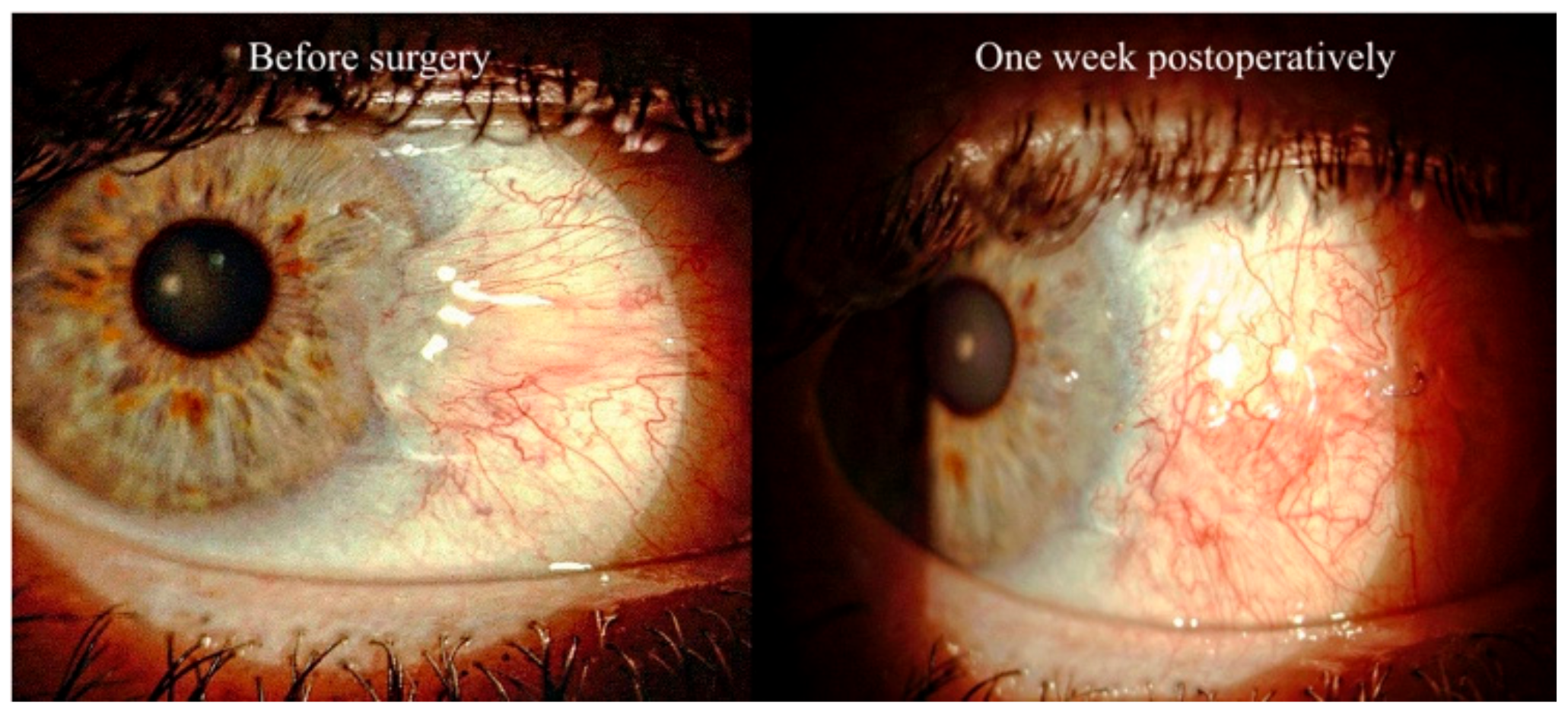
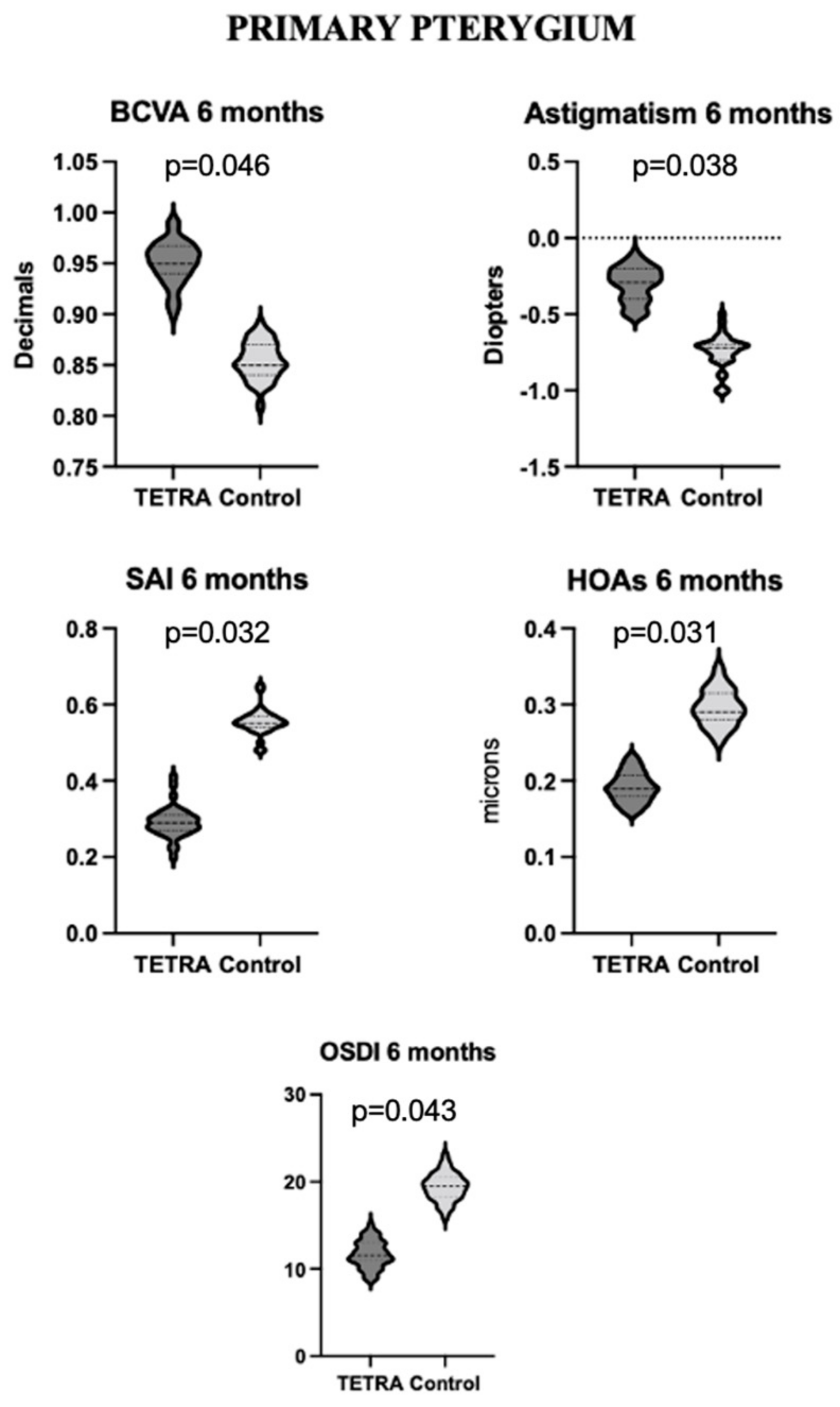
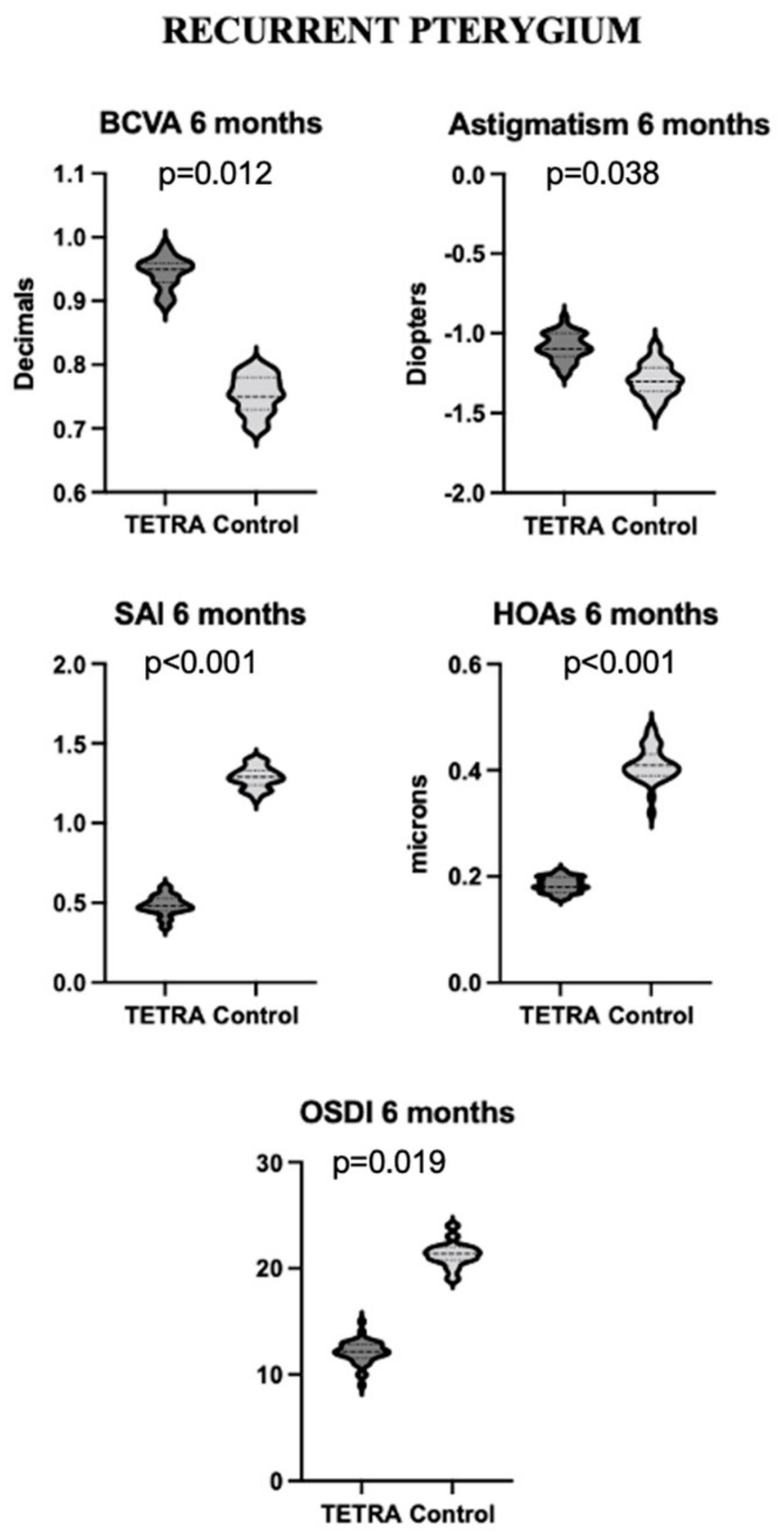
| TETRA Group | |||||
|---|---|---|---|---|---|
| Primary | Baseline | 6 Weeks | 6 Months | 1 Year | p |
| Age | 45.69 ± 14.27 | ||||
| Male sex | 16/36 (44.4%) | ||||
| Ethnicity | Caucasian = 22/36 (61.1%) Hispanic = 14/36 (38.9%) | ||||
| Sun exposure > 5 h/day | 16/36 (44.4%) | ||||
| Eye | RE = 18/36 (50%) | ||||
| BCVA (Decimals) | 0.79 ± 0.11 | 0.92 ± 0.06 | 0.96 ± 0.038 | 0.96 ± 0.038 | 0.037 * |
| Sphere (D) | 1.09 ± 1.34 | 1.08 ± 1.94 | 1.08 ± 1.94 | 1.08 ± 1.94 | 0.851 |
| Cylinder (D) | −1.17 ± 0.95 | −0.25 ± 0.73 | −0.25 ± 0.73 | −0.25 ± 0.73 | 0.021 * |
| SAI | 0.67 ± 0.49 | 0.41 ± 0.29 | 0.29 ± 0.25 | 0.24 ± 0.21 | 0.042 * |
| LSA | 0.95 ± 0.72 | 0.85 ± 0.71 | 0.78 ± 0.50 | 0.66 ± 0.43 | 0.063 |
| IC | 0.18 ± 0.12 | 0.16 ± 0.11 | 0.15 ± 0.09 | 0.14 ± 0.10 | 0.649 |
| RMS tot | 0.59 ± 0.48 | 0.47 ± 0.38 | 0.37 ± 0.29 | 0.24 ± 0.08 | 0.039 * |
| HOAs | 0.32 ± 0.27 | 0.20 ± 0.15 | 0.19 ± 0.15 | 0.13 ± 0.11 | 0.058 |
| OSDI | 26.85 ± 8.42 | 14.07 ± 6.15 | 11.50 ± 4.57 | 10.37 ± 3.66 | 0.025 * |
| Recurrence | 1/36 (2.77%) | ||||
| Recurrent | Baseline | 6 weeks | 6 months | 1 year | p |
| Age | 50.31 ± 9.62 | ||||
| Male sex | 10/24 (41.67%) | ||||
