Merging Two Models of One-Dimensional Convolutional Neural Networks to Improve the Differential Diagnosis between Acute Asthma and Bronchitis in Preschool Children
Abstract
1. Introduction
2. Literature Review
3. Dataset
3.1. Dataset Description
3.2. Influencing Factors
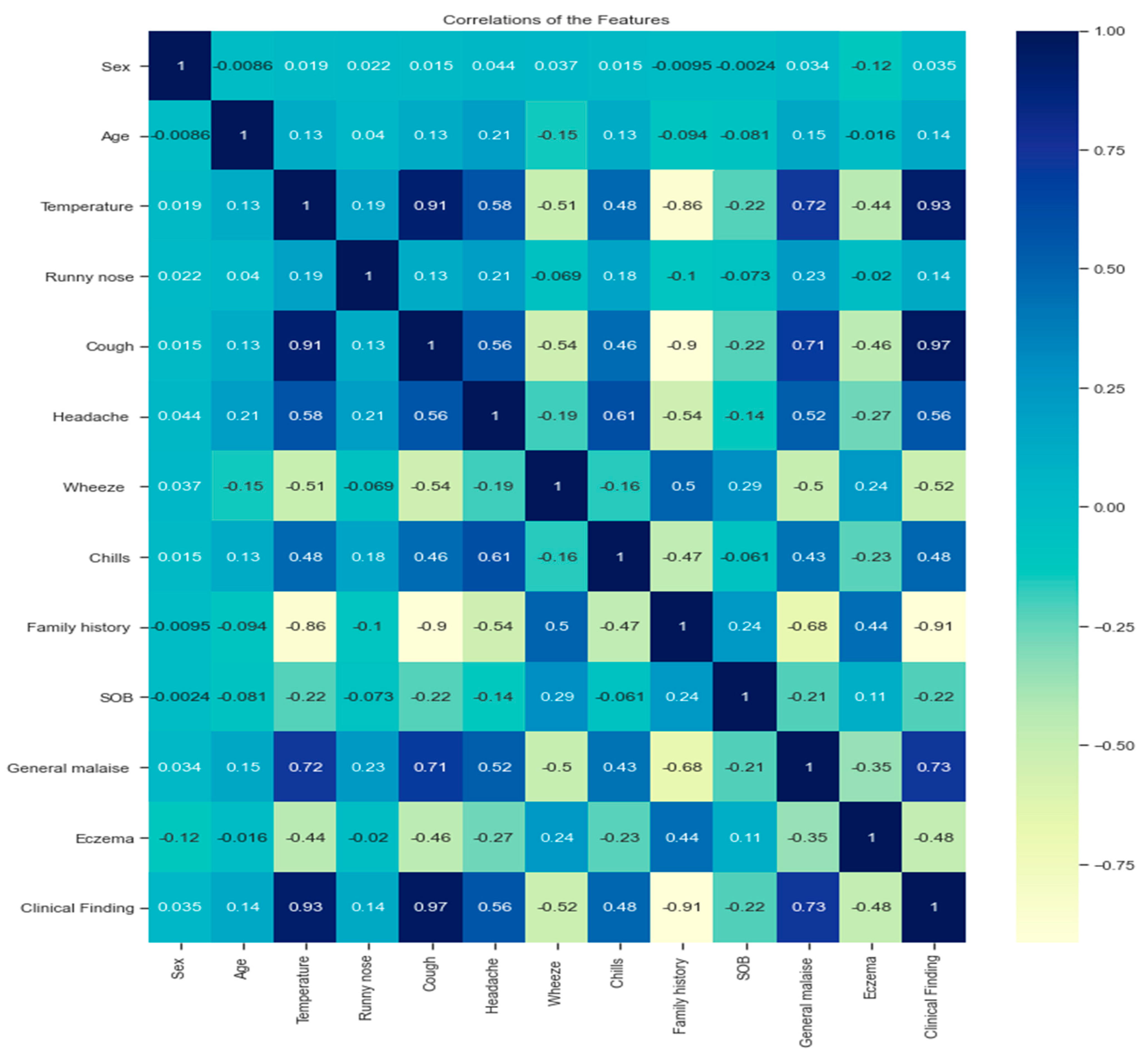
3.3. Dataset Analysis
- A strong positive correlation between the variables (cough, temperature; cough, general malaise; temperature and general malaise) can be explained by the existence of multiple linear relationships between the variables because the Pearson correlation coefficient is more significant than 0.7.
