Acoustic Radiation Forced Impulse of the Liver and the Spleen, Combined with Spleen Dimension and Platelet Count in New Ratio Scores, Identifies High-Risk Esophageal Varices in Well-Compensated Cirrhotic Patients
Abstract
1. Introduction
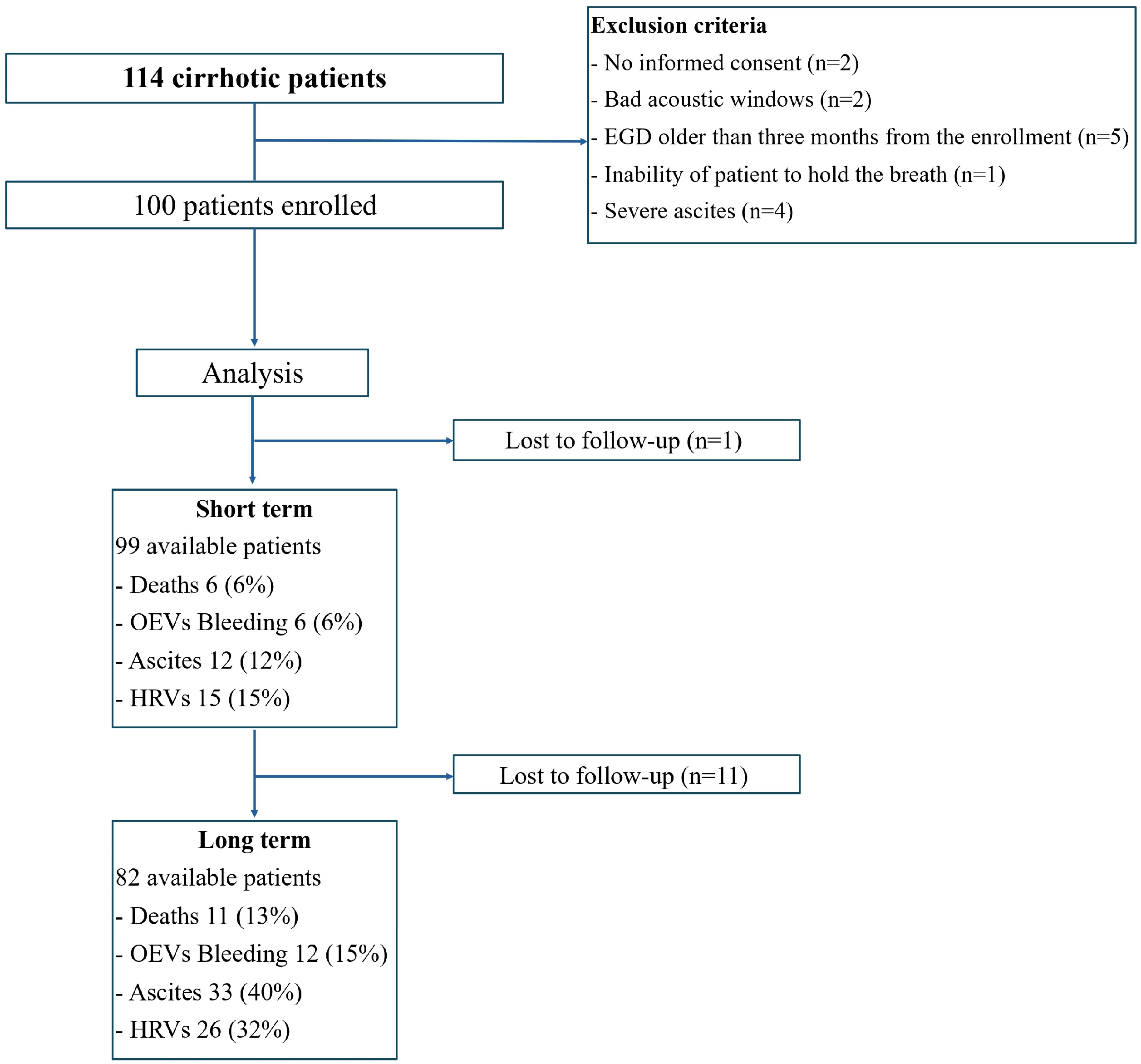
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALSAP | ARFI Liver–Spleen Area-to-Platelet Ratio Score |
| ALSDP | ARFI Liver–Spleen Diameter-to-Platelet Ratio Score |
| ARFI | acoustic radiation forced impulse |
| ASPS | ARFI Spleen–Spleen Diameter-to-Platelet Ratio Score |
| aRR | adjusted risk ratios |
| ASSAP | ARFI Spleen–Spleen Area-to-Platelet Ratio Score |
| ASSDP | ARFI Spleen–Spleen Diameter-to-Platelet ratio score |
| AUC | area under curve |
| CI | confidence intervals |
| CSPH | clinically significant portal hypertension |
| EGD | esophagogastroduodenoscopy |
| EASL | European Association for the Study of the Liver |
| GEVs | gastroesophageal varices |
| HVPG | hepatic venous pressure gradient |
| HVRs | high risk varices |
| IQR | interquartile range |
| LSM | liver stiffness measurement |
| TE | transient elastography |
| OEVs | esophageal varices |
| OR | odds ratio |
| PH | portal hypertension |
| pSWE | point shear wave elastography |
| ROI | region of interest |
| SSM | spleen stiffness measurement |
| SD | standard deviation |
References
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. J. Hepatol. 2016, 65, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Planas, R.; Escorsell, A.; Guarcia-Pagan, J.C.; Patch, D.; Matloff, D.; et al. Beta-Blockers to Prevent Gastroesophageal Varices in Patients with Cirrhosis. N. Engl. J. Med. 2005, 353, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Groszmann, R.; Garcia–Tsao, G.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Guarcia-Pagan, J.C.; Makuch, R.; Patch, D.S.; et al. Hepatic Venous Pressure Gradient Predicts Clinical Decompensation in Patients with Compensated Cirrhosis. Gastroenterology 2007, 133, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Guarcia-Pagan, J.C.; Makuch, R.; et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J. Hepatol. 