A Web-Based Dynamic Nomogram to Predict the Risk of Methicillin-Resistant Staphylococcal Infection in Patients with Pneumonia
Abstract
1. Introduction
2. Materials and Methods
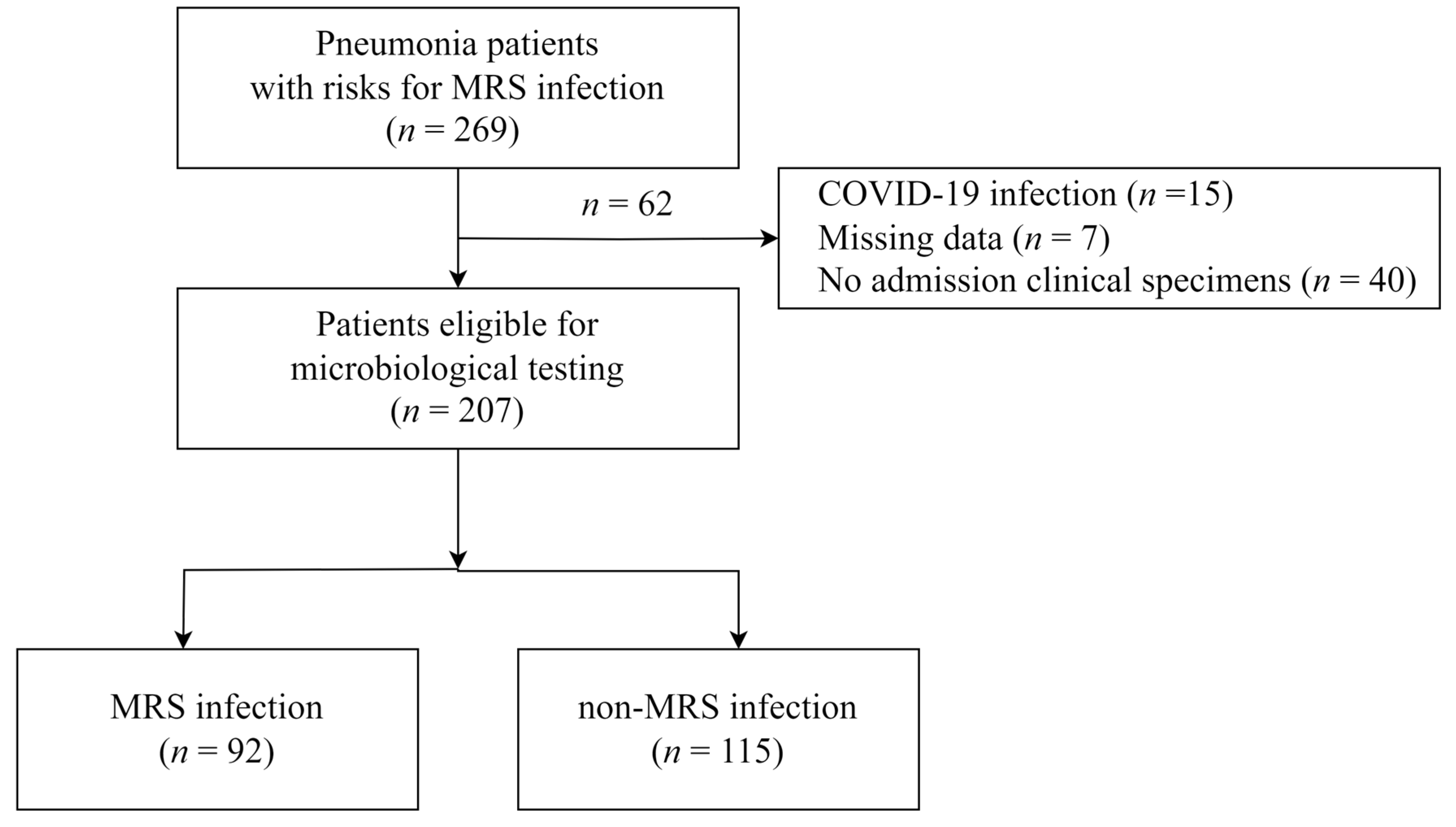
2.1. Study Population and Design
2.2. Data Collection