| Ethnicity | Caucasian = 4/24 (16.67%) Hispanic = 20/24 (83.33%) | ||||
| Sun exposure > 5 h/day | 4/24 (16.67%) | ||||
| Eye | RE = 14/24 (58.33%) | ||||
| BCVA | 0.56 ± 0.19 | 0.84 ± 0.058 | 0.95 ± 0.04 | 0.94 ± 0.06 | <0.001 * |
| Sphere | 1.67 ± 1.02 | 1.55 ± 1.13 | 1.71 ± 1.17 | 1.70 ± 1.12 | 0.714 |
| Cyl | −2.53 ± 0.83 | −1.18 ± 0.37 | −1.10 ± 0.22 | −1.15 ± 0.33 | 0.002 * |
| SAI | 1.31 ± 0.82 | 0.59 ± 0.28 | 0.47 ± 0.23 | 0.33 ± 0.26 | 0.004 * |
| LSA | 1.35 ± 1.08 | 0.88 ± 0.38 | 0.76 ± 0.37 | 0.64 ± 0.26 | 0.021 * |
| IC | 0.45 ± 0.16 | 0.25 ± 0.15 | 0.23 ± 0.12 | 0.20 ± 0.12 | 0.043 * |
| RMS tot | 1.11 ± 0.88 | 0.70 ± 0.38 | 0.60 ± 0.28 | 0.51 ± 0.25 | 0.036 * |
| HOAs | 0.49 ± 0.56 | 0.31 ± 0.41 | 0.18 ± 0.14 | 0.15 ± 0.07 | 0.041 * |
| OSDI | 24.0 ± 8.91 | 15.33 ± 6.38 | 12.17 ± 3.38 | 10.76 ± 2.73 | 0.005 * |
| Recurrence | 1/24 (4.16%) | ||||
| CONTROL Group | |||||
|---|---|---|---|---|---|
| Primary | Baseline | 6 Weeks | 6 Months | 1 Year | p |
| Age | 41.39 ± 13.17 | ||||
| Male sex | 15/33 (45.5%) | ||||
| Ethnicity | Caucasian = 21/33 (63.6%) Hispanic = 12/33 (34.6%) | ||||
| Sun exposure > 5 h/day | 13/33 (39.4%) | ||||
| Eye | RE = 16/33 (48.5%) | ||||
| BCVA (Decimals) | 0.75 ± 0.10 | 0.81 ± 0.09 | 0.86 ± 0.04 | 0.87 ± 0.05 | 0.045 * |
| Sphere (D) | 1.51 ± 0.95 | 1.39 ± 1.04 | 1.38 ± 1.06 | 1.39 ± 1.07 | 0.463 |