2009, 50, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; García-Pagán, J.C. Prevention of variceal rebleeding. Lancet 2003, 361, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Bosch, J.; Boyer, T.D. Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. J. Hepatol. 2013, 59, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Berzigotti, A.; Seijo, S.; Arena, U.; Abraldes, J.G.; Vizzutti, F.; García–Pagán, J.C.; Pinzani, M.; Bosch, J. Elastography, Spleen Size, and Platelet Count Identify Portal Hypertension in Patients with Compensated Cirrhosis. Gastroenterology 2013, 144, 102–111. [Google Scholar] [CrossRef]
- Kim, B.K.; Han, K.H.; Park, J.Y.; Ahn, S.H.; Kim, J.K.; Paik, Y.H.; Lee, K.S.; Chon, C.Y.; Kim, D.Y. A Liver Stiffness Measurement-Based, Noninvasive Prediction Model for High-Risk Esophageal Varices in B-Viral Liver Cirrhosis. Am. J. Gastroenterol. 2010, 105, 1382–1390. [Google Scholar] [CrossRef]
- Shi, K.Q.; Fan, Y.C.; Pan, Z.Z.; Lin, X.F.; Liu, W.Y.; Chen, Y.P.; Zheng, M.H. Transient elastography: A meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2012, 33, 62–71. [Google Scholar] [CrossRef]
- Castéra, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Calvaruso, V.; Cacopardo, B.; Alessi, N.; Attanasio, M.; Petta, S.; Fatuzzo, F.; Montineri, A.; Mazzola, A.; L’abbate, L.; et al. Comparison of Transient Elastography and Acoustic Radiation Force Impulse for Non-Invasive Staging of Liver Fibrosis in Patients with Chronic Hepatitis C. Am. J. Gastroenterol. 2011, 106, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Palmeri, M.L.; Bouchard, R.R.; Nightingale, R.W.; Nightingale, K.R. An Integrated Indenter-ARFI Imaging System for Tissue Stiffness Quantification. Ultrason. Imag. 2008, 30, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gallotti, A.; D’Onofrio, M.; Pozzi Mucelli, R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol. Med. 2010, 115, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Idezuki, Y. General rules for recording endoscopic findings of esophagogastric varices (1991). World. J. Surg. 1995, 19, 420–422. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2010, 53, 762–768. [Google Scholar] [CrossRef]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Augustin, S.; Pons, M.; Maurice, J.B.; Bureau, C.; Stefanescu, H.; Ney, M.; Blasco, H.; Procopet, B.; Tsochatzis, E.; Westbrook, R.H.; et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. J. Hepatol. 2017, 66, 1980–1988. [Google Scholar] [CrossRef]
- Colecchia, A.; Ravaioli, F.; Marasco, G.; Colli, A.; Dajti, E.; Di Biase, A.R.; Festi, D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J. Hepatol. 2018, 69, 308–317. [Google Scholar] [CrossRef]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Berzigotti, A.; Rossi, V.; Tiani, C.; Pierpaoli, L.; Zappoli, P.; Riili, A.; Serra, C.; Andreone, P.; Morelli, M.C.; Golfieri, R.; et al. Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J. Gastroenterol. 2010, 46, 687–695. [Google Scholar] [CrossRef]
- Jangouk, P.; Turco, L.; De Oliveira, A.; Schepis, F.; Villa, E.; Garcia-Tsao, G. Validating, deconstructing and refining Baveno criteria for ruling out high-risk varices in patients with compensated cirrhosis. Liver Int. 2017, 37, 1177–1183. [Google Scholar] [CrossRef]
- De Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; So, Y.H.; Kim, W.; Ahn, D.W.; Jin Jung, Y.; Woo, H.; Kim, D.; Kim, M.Y.; Baik, S.K. Noninvasive Response Prediction in Prophylactic Carvedilol Therapy for Cirrhotic Patients with Esophageal Varices. J. Hepatol. 2018, 70, 412–422. [Google Scholar] [CrossRef]
- Galati, G.; De Vincentis, A.; Guidi, A.; Gallo, P.; Vespasiani-Gentilucci, U.; Picardi, A. Spleen Stiffness Evaluated by Acoustic Radiation Force Impulse (ARFI) Elastography in Cirrhotic Patients is not a Useful Tool to Predict Esophageal Varices Needing Treatment. Ultrason. Med. Biol. 2017, 43, S102. [Google Scholar] [CrossRef][Green Version]