- A strong negative correlation between variables (temperature, family history; cough, family history).
- There is a strong positive correlation between some variables and the target variables (clinical findings), such as temperature, cough, and general malaise. Their effect on the target was more significant than 0.7.
- A strong negative correlation exists between family history and clinical finding.
3.4. Preprocessing Dataset
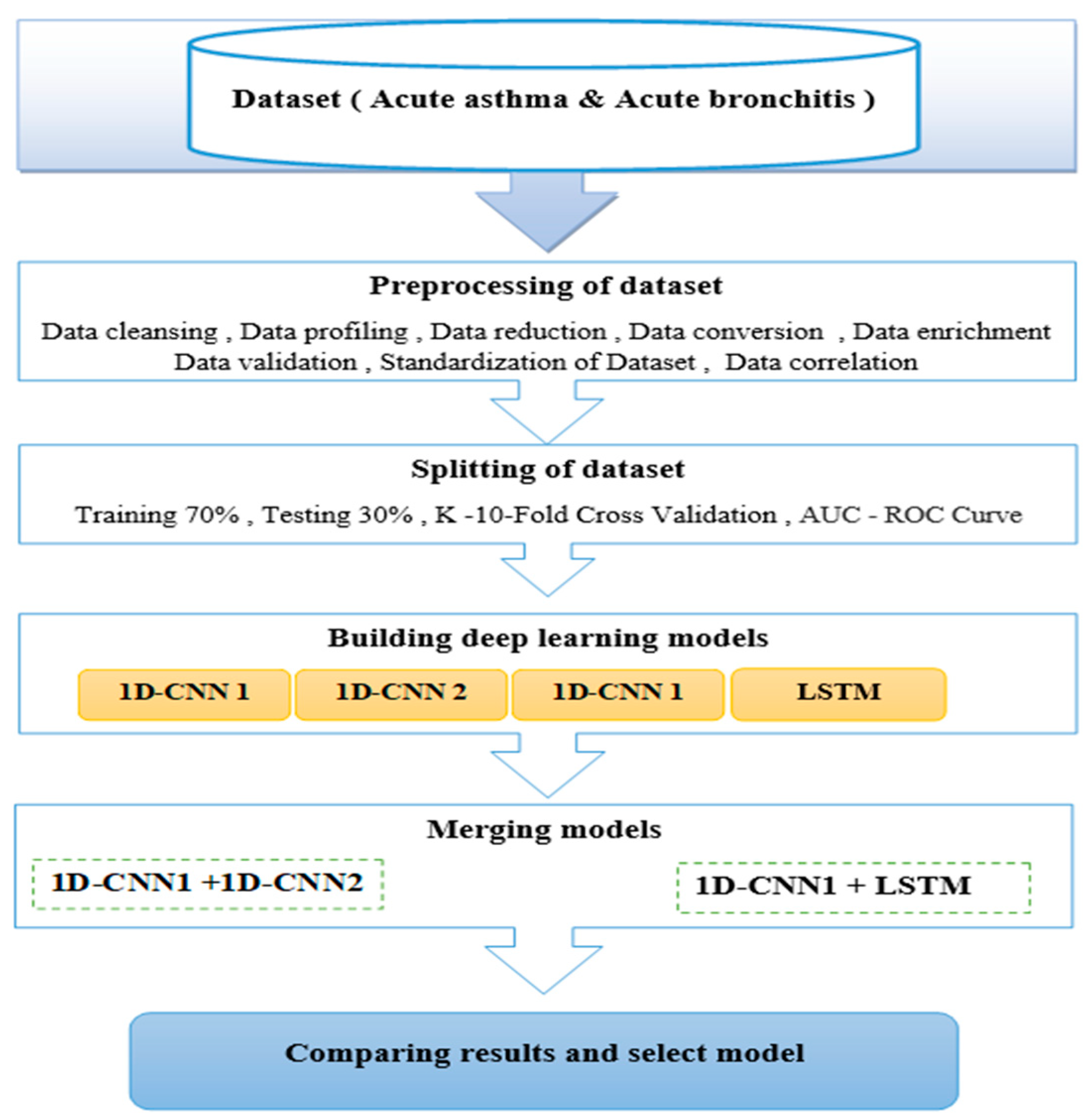
4. Methods
4.1. Proposed Method
4.2. Flowchart Architectures
4.3. One-Dimensional Neural Networks (1D-CNNs)
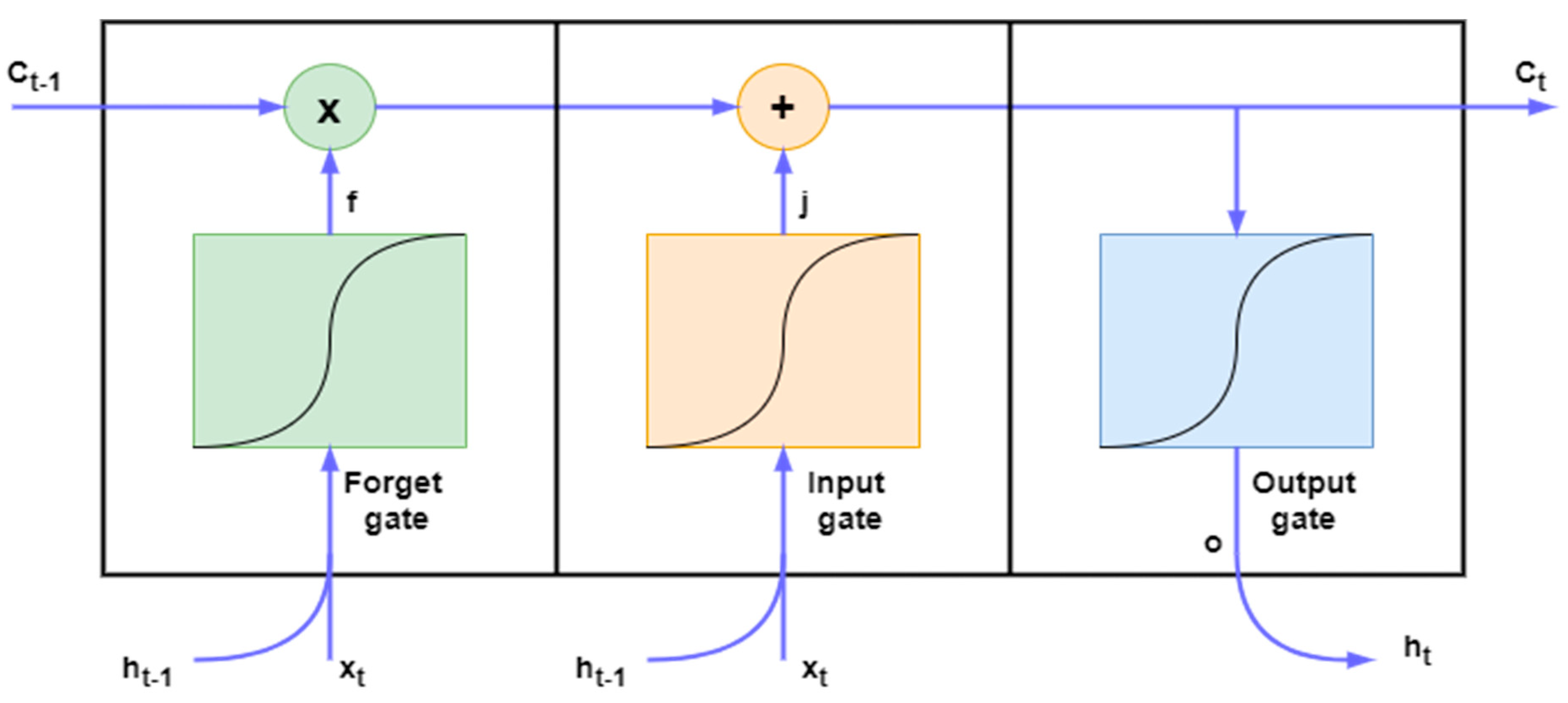
4.4. Long Short-Term Memory (LSTM)
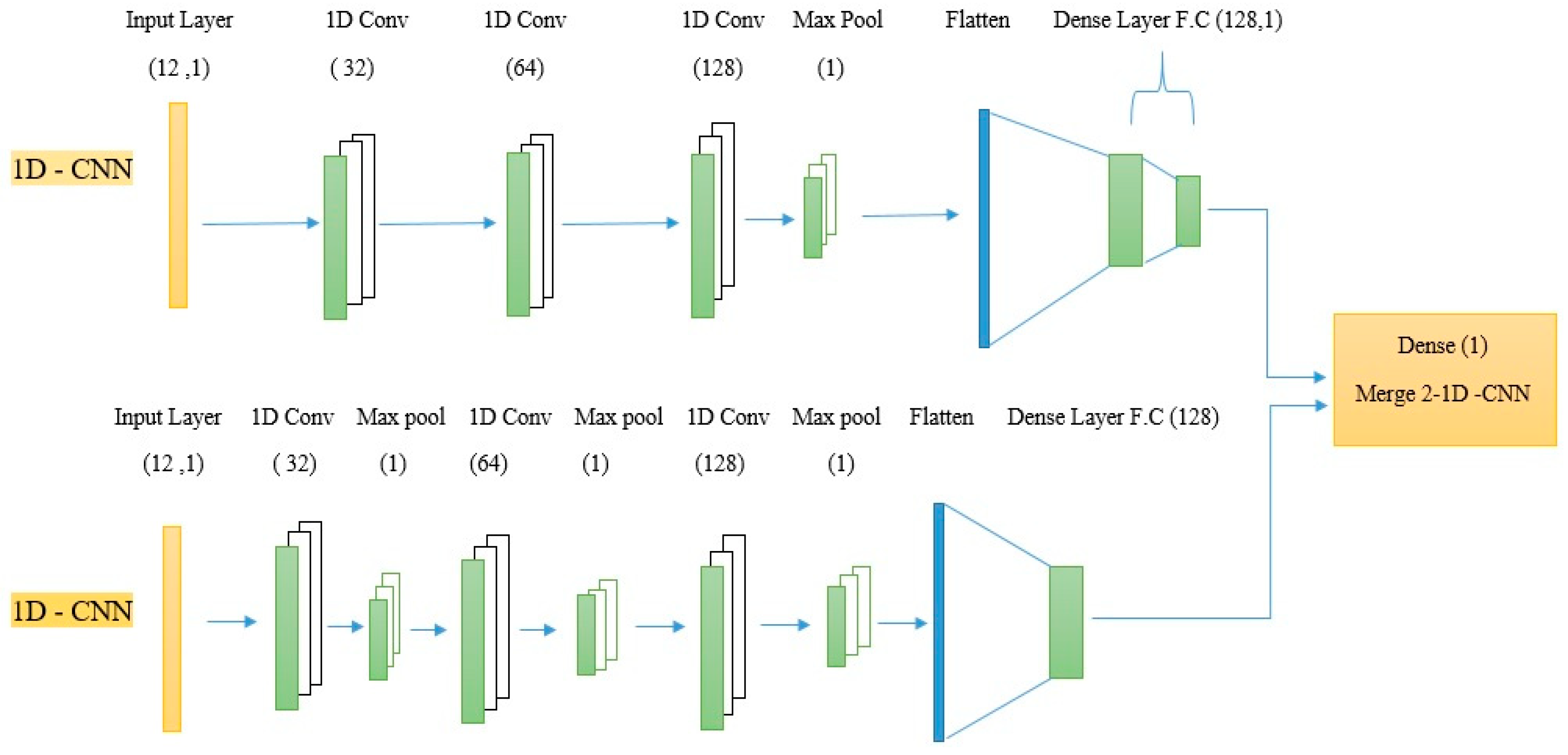
4.5. Merge 2-1D-CNNs
4.6. Merge 1D-CNNs + LSTM
5. Results and Discussion
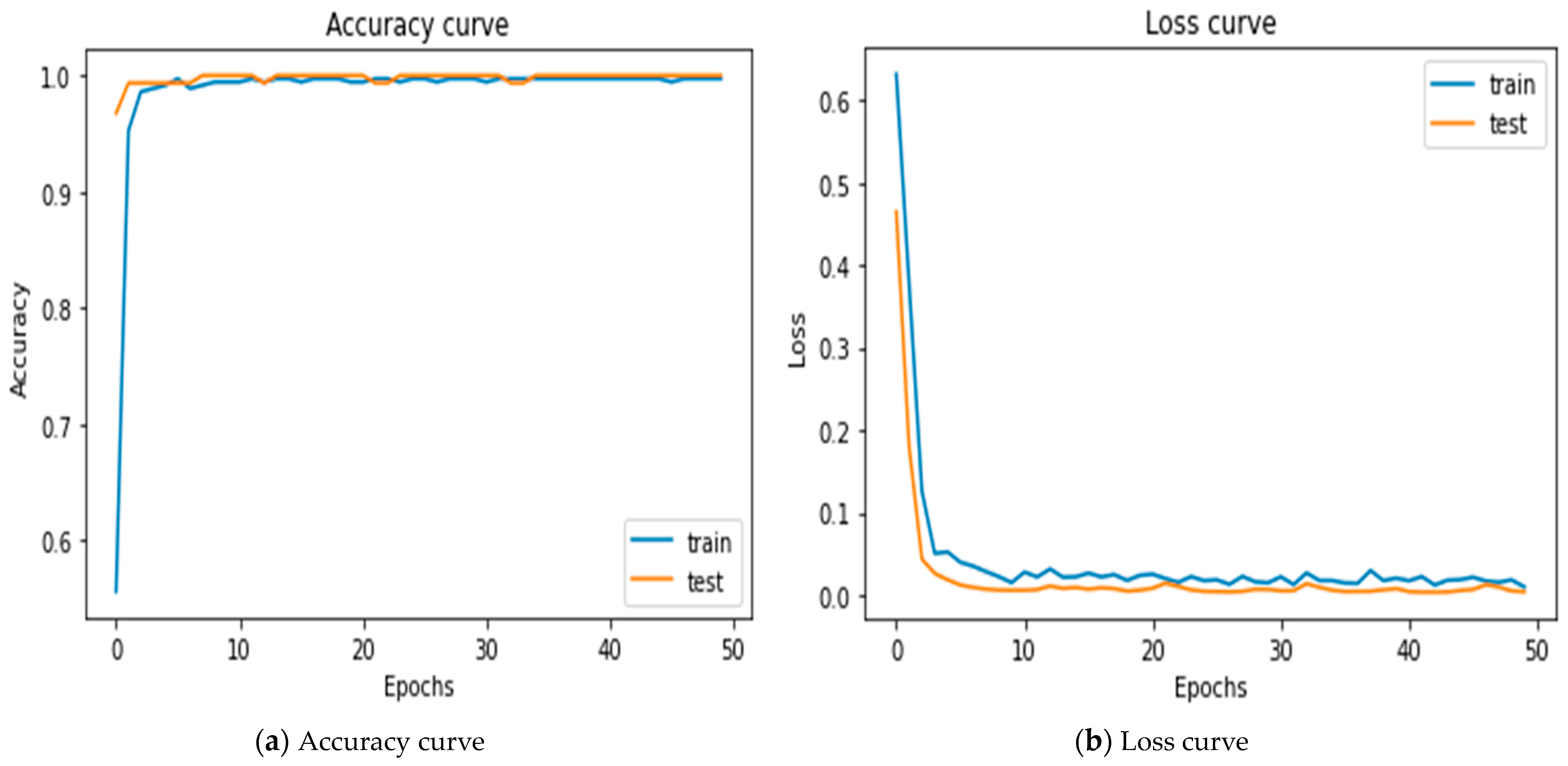
5.1. Results of Merging 2-1D-CNNs
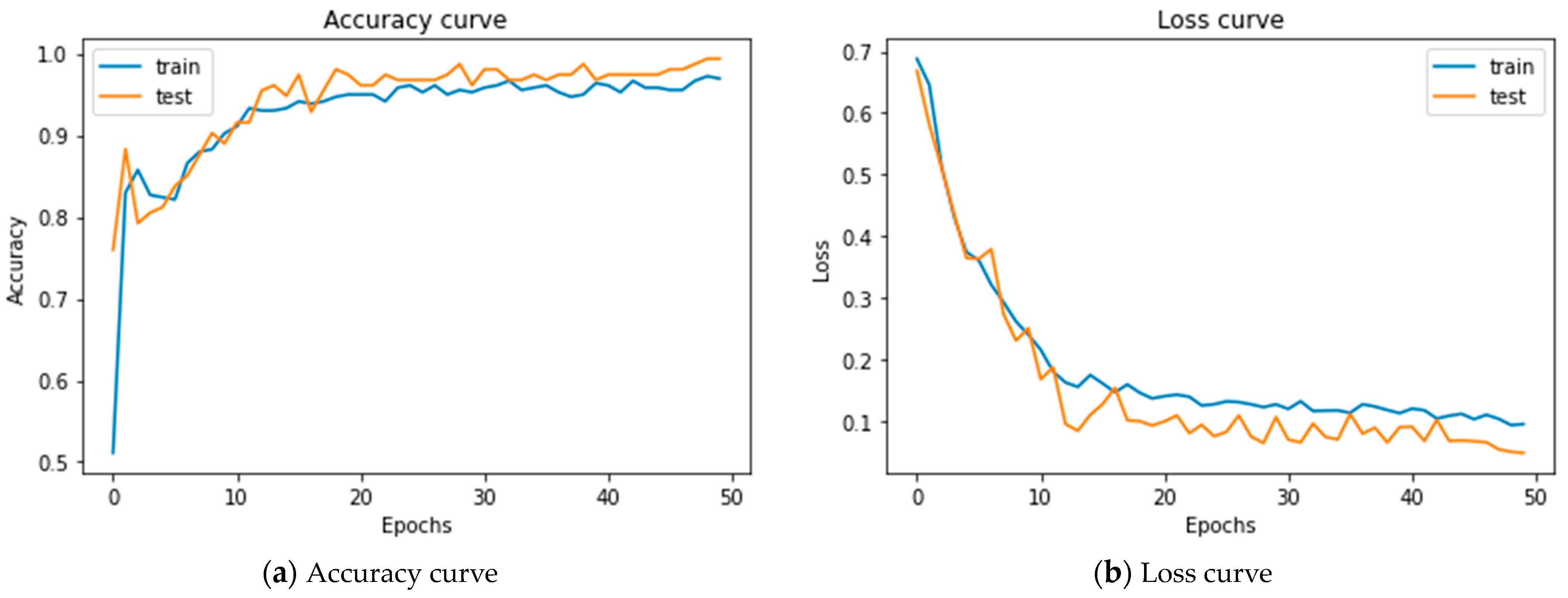
5.2. Results of Merging 1D-CNNs + LSTM
5.3. Results Comparison
5.4. Comparison of Existing Results with Previous Studies’ Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, D.; Hall, G.L.; Broadhurst, D.; Smith, A.; Schultz, A.; Foong, R.E. Does machine learning have a role in the prediction of asthma in children? Paediatr. Respir. Rev. 2022, 41, 51–60. [Google Scholar] [CrossRef]