2.3. Definition of Variables
2.4. Statistical Analysis
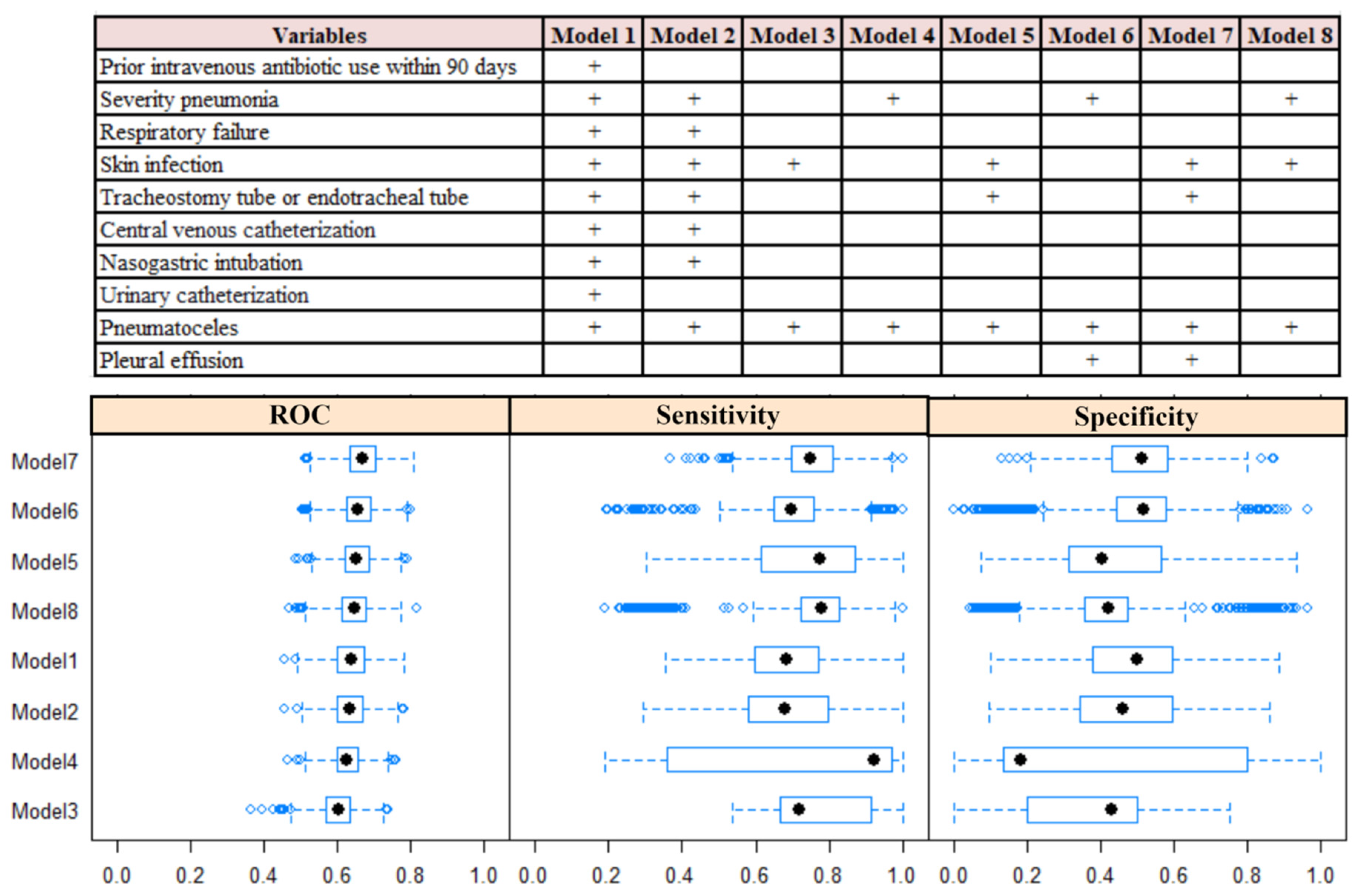
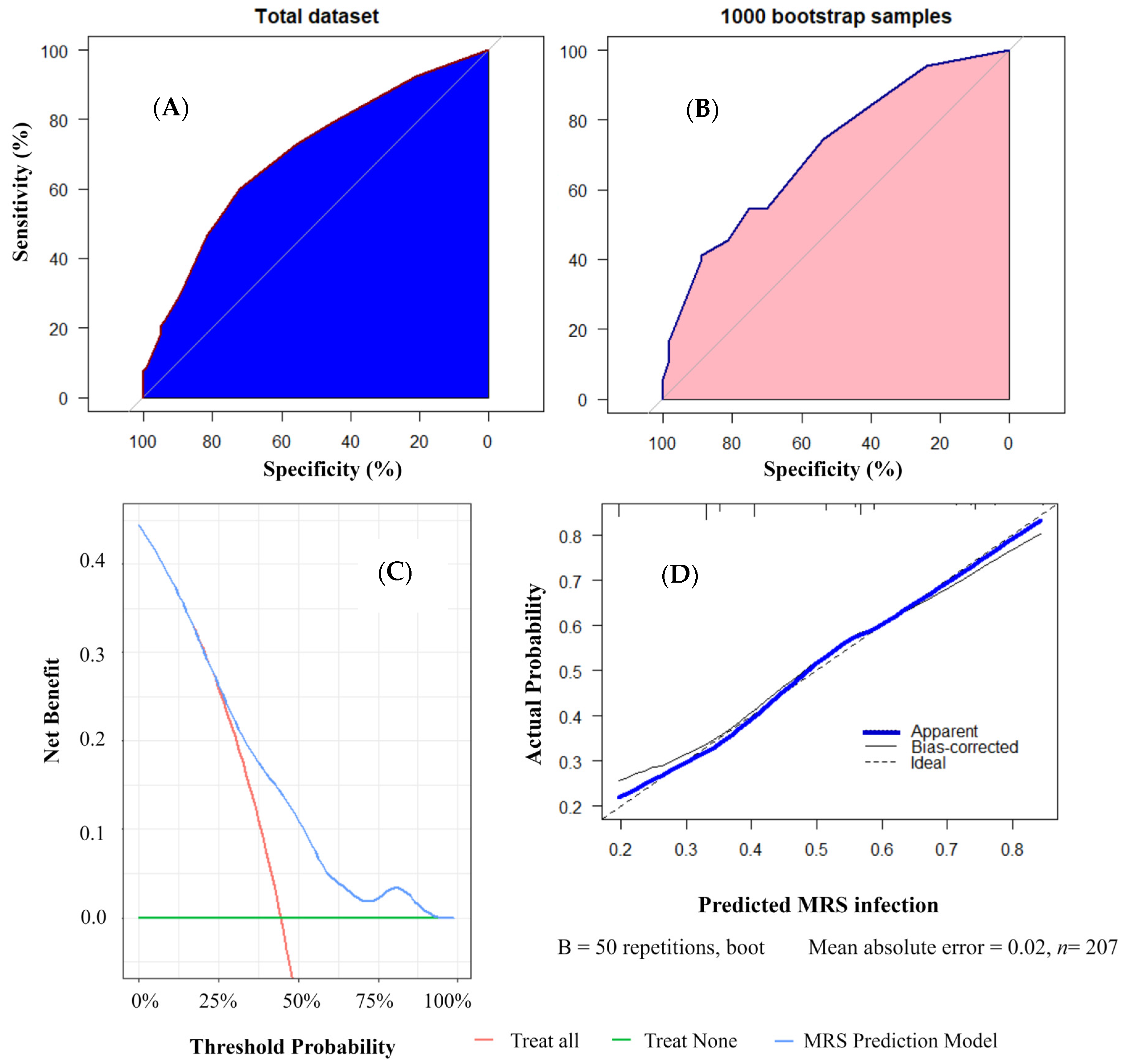
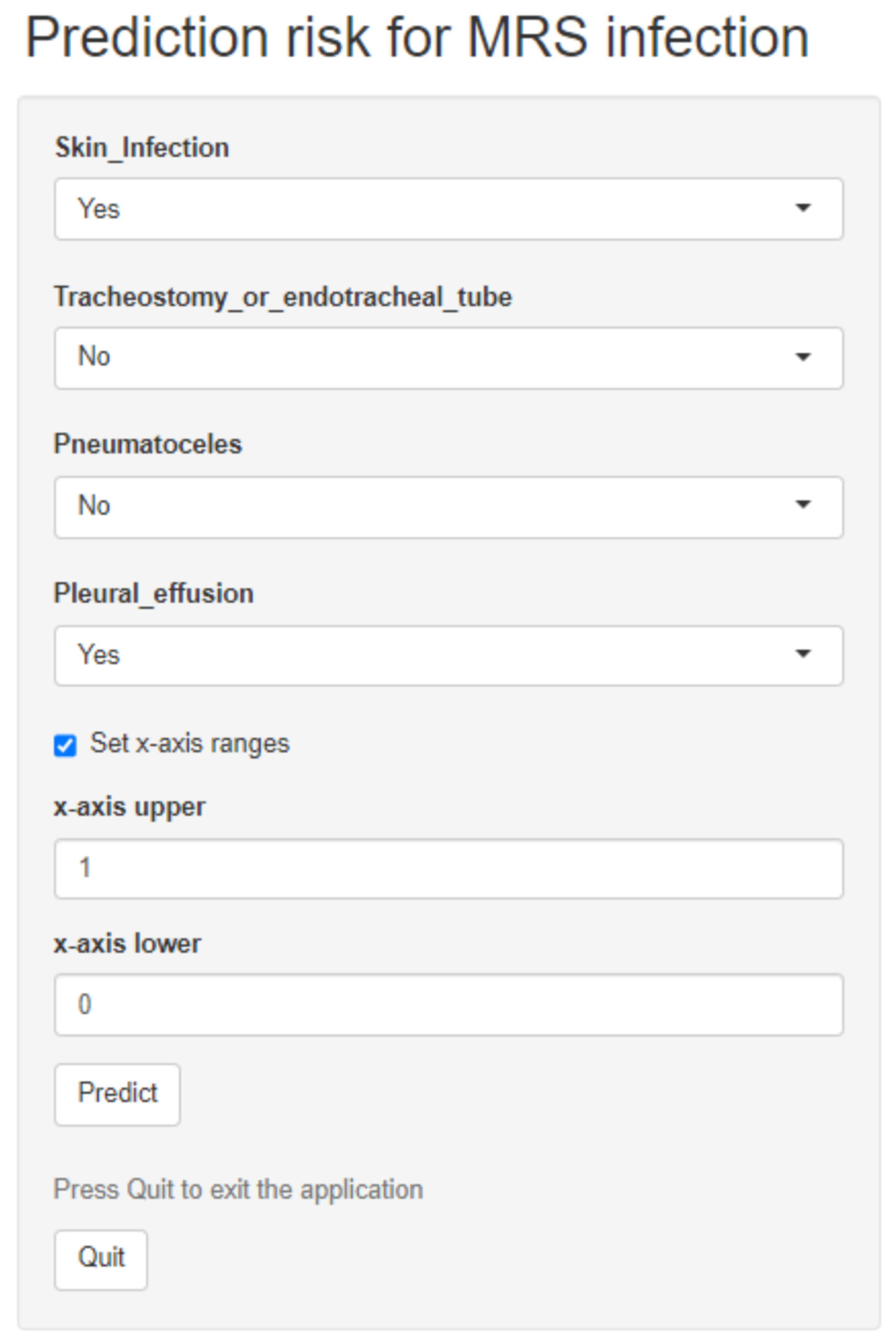
2.5. Predictive Model Development and Validation
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Predictive Model Development and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chastre, J.; Blasi, F.; Masterton, R.G.; Relio, J.; Torres, A.; Welte, T. European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014, 20, 19–36. [Google Scholar] [CrossRef]
- National Healthcare Safety Network. Pneumonia (Ventilator-Associated [VAP] and Non-Ventilator Associated Pneumonia [PNEU]) Event [Internet]. 2023. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf (accessed on 8 February 2024).