- Jain, A.K.; Bundiwal, A.K.; Jain, S.; Agrawal, P.; Jain, D.; Sircar, S. Evaluation of liver and splenic stiffness by acoustic radiation force impulse for assessment of esophageal varices. Indian J. Gastroenterol. 2023. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Wu, H.; Feng, Y.; Han, X.; Bu, H.; Zhu, Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS ONE 2016, 11, e0165786. [Google Scholar] [CrossRef] [PubMed]
- Manatsathit, W.; Samant, H.; Kapur, S.; Ingviya, T.; Esmadi, M.; Wijarnpreecha, K.; McCashland, T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J. Gastroenterol. Hepatol. 2018, 33, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, S.U.; Park, S.Y.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Tak, W.Y.; Kweon, Y.O.; Han, K.H. A Novel Model to Predict Esophageal Varices in Patients with Compensated Cirrhosis Using Acoustic Radiation Force Impulse Elastography. PLoS ONE 2015, 10, e0121009. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, L.; He, R.; Li, S.; Liu, C.; Qi, X.; Li, J. A strategy for varices screening based on acoustic radiation force impulse combined with platelet (CHESS2001): An alternative of Baveno VI criteria. Hepatol. Commun. 2022, 6, 3154–3162. [Google Scholar] [CrossRef]
- Wang, H.; Xi, R.; Song, J.; Wen, B.; Zhang, Y.; Zhou, L.; Zhang, X.; Li, Y.; Zhou, F.; Zhu, Y.; et al. Combined model with acoustic radiation force impulse to rule out high-risk varices in HBV-related cirrhosis with viral suppression. Dig. Liver Dis. 2023, 55, 1062–1071. [Google Scholar] [CrossRef]
- Mejias, M.; Garcia-Pras, E.; Gallego, J.; Mendez, R.; Bosch, J.; Fernandez, M. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J. Hepatol. 2010, 52, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fujii, L.L.; Murad, M.H.; Wang, Z.; Asrani, S.K.; Ehman, R.L.; Kamath, P.S.; Talwalkar, J.A. Liver Stiffness Is Associated with Risk of Decompensation, Liver Cancer, and Death in Patients with Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1573–1584.e2. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.J.; Belfield, K.J.; Orr, J.G.; Gordon, F.H.; Abeysekera, K.W. EASL clinical practice guidelines: Non-invasive liver tests for evaluation of liver disease severity and prognosis. Front. Gastroenterol. 2022, 13, 436–439. [Google Scholar] [CrossRef]