- Stokes, K.; Castaldo, R.; Franzese, M.; Salvatore, M.; Fico, G.; Pokvic, L.G.; Badnjevic, A.; Pecchia, L. A machine learning model for supporting symptom-based referral and diagnosis of bronchitis and pneumonia in limited resource settings. Biocybern. Biomed. Eng. 2021, 41, 1288–1302. [Google Scholar] [CrossRef]
- Tam, Y.; Johansson, E.; Mersha, T.B. Multi-Omics Profiling Approach to Asthma: An Evolving Paradigm. J. Pers. Med. 2022, 12, 66. [Google Scholar]
- De Rose, C.; Sopo, S.M.; Valentini, P.; Morello, R.; Biasucci, D.; Buonsenso, D. Potential Application of Lung Ultrasound in Children with Severe Uncontrolled Asthma: Preliminary Hypothesis Based on a Case Series. Medicines 2022, 9, 11. [Google Scholar] [CrossRef]
- Olayemi, O.C.; Sunday, A.O.; Olasehinde, O.O.; Ojokoh, B.A.; Adetunmbi, A.O. Application of Machine Learning to the Diagnosis of Lower Respiratory Tract Infections in Paediatric Patients. I-Manag. J. Pattern Recognit. 2018, 5, 1–46. [Google Scholar]
- McCune, J.P.D. Reducing Unnecessary Antibiotic Treatment for Acute Bronchitis Using Real-Time, Text-Based Primary Care. Telehealth Med. Today 2021, 6, 1–9. [Google Scholar]