- Meyer, E.; Schwab, F.; Gastmeier, P. Nosocomial methicillin resistant Staphylococcus aureus pneumonia—Epidemiology and trends based on data of a network of 586 German ICUs (2005–2009). Eur. J. Med. Res. 2010, 15, 514–524. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Zhang, M.; Sun, X.; Li, Y.; Hu, X.; Gu, S.; Gu, Y.; Wei, J.; Dong, H. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: A multicentre study in China. Sci. Rep. 2020, 10, 3900. [Google Scholar] [CrossRef]
- Leem, A.Y.; Jung, W.J.; Kang, Y.A.; Park, S.C.; Kim, Y.J.; Hwang, E.D.; Kim, E.Y.; Jung, K.S.; Park, M.S.; Kim, S.Y.; et al. Comparison of Methicillin-Resistant Staphylococcus aureus Community-Acquired and Healthcare-Associated Pneumonia. Yonsei Med. J. 2014, 55, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Torre-Cisneros, J.; Natera, C.; Trikic, M.; Rodríguez-Baño, J. Clinical predictors of methicillin-resistant Staphylococcus aureus in nosocomial and healthcare-associated pneumonia: A multicenter, matched case–control study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Myers, D.E.; Huang, D.B.; Nathanson, B.H.; Emons, M.F.; Kollef, M.H. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect. Dis. 2013, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.; Frei, C.R.; Mortensen, E.M. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology 2016, 21, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Jeune, I.L.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64, iii1–iii55. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Zheng, Q.; Ren, C.; Ma, W.; Yang, Y. A dynamic nomogram for predicting the risk of asthma: Development and validation in a database study. J. Clin. Lab. Anal. 2021, 35, e23820. [Google Scholar] [CrossRef]
- Feretzakis, G.; Sakagianni, A.; Loupelis, E.; Kalles, D.; Skarmoutsou, N.; Martsoukou, M.; Christopoulos, C.; Lada, M.; Petropoulou, S.; Velentza, A.; et al. Machine Learning for Antibiotic Resistance Prediction: A Prototype Using Off-the-Shelf Techniques and Entry-Level Data to Guide Empiric Antimicrobial Therapy. Healthc. Inform. Res. 2021, 27, 214–221. [Google Scholar] [CrossRef]
- American Thoracic Society. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Thu, V.P.M.; Van, D.T.T.; Thang, N.; Quyen, P.T.X.; Hoang, P.T.; Tram, B.A.; Phuoc, D.T.; Sy, D.Q. The Impact of Risk Factors on Treatment Outcomes of Nosocomial Pneumonia Due to Gram-Negative Bacteria in the Intensive Care Unit. Pulm. Ther. 2021, 7, 563–574. [Google Scholar]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; See, I.; Edwards, J.R. Bayesian model averaging: Improved variable selection for matched case-control studies. Epidemiol. Biostat. Public Health 2019, 16, e13048. [Google Scholar] [CrossRef] [PubMed]
- Van Walraven, C.; Manuel, D.G.; Desjardins, M.; Forster, A.J. Derivation and Internal Validation of a Model to Predict the Probability of Severe Acute Respiratory Syndrome Coronavirus-2 Infection in Community People. J. Gen. Intern. Med. 2021, 36, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.; Silva, M.C.; Ribeiro, C.; Silva, E.; Dias, M.; Coutinho, D.P.; Shiang, T.; Abreu, G.; Sanches, I.; Vanzeller, M. Predictive factors for methicillin-resistant Staphylococcus aureus (MRSA) infection in a MRSA screening protocol. Eur. Respir. J. 2018, 52, PA4716. [Google Scholar]