| Cylinder (D) | −1.27 ± 0.82 | −1.15 ± 0.71 | −0.75 ± 0.53 | −0.25 ± 0.73 | 0.028 * |
| SAI | 0.59 ± 0.22 | 0.57 ± 0.27 | 0.55 ± 0.36 | 0.58 ± 0.21 | 0.561 |
| LSA | 0.93 ± 0.56 | 0.89 ± 0.64 | 0.86 ± 0.61 | 0.84 ± 0.63 | 0.499 |
| IC | 0.17 ± 0.11 | 0.15 ± 0.10 | 0.15 ± 0.09 | 0.16 ± 0.11 | 0.672 |
| RMS tot | 0.61 ± 0.37 | 0.57 ± 0.38 | 0.41 ± 0.26 | 0.39 ± 0.13 | 0.046 * |
| HOAs | 0.33 ± 0.21 | 0.28 ± 0.17 | 0.29 ± 0.15 | 0.28 ± 0.13 | 0.112 |
| OSDI | 25.32 ± 6.81 | 22.97 ± 7.35 | 19.58 ± 6.17 | 17.11 ± 5.26 | 0.046 * |
| Recurrence | 3/33 (9.09%) | ||||
| Recurrent | Baseline | 6 weeks | 6 months | 1 year | p |
| Age | 52.41 ± 7.11 | ||||
| Male Sex | 8/19 (42.11%) | ||||
| Ethnicity | Caucasian = 4/19 (21.05%) Hispanic = 15/19 (78.9%) | ||||
| Sun exposure > 5 h/day | 4/19 (21.05%) | ||||
| Eye | RE = 9/19 (47.36%) | ||||
| BCVA (decimals) | 0.59 ± 0.13 | 0.72 ± 0.09 | 0.75 ± 0.12 | 0.81 ± 0.09 | 0.027 * |
| Sphere (D) | 1.08 ± 0.75 | 1.21± 0.69 | 1.31 ± 0.54 | 1.10 ± 0.74 | 0.438 |
| Cylinder (D) | −2.27 ± 1.03 | −1.62 ± 0.41 | −1.30 ± 0.34 | −1.35 ± 0.33 | 0.029 * |
| SAI | 1.45 ± 0.73 | 1.29 ± 0.68 | 1.27 ± 0.53 | 1.33 ± 0.61 | 0.516 |
| LSA | 1.25 ± 0.17 | 1.08 ± 0.22 | 0.96 ± 0.34 | 1.04 ± 0.36 | 0.213 |
| IC | 0.41 ± 0.11 | 0.35 ± 0.12 | 0.37 ± 0.11 | 0.38 ± 0.12 | 0.395 |