| N | 100 |
|---|---|
| Age (years), mean (SD) | 66.7 (±10.5) |
| Female, n (%) | 50 (50%) |
| Etiology | |
| Viral, n (%) | 33 (33%) |
| Metabolic, n (%) | 31 (31%) |
| Alcoholic, n (%) | 17 (17%) |
| Other, n (%) | 19 (19%) |
| Child–Pugh score, n (%) | |
| A | 86 (86%) |
| B | 12 (12%) |
| C | 2 (2%) |
| Previous OEV bleeding, n (%) | 10 (10%) |
| HRVs, n (%) | 20 (20%) |
| Ascites, n (%) | 20 (20%) |
| Encephalopathy, n (%) | 9 (9%) |
| β-blockers therapy, n (%) | 39 (39%) |
| Hepatocellular carcinoma, n (%) | 5 (5%) |
| ARFI liver, median (IQR) | 2.1 m/s (1.7–2.8) |
| ARFI spleen, median (IQR) | 3.3 m/s (3–3.7) |
| ASSDP, median (IQR) | 4.1 (2.4–6.7) |
| ASSAP, median (IQR) | 17.1 (9.3–35.4) |
| ALSDP, median (IQR) | 2.5 (1.4–5.2) |
| ALSAP, median (IQR) | 10.9 (5.7–30.2) |
| N | 99 |
| Short-term follow-up, median (IQR) (in months) | 14 (13–17) |
| Deaths, n (%) | 6 (6%) |
| OEV bleeding, n (%) | 6 (6%) |
| Ascites, n (%) | 12 (12%) |
| HRVs, n (%) | 15 (15%) |
| N | 82 |
| Long-term follow-up, median (IQR) (in months) | 46 (44–48) |
| Deaths, n (%) | 11 (13%) |
| OEV bleeding, n (%) | 12 (15%) |
| Ascites, n (%) | 33 (40%) |
| HRVs, n (%) | 26 (32%) |
| Prospective Study | Cross-Sectional Study | ||
|---|---|---|---|
| Short-Term Follow-Up | Long-Term Follow-Up | ||
| aRR (95% CI), p | aRR (95% CI), p | aOR (95% CI), p | |
| Death | |||
| ARFI liver | 0.89 (0.41–1.93), 0.772 | 1.35 (0.81–2.26), 0.248 | - |
| ARFI spleen | 1.65 (0.71–3.88), 0.247 | 1.37 (0.68–2.77), 0.378 | - |
| ASSDP | 0.99 (0.83–1.19), 0.911 | 1.04 (0.97–1.12), 0.264 | - |
| ASSAP | 1.00 (0.98–1.02), 0.965 | 1.00 (0.99–1.01), 0.423 | - |
| ALSDP | 0.91 (0.70–1.19), 0.511 | 1.05 (0.97–1.15), 0.240 | - |
| ALSAP | 0.99 (0.95–1.03), 0.629 | 1.01 (0.99–1.02), 0.439 | - |
| Ascites | |||
| ARFI liver | 1.17 (0.62–2.22), 0.622 | 1.42 (1.02–1.98), 0.039 | - |
| ARFI spleen | 2.58 (1.27–5.24), 0.009 | 1.07 (0.68–1.67), 0.776 | - |
