Computed Tomography to Exclude Cardiac Thrombus in Atrial Fibrillation—An 11-Year Experience from an Academic Emergency Department
Abstract
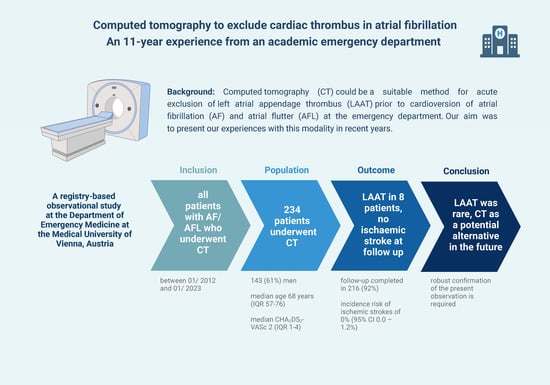
1. Background
2. Methods
2.1. Study Centre and AF Registry
2.2. Follow-Up as Part of the Registry Database
2.3. Study Population and CT Scan Protocol
2.4. Statistical Analysis
3. Results
3.1. LAAT Prevalence and AF Characteristics
3.2. Rate of Ischemic Stroke and Mortality
3.3. Risk Factors for the Presence of LAAT
3.4. Temporal Trend of CT Scans Performed in the ED
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milman, B.; Burns, B.D. Atrial fibrillation: An approach to diagnosis and management in the emergency department. Emerg. Med. Pract. 2021, 23, 1–28. [Google Scholar] [PubMed]
- Boriani, G.; Diemberger, I.; Martignani, C.; Biffi, M.; Branzi, A. The epidemiological burden of atrial fibrillation: A challenge for clinicians and health care systemsThe opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology. Eur. Heart J. 2006, 27, 893–894. [Google Scholar] [CrossRef]
- Costantino, G.; Solbiati, M. Atrial fibrillation cardioversion in the emergency department. Lancet 2020, 395, 313–314. [Google Scholar] [CrossRef]
- Grönberg, T.; Hartikainen, J.E.K.; Nuotio, I.; Biancari, F.; Ylitalo, A.; Airaksinen, K.E.J. Anticoagulation, CHA2DS2VASc Score, and Thromboembolic Risk of Cardioversion of Acute Atrial Fibrillation (from the FinCV Study). Am. J. Cardiol. 2016, 117, 1294–1298. [Google Scholar] [CrossRef]
- Garg, A.; Khunger, M.; Seicean, S.; Chung, M.K.; Tchou, P.J. Incidence of Thromboembolic Complications within 30 Days of Electrical Cardioversion Performed within 48 Hours of Atrial Fibrillation Onset. JACC Clin. Electrophysiol. 2016, 2, 487–494. [Google Scholar] [CrossRef]
- Beigel, R.; Wunderlich, N.C.; Ho, S.Y.; Arsanjani, R.; Siegel, R.J. The Left Atrial Appendage: Anatomy, Function, and Noninvasive Evaluation. JACC Cardiovasc. Imaging 2014, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.I.; Manning, W.J. Role of Echocardiography in Patients Undergoing Elective Cardioversion of Atrial Fibrillation. Circulation 1998, 98, 479–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Daniel, W.G.; Erbel, R.; Kasper, W.; Visser, C.A.; Engberding, R.; Sutherland, G.R.; Grube, E.; Hanrath, P.; Maisch, B.; Dennig, K. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991, 83, 817–821. [Google Scholar] [CrossRef]
- Patel, K.M.; Desai, R.G.; Trivedi, K.; Neuburger, P.J.; Krishnan, S.; Potestio, C.P. Complications of Transesophageal Echocardiography: A Review of Injuries, Risk Factors, and Management. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3292–3302. [Google Scholar] [CrossRef]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic Examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef]