- Kleiman, R.; Kuusisto, F.; Ross, I.; Peissig, P.L.; Stewart, R.; Page, C.D.; Weiss, J. Machine Learning Assisted Discovery of Novel Predictive Lab Tests Using Electronic Health Record Data. AMIA Summits Transl. Sci. Proc. 2019, 2019, 572–581. [Google Scholar]
- Kim, M.; Jang, J.; Jeon, S.; Youm, S. A Study on Customized Prediction of Daily Illness Risk Using Medical and Meteorological Data. Appl. Sci. 2022, 12, 6060. [Google Scholar] [CrossRef]
- Shurrab, S.; Duwairi, R. Self-supervised learning methods and applications in medical imaging analysis: A survey. PeerJ Comput. Sci. 2022, 8, e1045. [Google Scholar] [CrossRef]
- Tong, Y.; Lin, B.; Chen, G.; Zhang, Z. Predicting Continuity of Asthma Care Using a Machine Learning Model: Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 1237. [Google Scholar] [CrossRef]
- Raita, Y.; Camargo, C.A.; Macias, C.G.; Mansbach, J.M.; Piedra, P.A.; Porter, S.C.; Teach, S.J.; Hasegawa, K. Machine learning-based prediction of acute severity in infants hospitalized for bronchiolitis: A multicenter prospective study. Sci. Rep. 2020, 10, 10979. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Avci, O.; Abdeljaber, O.; Ince, T.; Gabbouj, M.; Inman, D.J. 1D convolutional neural networks and applications: A survey. Mech. Syst. Signal Process. 2021, 151, 107398. [Google Scholar] [CrossRef]