| RMS tot | 1.27 ± 0.53 | 1.00 ± 0.48 | 0.92 ± 0.31 | 0.81 ± 0.29 | 0.041 * |
| HOAs | 0.52 ± 0.31 | 0.46 ± 0.39 | 0.41 ± 0.26 | 0.42 ± 0.23 | 0.225 |
| OSDI | 27.33 ± 6.11 | 25.09 ± 6.35 | 21.28 ± 5.88 | 20.93 ± 5.83 | 0.072 * |
| Recurrence | 3/19 (15.79%) | ||||
| TETRA Group (60 Eyes) | CONTROL Group (52 Eyes) | p | |
|---|---|---|---|
| Age (years) | 47.6 ± 7.22 | 46.9 ± 6.03 | 0.890 |
| Sex | 26/60 (43.3%) | 23/52 (44.2%) | 0.923 |
| Hispanic ethnicity | 34/60 (56.7%) | 27/52 (51.9%) | 0.615 |
| Outdoor working and/or sun exposure > 5 h/day | 20/60 (33.3%) | 17/52 (32.7%) | 0.942 |
| Preoperative DED | Mild = 15/60 (25.0%) Moderate = 11/60 (18.3%) Severe = 4/60 (6.7%) | Mild = 11/52 (21.2%) Moderate = 10/52 (19.2%) Severe = 3/52 (5.7%) | 0.959 |
| Preoperative treatment with artificial tears | 37/60 (61.6%) | 30/52 (57.7%) | 0.668 |
| Preoperative inflammation/pterygium congestion | 8/60 (13.3%) | 10/52 (19.2%) | 0.396 |
| Pterygium largest diameter (mm) | 2.8 ± 1.3 | 2.7 ± 1.7 | 0.299 |
| Visual axis involvement | 22/60 (36.7%) | 18/52 (34.6%) | 0.821 |
| Chronic pterygium | 17/60 (28.3%) | 19/52 (36.5%) | 0.353 |
| Recurrent pterygium | 24/60 (40.0%) | 19/52 (36.5%) | 0.707 |
| Surgical technique | Autograft = 22/60 (36.7%) Transposition = 38/60 (63.3%) | Autograft = 17/52 (32.7%) Transposition = 35/52 (67.3%) | 0.659 |