- Zhan, Y.; Joza, J.; Al Rawahi, M.; Barbosa, R.S.; Samuel, M.; Bernier, M.; Huynh, T.; Thanassoulis, G.; Essebag, V. Assessment and Management of the Left Atrial Appendage Thrombus in Patients with Nonvalvular Atrial Fibrillation. Can. J. Cardiol. 2018, 34, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, W.; Sheikh, A.; Said, Z.; Ahmed, M.A.; Gadde, S.; Shah, T.; Wilson, M.F.; Beck, H.; Kim, C.; Sharma, U.C. The use of cardiac-CT alone to exclude left atrial thrombus before atrial fibrillation ablation: Efficiency, safety, and cost analysis. Pacing Clin. Electrophysiol. 2018, 41, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Pathan, F.; Hecht, H.; Narula, J.; Marwick, T.H. Roles of Transesophageal Echocardiography and Cardiac Computed Tomography for Evaluation of Left Atrial Thrombus and Associated Pathology. JACC Cardiovasc. Imaging 2018, 11, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Husain, S.A.; Kelesidis, I.; Sanz, J.; Medina, H.M.; Garcia, M.J. Detection of Left Atrial Appendage Thrombus by Cardiac Computed Tomography in Patients with Atrial Fibrillation: A Meta-Analysis. Circ. Cardiovasc. Imaging 2013, 6, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lutnik, M.; Niederdöckl, J.; Schnaubelt, S. From Bench to Bedside—Implementing the New ABC Approach for Atrial Fibrillation in an Emergency Department Setting. Int. J. Environ. Res. Public Health 2022, 19, 4797. [Google Scholar] [CrossRef]
- Jakobs, T.F.; Becker, C.R.; Ohnesorge, B.; Flohr, T.; Suess, C.; Schoepf, U.J.; Reiser, M.F. Multislice helical CT of the heart with retrospective ECG gating: Reduction of radiation exposure by ECG-controlled tube current modulation. Eur. Radiol. 2002, 12, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Gulizia, M.M.; Cemin, R.; Colivicchi, F.; De Luca, L.; Di Lenarda, A.; Boriani, G.; Di Pasquale, G.; Nardi, F.; Scherillo, M.; Lucci, D.; et al. Management of atrial fibrillation in the emergency room and in the cardiology ward: The BLITZ AF study. EP Eur. 2019, 21, 230–238. [Google Scholar] [CrossRef]
- Niederdöckl, J.; Schwameis, M.; Herkner, H.; Domanovits, H. Excess short-term mortality in noncritical patients with atrial fibrillation presenting to the emergency department. Wien. Klin. Wochenschr. 2021, 133, 802–805. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Qiu, C.; Davis, P.J.; Jhaveri, M.; Prystowsky, E.N.; Kowey, P.; Weintraub, W.S. Predictors of Progression of Recently Diagnosed Atrial Fibrillation in REgistry on Cardiac Rhythm DisORDers Assessing the Control of Atrial Fibrillation (RecordAF)–United States Cohort. Am. J. Cardiol. 2013, 112, 79–84. [Google Scholar] [CrossRef]
- Bunch, T.J.; May, H.T.; Bair, T.L.; Johnson, D.L.; Weiss, J.P.; Crandall, B.G.; Osborn, J.S.; Anderson, J.L.; Muhlestein, J.B.; Lappe, D.L.; et al. Increasing time between first diagnosis of atrial fibrillation and catheter ablation adversely affects long-term outcomes. Heart Rhythm 2013, 10, 1257–1262. [Google Scholar] [CrossRef]
- Sandhu, R.K.; Smigorowsky, M.; Lockwood, E.; Savu, A.; Kaul, P.; McAlister, F.A. Impact of Electrical Cardioversion on Quality of Life for the Treatment of Atrial Fibrillation. Can. J. Cardiol. 2017, 33, 450–455. [Google Scholar] [CrossRef]
- Jagadish, P.S.; Kabra, R. Stroke Risk in Atrial Fibrillation: Beyond the CHA2DS2-VASc Score. Curr. Cardiol. Rep. 2019, 21, 95. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Shrader, P.; Thomas, L.; Ansell, J.; Fonarow, G.C.; Gersh, B.J.; Kowey, P.R.; Mahaffey, K.W.; Naccarelli, G.; Reiffel, J.; et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes. J. Am. Coll. Cardiol. 2016, 68, 2597–2604. [Google Scholar] [CrossRef]