- Balaji, E.; Brindha, D.; Elumalai, V.K.; Vikrama, R. Automatic and non-invasive Parkinson’s disease diagnosis and severity rating using LSTM network. Appl. Soft Comput. 2021, 108, 107463. [Google Scholar] [CrossRef]
- Chen, C.; Hua, Z.; Zhang, R.; Liu, G.; Wen, W. Automated arrhythmia classification based on a combination network of CNN and LSTM. Biomed. Signal Process. Control 2020, 57, 101819. [Google Scholar] [CrossRef]
- Alkhatib, M.; Hafiane, A.; Vieyres, P. Merged 1D-2D deep convolutional neural networks for nerve detection in ultrasound images. In Proceedings of the 2020 25th International Conference on Pattern Recognition (ICPR), Milan, Italy, 10–15 January 2021; pp. 4774–4780. [Google Scholar] [CrossRef]
- El-shafiey, M.G.; Hagag, A.; Ismail, M.A. A Hybrid Bidirectional LSTM and 1D CNN for Heart Disease Prediction. Int. J. Comput. Sci. Netw. Secur. 2021, 21, 135. [Google Scholar] [CrossRef]
- Ghennam, K.; Attou, F.; Abdoun, F. Impact of atmospheric pollution on asthma and bronchitis based on lichen biomonitoring using IAP, IHI and GIS in Algiers Bay (Algeria). Environ. Monit. Assess. 2021, 193, 198. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Nabeel, M.; Masood, U.; Mahmood, T.; Hashmi, M.S.; Posokhova, I.; Rizwan, A.; Imran, A. Informatics in Medicine Unlocked Towards using cough for respiratory disease diagnosis by leveraging Artificial Intelligence: A survey. Inform. Med. Unlocked 2022, 29, 100832. [Google Scholar] [CrossRef]
- Jesu, A.; Lazar, P.; Sharma, D.K.; Guna, T. Fetal Health Classification from Cardiotocographic Data Using Machine Learning. Expert Syst. 2022, 39, e12899. [Google Scholar]
- Buyrukoğlu, S.; Akbaş, A. Machine Learning based Early Prediction of Type 2 Diabetes: A New Hybrid Feature Selection Approach using Correlation Matrix with Heatmap and SFS. Balk. J. Electr. Comput. Eng. 2022, 10, 110–117. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Ince, T.; Hamila, R.; Gabbouj, M. Convolutional Neural Networks for patient-specific ECG classification. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 2608–2611. [Google Scholar] [CrossRef]
- Demir, F.; Tașcı, B. An Effective and Robust Approach Based on R-CNN+LSTM Model and NCAR Feature Selection for Ophthalmological Disease Detection from Fundus Images. J. Pers. Med. 2021, 11, 1276. [Google Scholar] [CrossRef]