- Souvenir, D.; Anderson, D.E., Jr.; Palpant, S.; Mroch, H.; Askin, S.; Anderson, J.; Claridge, J.; Eiland, J.; Malone, C.; Garrison, M.W.; et al. Blood Cultures Positive for Coagulase-Negative Staphylococci: Antisepsis, Pseudobacteremia, and Therapy of Patients. J. Clin. Microbiol. 1998, 36, 1923–1926. [Google Scholar] [CrossRef] [PubMed]
- Ścibik, Ł.; Ochońska, D.; Gołda-Cępa, M.; Brzychczy-Włoch, M.; Kotarba, A. Microbiological analysis of tracheostomy tube biofilms and antibiotic resistance profiles of potentially pathogenic microorganisms. Otolaryngol. Pol. 2022, 76, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Defres, S.; Marwick, C.; Nathwani, D. MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia. Eur. Respir. J. 2009, 34, 1470–1476. [Google Scholar] [CrossRef]
- Farkas, A.; Lin, F.; Bui, K.; Liu, F.; An, G.L.; Pakholskiy, A.; Stavropoulos, C.F.; Lantis, J.C.; Yassin, A. Development of predictive nomograms for clinical use to quantify the risk of isolating resistance prone organisms in patients with infected foot ulcers. Epidemiol. Infect. 2019, 147, e157. [Google Scholar] [CrossRef]
- Wozniak, T.M.; Dyda, A.; Merlo, G.; Hall, L. Disease burden, associated mortality and economic impact of antimicrobial resistant infections in Australia. Lancet Reg. Health—West. Pac. 2022, 27, 100521. [Google Scholar] [CrossRef]
- Bostwick, A.D.; Jones, B.E.; Goetz, M.B.G.; Samore, M.; Jones, M. Potential Impact of Hospital-acquired Pneumonia Guidelines on Empiric Antibiotics. An Evaluation of 113 Veterans Affairs Medical Centers. Ann. Am. Thorac. Soc. 2019, 16, 1392–1398. [Google Scholar] [CrossRef]
- Jones, B.E.; Ying, J.; Ying, V.; Haroldsen, C.; He, T.; Nevers, M.; Christensen, M.A.; Nelson, R.E.; Stoddard, G.J.; Sauer, B.C.; et al. Empirical Anti-MRSA vs Standard Antibiotic Therapy and Risk of 30-Day Mortality in Patients Hospitalized for Pneumonia. JAMA Intern. Med. 2020, 180, 552–560. [Google Scholar] [CrossRef]
- Case, A.; Charalambous, L.T.; Seidelman, J.; Hendershot, E.; Jiranek, W.; Bolognesi, M.; Seyler, T. Poor Outcomes in the Treatment of Coagulase-Negative Staphylococci Periprosthetic Joint Infections. Open Forum Infect. Dis. 2021, 8 (Suppl. 1), S233. [Google Scholar] [CrossRef]
- Sagan, A. Sample size in multilevel structural equation modeling—The monte carlo approach. Adv. Appl. Data Anal. 2019, 23, 63–79. [Google Scholar] [CrossRef]
- VanVoorhis, C.R.W.; Morgan, B.L. Understanding Power and Rules of Thumb for Determining Sample Sizes. Tutor. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Beleites, C.; Neugebauer, U.; Bocklitz, T.; Krafft, C.; Popp, J. Sample size planning for classification models. Anal. Chim. Acta 2013, 760, 25–33. [Google Scholar] [CrossRef]
- Osama, S.; Zafar, K.; Sadiq, M.U. Predicting Clinical Outcome in Acute Ischemic Stroke Using Parallel Multi-parametric Feature Embedded Siamese Network. Diagnostics 2020, 10, 858. [Google Scholar] [CrossRef]