- Giustozzi, M.; Agnelli, G.; Quattrocchi, S.; Acciarresi, M.; Alberti, A.; Caso, V.; Vedovati, M.C.; Venti, M.; Paciaroni, M. Rates and Determinants for the Use of Anticoagulation Treatment before Stroke in Patients with Known Atrial Fibrillation. Cerebrovasc. Dis. Extra 2020, 10, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J. Accuracy of Transesophageal Echocardiography for Identifying Left Atrial Thrombi: A Prospective, Intraoperative Study. Ann. Intern. Med. 1995, 123, 817. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-J.; Chen, J.-J.; Lin, S.-C.; Tseng, Y.-Z.; Kuan, P.; Lien, W.-P.; Lin, F.-Y.; Chu, S.-H.; Hung, C.-R.; How, S.-W. Diagnostic accuracy of transesophageal echocardiography for detecting left atrial thrombi in patients with rheumatic heart disease having undergone mitral valve operations. Am. J. Cardiol. 1993, 72, 677–681. [Google Scholar] [CrossRef]
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.A.; et al. Use of Transesophageal Echocardiography to Guide Cardioversion in Patients with Atrial Fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Hilberath, J.N.; Oakes, D.A.; Shernan, S.K.; Bulwer, B.E.; D’Ambra, M.N.; Eltzschig, H.K. Safety of Transesophageal Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 1115–1127. [Google Scholar] [CrossRef]
- Spagnolo, P.; Giglio, M.; Di Marco, D.; Cannaò, P.M.; Agricola, E.; Della Bella, P.E.; Monti, C.B.; Sardanelli, F. Diagnosis of left atrial appendage thrombus in patients with atrial fibrillation: Delayed contrast-enhanced cardiac CT. Eur. Radiol. 2021, 31, 1236–1244. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Li, H. Cardiac Computed Tomography Versus Transesophageal Echocardiography for the Detection of Left Atrial Appendage Thrombus: A Systemic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e022505. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S. Electron beam computed tomography for the detection of left atrial thrombi in patients with atrial fibrillation. Heart 2004, 90, 1477–1478. [Google Scholar] [CrossRef][Green Version]
- Hansen, M.L.; Jepsen, R.M.H.G.; Olesen, J.B.; Ruwald, M.H.; Karasoy, D.; Gislason, G.H.; Hansen, J.; Køber, L.; Husted, S.; Torp-Pedersen, C. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. EP Eur. 2015, 17, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Haeusler, K.G.; Oeff, M.; Treszl, A.; Andresen, D.; Borggrefe, M.; Lip, G.Y.H.; Meinertz, T.; Parade, U.; Samol, A.; et al. Low stroke risk after elective cardioversion of atrial fibrillation: An analysis of the Flec-SL trial. Int. J. Cardiol. 2013, 168, 3977–3981. [Google Scholar] [CrossRef] [PubMed]
- Pluymaekers, N.A.H.A.; Dudink, E.A.M.P.; Luermans, J.G.L.M.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.J.J.; Rienstra, M.; Kamp, O.; Van Opstal, J.M.; et al. Early or Delayed Cardioversion in Recent-Onset Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1499–1508. [Google Scholar] [CrossRef]
- A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [CrossRef]

| Baseline Characteristics | LAAT (n = 8) | No LAAT (n = 226) | Overall (n = 234) |
|---|---|---|---|
| male, n % | 5 (63) | 138 (61) | 143 (61) |