- Wang, P.; Song, L.; Hao, Y.; Wang, H.; Li, S.; Cui, L. A light intelligent diagnosis model based on improved Online Dictionary Learning sample-making and simplified convolutional neural network. Measurement 2021, 183, 109813. [Google Scholar] [CrossRef]
- Zhang, Y.; Technology, S. Traitement du Signal Seedlings Supplement Device and Seedling Recognition Based on Convolution Neural Network. Trait. Signal 2022, 39, 1567–1575. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathy, H.K.; Mallick, P.K. EAGA-MLP—An Enhanced and Adaptive Hybrid. Sensors 2020, 20, 4036. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]



| Features | Acute Asthma | Acute Bronchitis |
|---|---|---|
| Sex | Male/Female | Ma/leFemale |
| Age | Under six years | Under six years |
| Temperature | Normal | Low-grade fever |
| Runny nose | +ve/−ve | +ve |
| Headage | −ve | +ve |
| Cough | +ve/dry | +ve/productive |
| Wheeze | +ve | +ve/−ve |
| Chills | −ve | +ve/−ve |
| Family history | +ve | −ve |
| Shortness of breath | +ve | +ve |
| General malaise | −ve | +ve |
| Eczema (allergic) | +ve | −ve |
| Month | Acute Asthma | Acute Bronchitis |
|---|---|---|
| March | 39 | 94 |
| April | 63 | 78 |
| May | 77 | 49 |
| June | 69 | 43 |
| Sum | 248 | 264 |
| Percentage Values | 48.4375% | 51.5625% |
| Layers | Number Neurons | Kernel Size | Activation Function |