| Variables, n (%) | Total | Non-MRS Infection (n = 115) | MRS Infection (n = 92) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 61 (29.47) | 33 (28.70) | 28 (30.43) | 0.905 |
| Male | 146 (70.53) | 82 (71.30) | 64 (69.57) | |
| Comorbidities | ||||
| Myocardial infartion | 4 (1.93) | 1 (0.87) | 3 (3.26) | 0.463 |
| Congestive heart failure | 21 (10.14) | 9 (7.83) | 12 (13.04) | 0.316 |
| Peripheral vascular disease | 3 (1.45) | 2 (1.74) | 1 (1.09) | 1.000 |
| Solid tumor | 6 (2.90) | 1 (0.87) | 5 (5.43) | 0.126 |
| Cerebrovascular disease | 7 (3.38) | 5 (4.35) | 2 (2.17) | 0.636 |
| Ulcer disease | 13 (6.28) | 6 (5.22) | 7 (7.61) | 0.677 |
| Diabetes | 31 (14.98) | 16 (13.91) | 15 (16.30) | 0.777 |
| Chronic obstructive pulmonary disease | 33 (15.94) | 19 (16.52) | 14 (15.22) | 0.949 |
| Moderate or severe renal disease | 37 (17.87) | 22 (19.13) | 15 (16.30) | 0.730 |
| Diabetes with complications | 42 (20.29) | 20 (17.39) | 22 (23.91) | 0.324 |
| Hemiplegia | 18 (8.70) | 11 (9.57) | 7 (7.61) | 0.804 |
| Moderate or severe liver disease | 16 (7.73) | 8 (6.96) | 8 (8.70) | 0.839 |
| Metastatic solid tumor | 13 (6.28) | 9 (7.83) | 4 (4.35) | 0.461 |
| AIDS | 4 (1.93) | 1 (0.87) | 3 (3.26) | 0.463 |
| Previously infected with MRSA | 6 (2.90) | 1 (0.87) | 5 (5.43) | 0.126 |
| Prior intravenous antibiotic use within 90 days | 153 (73.91) | 79 (68.70) | 74 (80.43) | 0.080 |
| After surgery | 25 (12.08) | 13 (11.30) | 12 (13.04) | 0.867 |
| Immunodeficiency | 38 (18.36) | 21 (18.26) | 17 (18.48) | 1.000 |
| Severity of pneumonia | 148 (71.50) | 72 (62.61) | 76 (82.61) | 0.003 |
| Fever | 17 (8.21) | 9 (7.83) | 8 (8.70) | 1.000 |
| Sputum | 197 (95.17) | 112 (97.39) | 85 (92.39) | 0.180 |
| Dyspnea | 179 (86.47) | 95 (82.61) | 84 (91.30) | 0.107 |
| Chest pain | 38 (18.36) | 20 (17.39) | 18 (19.57) | 0.825 |
| Skin infection | 67 (32.37) | 29 (25.22) | 38 (41.30) | 0.021 |
| Respiratory failure | 151 (72.95) | 76 (66.09) | 75 (81.52) | 0.020 |
| Sedative drug use | 23 (11.11) | 12 (10.43) | 11 (11.96) | 0.902 |
| Shock | 36 (17.39) | 20 (17.39) | 16 (17.39) | 1.000 |
| Dialysis | 13 (6.28) | 5 (4.35) | 8 (8.70) | 0.321 |
| Tracheostomy tube or endotracheal tube | 88 (42.51) | 39 (33.91) | 49 (53.26) | 0.008 |
| Central venous catheterization | 87 (42.03) | 40 (34.78) | 47 (51.09) | 0.026 |
| Urinary catheterization | 87 (42.03) | 41 (35.65) | 46 (50.00) | 0.053 |
| Nasogastric intubation | 90 (43.48) | 41 (35.65) | 49 (53.26) | 0.016 |
| Pleural drainage | 15 (7.25) | 5 (4.35) | 10 (10.87) | 0.126 |
| Insulin therapy | 52 (25.12) | 25 (21.74) | 27 (29.35) | 0.274 |
| Consolidation | 201 (97.10) | 113 (98.26) | 88 (95.65) | 0.487 |
| Pulmonary cavities | 20 (9.66) | 13 (11.30) | 7 (7.61) | 0.511 |
| Pneumatoceles | 21 (10.14) | 6 (5.22) | 15 (16.30) | 0.017 |
| Pleural effusion | 101 (48.79) | 50 (43.48) | 51 (55.43) | 0.116 |
| Levels of BMI | ||||
| Underweight | 55 (26.57) | 31 (26.96) | 24 (26.09) | 0.526 |
| Normal range | 95 (45.89) | 55 (47.83) | 40 (43.48) | |
| Overweight | 32 (15.46) | 14 (12.17) | 18 (19.57) | |
| Obese | 25 (12.08) | 15 (13.04) | 10 (10.87) | |
| Types of nosocomial pneumonia | ||||
| HAP | 113 (54.59) | 67 (58.26) | 46 (50.00) | 0.493 |
| VAP | 31 (14.98) | 16 (13.91) | 15 (16.30) | |