| age, median (IQR) | 72 (64–75) | 67 (57–76) | 68 (57–76) |
| bmi, median (IQR) | 29 (29–32) | 28 (24–31) | 28 (24–31) |
| Vitals | |||
| heart rate, median (IQR) | 132 (122–167) | 140 (123–160) | 140 (122–160) |
| blood pressure, systolic, median (IQR) | 164 (162–180) | 129 (116–142) | 130 (116–147) |
| blood pressure, diastolic, median (IQR) | 113 (70–120) | 82 (73–95) | 83 (72–96) |
| Medical history | |||
| heart failure, n (%) | 1 (13) | 29 (13) | 30 (13) |
| arterial hypertension, n (%) | 3 (38) | 126 (56) | 129 (55) |
| chd, n (%) | 2 (25) | 41 (18) | 43 (18) |
| myocardial infarction, n (%) | 1 (13) | 15 (7) | 16 (7) |
| stroke, n (%) | 2 (25) | 5 (2) | 7 (3) |
| cerebral vascular disease, n (%) | 1 (13) | 8 (4) | 9 (4) |
| diabetes mellitus, n (%) | 1 (13) | 27 (12) | 28 (12) |
| copd, n (%) | 1 (13) | 19 (8) | 20 (9) |
| hyperlipidemia, n (%) | 3 (38) | 65 (29) | 68 (29) |
| AF Characteristics | LAAT (n = 8) | No LAAT (n = 226) | Overall (n = 234) |
|---|---|---|---|
| onset unknown, n (%) | 6 (75) | 135 (60) | 141 (60) |
| first episode, n (%) | 3 (38) | 105 (46) | 108 (46) |
| CHA2DS2-VASc, median (IQR) | 3 (3–4) | 2 (1–4) | 2 (1–4) |
| Oac and/or antiplatelet therapy at admission, n (%) | 3 (38) | 87 (38) | 90 (38) |
| sinus rhythm restored, n (%) | 1 (13) | 163 (72) | 164 (70) |
| Outcomes and Follow Up | LAAT (n = 8) | No LAAT (n = 226) | Overall (n = 234) |
|---|---|---|---|
| stroke rate at follow up, n (%) | 0 (0) | 0 (0) | 0 (0) |
| death, n (%) | 0 (0) | 14 (6) | 14 (6) |
| follow up completed, n (%) | 8 (100) | 208 (92) | 216 (92) |
| follow up time, median (IQR) | 376 (315–1029) | 523 (158–1396) | 506 (159–1391) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Lutnik, M.; Cacioppo, F.; Lindmayr, T.; Schuetz, N.; Tumnitz, E.; Friedl, L.; Boegl, M.; Schnaubelt, S.; Domanovits, H.; et al. Computed Tomography to Exclude Cardiac Thrombus in Atrial Fibrillation—An 11-Year Experience from an Academic Emergency Department. Diagnostics 2024, 14, 699. https://doi.org/10.3390/diagnostics14070699
Gupta S, Lutnik M, Cacioppo F, Lindmayr T, Schuetz N, Tumnitz E, Friedl L, Boegl M, Schnaubelt S, Domanovits H, et al. Computed Tomography to Exclude Cardiac Thrombus in Atrial Fibrillation—An 11-Year Experience from an Academic Emergency Department. Diagnostics. 2024; 14(7):699. https://doi.org/10.3390/diagnostics14070699
Chicago/Turabian StyleGupta, Sophie, Martin Lutnik, Filippo Cacioppo, Teresa Lindmayr, Nikola Schuetz, Elvis Tumnitz, Lena Friedl, Magdalena Boegl, Sebastian Schnaubelt, Hans Domanovits, and et al. 2024. "Computed Tomography to Exclude Cardiac Thrombus in Atrial Fibrillation—An 11-Year Experience from an Academic Emergency Department" Diagnostics 14, no. 7: 699. https://doi.org/10.3390/diagnostics14070699
APA StyleGupta, S., Lutnik, M., Cacioppo, F., Lindmayr, T., Schuetz, N., Tumnitz, E., Friedl, L., Boegl, M., Schnaubelt, S., Domanovits, H., Spiel, A., Toth, D., Varga, R., Raudner, M., Herkner, H., Schwameis, M., & Niederdoeckl, J. (2024). Computed Tomography to Exclude Cardiac Thrombus in Atrial Fibrillation—An 11-Year Experience from an Academic Emergency Department. Diagnostics, 14(7), 699. https://doi.org/10.3390/diagnostics14070699