| Postoperative inflammatory episodes (number of episodes during follow-up) | 0.4 ± 0.6 | 0.8 ± 1.3 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catania, F.; Vinciguerra, P.; Di Maria, A. Low Rate of Postoperative Pterygium Recurrence in Patients under Treatment with Low-Dose Oral Doxycycline for Chronic Blepharitis: A First Report. Diagnostics 2024, 14, 715. https://doi.org/10.3390/diagnostics14070715
Catania F, Vinciguerra P, Di Maria A. Low Rate of Postoperative Pterygium Recurrence in Patients under Treatment with Low-Dose Oral Doxycycline for Chronic Blepharitis: A First Report. Diagnostics. 2024; 14(7):715. https://doi.org/10.3390/diagnostics14070715
Chicago/Turabian StyleCatania, Fiammetta, Paolo Vinciguerra, and Alessandra Di Maria. 2024. "Low Rate of Postoperative Pterygium Recurrence in Patients under Treatment with Low-Dose Oral Doxycycline for Chronic Blepharitis: A First Report" Diagnostics 14, no. 7: 715. https://doi.org/10.3390/diagnostics14070715
APA StyleCatania, F., Vinciguerra, P., & Di Maria, A. (2024). Low Rate of Postoperative Pterygium Recurrence in Patients under Treatment with Low-Dose Oral Doxycycline for Chronic Blepharitis: A First Report. Diagnostics, 14(7), 715. https://doi.org/10.3390/diagnostics14070715