| CAP | 63 (30.43) | 32 (27.83) | 31 (33.70) | |
| Levels of age | ||||
| Under 60 | 91 (43.96) | 52 (45.22) | 39 (42.39) | 0.790 |
| Above 60 | 116 (56.04) | 63 (54.78) | 53 (57.61) | |
| Age (mean [SD]) | 60.47 ± 16.03 | 60.23 ± 15.85 | 60.78 ± 16.32 | 0.805 |
| Commodity Channel Index | 2 (0–8.85) | 2 (0–9) | 2 (0–6.73) | 0.982 |
| Variables, n (%) | OR | 95%Cl | p-Value |
|---|---|---|---|
| Prior intravenous antibiotic use within 90 days | 1.71 | 0.83–3.65 | 0.153 |
| Severity of pneumonia | 1.72 | 0.65–4.71 | 0.279 |
| Skin infection | 1.89 | 1.00–3.62 | 0.052 |
| Respiratory failure | 1.27 | 0.46–3.45 | 0.643 |
| Tracheostomy tube or endotracheal tube | 1.26 | 0.50–3.19 | 0.626 |
| Central venous catheterization | 1.32 | 0.47–3.72 | 0.594 |
| Nasogastric intubation | 3.12 | 0.66–15.92 | 0.154 |
| Urinary catheterization | 0.36 | 0.07–1.49 | 0.175 |
| Pneumatoceles | 5.11 | 1.81–16.23 | 0.003 |
| Threshold | Dataset | Bootstrap | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| 0.1 | - | - | - | - |
| 0.15 | - | - | - | - |
| 0.2 | 92.39 | 20.87 | 91.11 | 19.66 |
| 0.25 | 92.39 | 20.87 | 91.11 | 19.66 |
| 0.3 | 92.39 | 20.87 | 91.11 | 19.66 |
| 0.35 | 72.83 | 55.65 | 63.33 | 47.86 |
| 0.4 | 59.78 | 72.17 | 46.67 | 61.54 |
| 0.45 | 59.78 | 72.17 | 46.67 | 61.54 |
| 0.5 | 59.78 | 72.17 | 46.67 | 61.54 |
| 0.55 | 51.09 | 78.26 | 38.89 | 68.38 |
| 0.6 | 21.74 | 93.91 | 15.56 | 88.89 |
| 0.65 | 21.74 | 93.91 | 15.56 | 88.89 |
| 0.7 | 21.74 | 93.91 | 15.56 | 88.89 |
| 0.75 | 8.70 | 99.13 | 3.33 | 94.87 |
| 0.8 | 7.61 | 100.0 | 2.22 | 95.73 |
| 0.85 | 4.35 | 100.0 | 1.11 | 97.44 |
| 0.9 | 1.09 | 100.0 | 0 | 99.15 |
| 0.95 | - | - | - | - |
| 1 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong-Thi-Thanh, V.; Truong-Quang, B.; Tran-Nguyen-Trong, P.; Le-Phuong, M.; Truong-Thien, P.; Lam-Quoc, D.; Dang-Vu, T.; Nguyen, M.-L.; Le-Thuong, V. A Web-Based Dynamic Nomogram to Predict the Risk of Methicillin-Resistant Staphylococcal Infection in Patients with Pneumonia. Diagnostics 2024, 14, 633. https://doi.org/10.3390/diagnostics14060633
Duong-Thi-Thanh V, Truong-Quang B, Tran-Nguyen-Trong P, Le-Phuong M, Truong-Thien P, Lam-Quoc D, Dang-Vu T, Nguyen M-L, Le-Thuong V. A Web-Based Dynamic Nomogram to Predict the Risk of Methicillin-Resistant Staphylococcal Infection in Patients with Pneumonia. Diagnostics. 2024; 14(6):633. https://doi.org/10.3390/diagnostics14060633
Chicago/Turabian StyleDuong-Thi-Thanh, Van, Binh Truong-Quang, Phu Tran-Nguyen-Trong, Mai Le-Phuong, Phu Truong-Thien, Dung Lam-Quoc, Thong Dang-Vu, Minh-Loi Nguyen, and Vu Le-Thuong. 2024. "A Web-Based Dynamic Nomogram to Predict the Risk of Methicillin-Resistant Staphylococcal Infection in Patients with Pneumonia" Diagnostics 14, no. 6: 633. https://doi.org/10.3390/diagnostics14060633
APA StyleDuong-Thi-Thanh, V., Truong-Quang, B., Tran-Nguyen-Trong, P., Le-Phuong, M., Truong-Thien, P., Lam-Quoc, D., Dang-Vu, T., Nguyen, M.-L., & Le-Thuong, V. (2024). A Web-Based Dynamic Nomogram to Predict the Risk of Methicillin-Resistant Staphylococcal Infection in Patients with Pneumonia. Diagnostics, 14(6), 633. https://doi.org/10.3390/diagnostics14060633