| ASSDP | 1.01 (0.9–1.13), 0.913 | 1.03 (0.98–1.08), 0.231 | - |
| ASSAP | 1 (0.98–1.01), 0.837 | 1 (1–1.01), 0.638 | - |
| ALSDP | 0.95 (0.84–1.08), 0.463 | 1.05 (0.98–1.12), 0.176 | - |
| ALSAP | 0.99 (0.97–1.01), 0.3 | 1 (0.99–1.01), 0.597 | - |
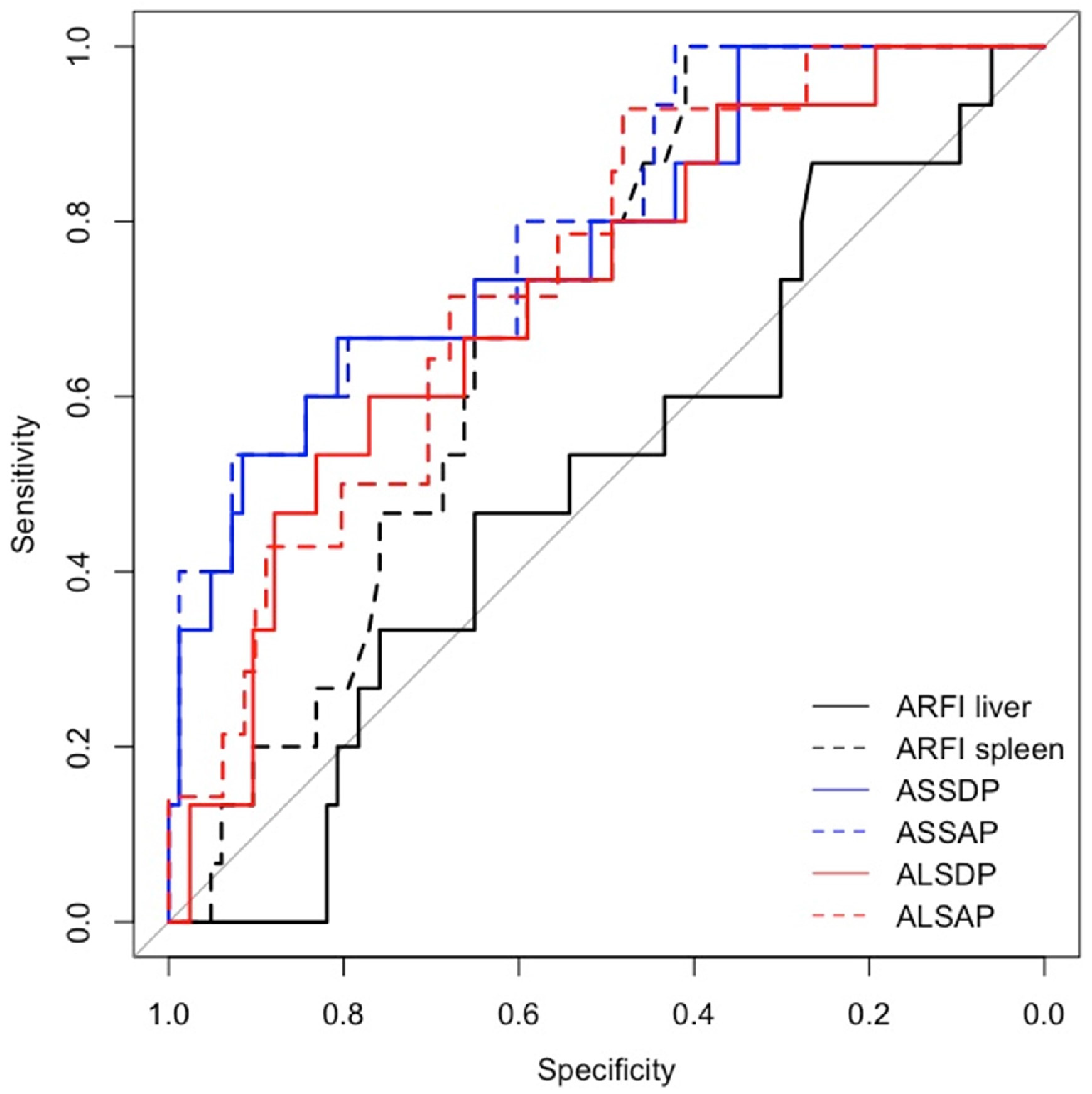
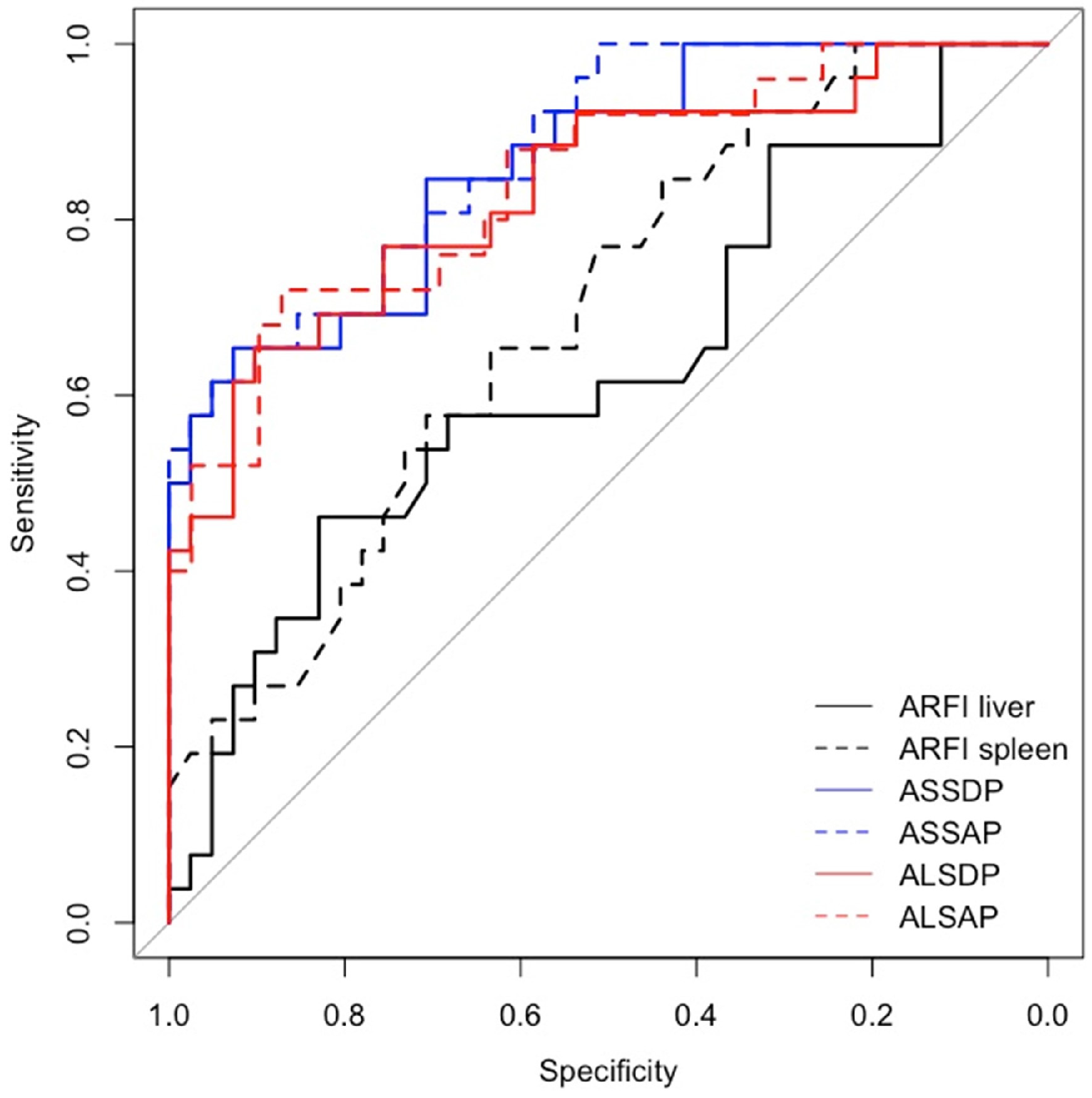
| HRVs | |||
| ARFI liver | 0.73 (0.45–1.19), 0.204 | 1.11 (0.79–1.56), 0.561 | 1.65 (0.9–3.1), 0.111 |
| ARFI spleen | 2.06 (1.06–4.03), 0.034 | 1.81 (1.11–2.96), 0.018 | 3.04 (1.23–8.64), 0.024 |
| ASSDP | 1.12 (1.02–1.22), 0.016 | 1.09 (1.04–1.15), <0.001 | 1.22 (1.09–1.39), 0.001 |
| ASSAP | 1.01 (1–1.02), 0.014 | 1.01 (1–1.02), 0.001 | 1.03 (1.01–1.05), 0.002 |
| ALSDP | 1.07 (0.97–1.18), 0.172 | 1.13 (1.05–1.22), 0.002 | 1.21 (1.06–1.4), 0.005 |
| ALSAP | 1.01 (1–1.02), 0.165 | 1.01 (1–1.02), 0.007 | 1.03 (1.01–1.05), 0.004 |
| OEV Bleeding | |||
| ARFI liver | 0.43 (0.18–1.03), 0.057 | 0.6 (0.29–1.25), 0.176 | - |
| ARFI spleen | 0.95 (0.38–2.35), 0.907 | 1.65 (0.71–3.84), 0.244 | - |
| ASSDP | 1.05 (0.91–1.21), 0.477 | 1.03 (0.93–1.13), 0.612 | - |
| ASSAP | 1.01 (0.99–1.02), 0.411 | 1 (0.99–1.02), 0.606 | - |
| ALSDP | 0.99 (0.85–1.14), 0.843 | 0.96 (0.84–1.11), 0.611 | - |
| ALSAP | 1 (0.98–1.02), 0.875 | 1 (0.98–1.02), 0.732 | - |
| T 0 | Short-Term Follow-Up | Long-Term Follow-Up | |
|---|---|---|---|
| AUC (CI 95%) | AUC (95% CI) | AUC (95% CI) | |
| ARFI liver | 0.63 (0.48–0.78) | 0.5 (0.35–0.66) | 0.63 (0.49–0.77) |
| ARFI spleen | 0.67 (0.55–0.8) | 0.69 (0.57–0.81) | 0.69 (0.56–0.82) |
| ASSDP | 0.77 (0.66–0.88) | 0.78 (0.65–0.91) | 0.86 (0.78–0.95) |
| ASSAP | 0.78 (0.68–0.89) | 0.8 (0.68–0.92) | 0.88 (0.79–0.96) |
| ALSDP | 0.75 (0.63–0.87) | 0.72 (0.58–0.86) | 0.83 (0.73–0.94) |
| ALSAP | 0.76 (0.65–0.88) | 0.74 (0.61–0.87) | 0.84 (0.74–0.94) |
| Child–Pugh score | 0.53 (0.44–0.63) | 0.58 (0.46–0.69) | 0.58 (0.49–0.67) |
| Baveno VII criteria | 0.61 (0.48–0.73) | 0.5 (0.37–0.64) | 0.59 (0.48–0.7) |
| Expanded Baveno VI criteria | 0.61 (0.5–0.73) | 0.52 (0.41–0.63) | 0.58 (0.49–0.67) |
| Colecchia criteria | 0.54 (0.47–0.61) | 0.48 (0.45–0.5) | 0.54 (0.49–0.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vainieri, A.F.M.; Brando, E.; De Vincentis, A.; Di Pasquale, G.; Flagiello, V.; Gallo, P.; Barone, F.; Massaro Cenere, T.; Di Matteo, E.; Picardi, A.; et al. Acoustic Radiation Forced Impulse of the Liver and the Spleen, Combined with Spleen Dimension and Platelet Count in New Ratio Scores, Identifies High-Risk Esophageal Varices in Well-Compensated Cirrhotic Patients. Diagnostics 2024, 14, 685. https://doi.org/10.3390/diagnostics14070685
Vainieri AFM, Brando E, De Vincentis A, Di Pasquale G, Flagiello V, Gallo P, Barone F, Massaro Cenere T, Di Matteo E, Picardi A, et al. Acoustic Radiation Forced Impulse of the Liver and the Spleen, Combined with Spleen Dimension and Platelet Count in New Ratio Scores, Identifies High-Risk Esophageal Varices in Well-Compensated Cirrhotic Patients. Diagnostics. 2024; 14(7):685. https://doi.org/10.3390/diagnostics14070685
Chicago/Turabian StyleVainieri, Antonio F. M., Elisa Brando, Antonio De Vincentis, Giulia Di Pasquale, Valentina Flagiello, Paolo Gallo, Francesca Barone, Teresa Massaro Cenere, Evelyn Di Matteo, Antonio Picardi, and et al. 2024. "Acoustic Radiation Forced Impulse of the Liver and the Spleen, Combined with Spleen Dimension and Platelet Count in New Ratio Scores, Identifies High-Risk Esophageal Varices in Well-Compensated Cirrhotic Patients" Diagnostics 14, no. 7: 685. https://doi.org/10.3390/diagnostics14070685
APA StyleVainieri, A. F. M., Brando, E., De Vincentis, A., Di Pasquale, G., Flagiello, V., Gallo, P., Barone, F., Massaro Cenere, T., Di Matteo, E., Picardi, A., & Galati, G., on behalf of Ultrasound Research Group (URG) of Fondazione Policlinico Universitario Campus Bio-Medico of Rome. (2024). Acoustic Radiation Forced Impulse of the Liver and the Spleen, Combined with Spleen Dimension and Platelet Count in New Ratio Scores, Identifies High-Risk Esophageal Varices in Well-Compensated Cirrhotic Patients. Diagnostics, 14(7), 685. https://doi.org/10.3390/diagnostics14070685