|---|---|---|---|
| 1D Conv | 32 | 2 | Relu |
| 1D Conv | 64 | 2 | Relu |
| 1D Conv | 128 | 2 | Relu |
| 1D pool | 1 | - | - |
| Flatten | - | - | - |
| Dense | 128 | Relu | |
| Dense | 1 | - | Sigmoid |
| 1D Conv | 32 | 2 | Relu |
| 1D pool | 1 | - | - |
| 1D Conv | 64 | 2 | Relu |
| 1D pool | 1 | - | - |
| 1D Conv | 128 | 2 | Relu |
| Flatten | - | - | - |
| Dense | 128 | - | Relu |
| Dense | 1 | - | Sigmoid |
| Layers | Number Neurons | Kernel Size | Activation Function |
|---|---|---|---|
| 1D Conv | 32 | 2 | Relu |
| 1D Conv | 64 | 2 | Relu |
| 1D Conv | 128 | 2 | Relu |
| 1D pool | 1 | - | - |
| Flatten | - | - | - |
| Dense | 128 | Relu | |
| Dense | 1 | - | Sigmoid |
| LSTM | 100 | - | |
| LSTM | 100 | - | Relu |
| Dense | 128 | - | Relu |
| Dense | 64 | - | Relu |
| Dense | 32 | - | Relu |
| Dense | 1 | - | Sigmoid |
| Classifier | AUC | Parameters | Test Loss | Accuracy |
|---|---|---|---|---|
| 1D-CNN | 99.32 | 80.449 | 0.043 | 99.35 |
| LSTM | 99.86 | 144.497 | 0.056 | 98.701 |
| 1D-CNN + LSTM | 99.96 | 312.979 | 0.048 | 99.44 |
| 1D-CNN + 1D-CNN | 1.0 | 336.963 | 0.0039 | 99.72 |
| Classifier | Sensitivity | Specificity | Precision | F1-Score |
|---|---|---|---|---|
| 1D-CNNs + LSTM | 1.0 | 98.73 | 99 | 99 |
| 2-1D-CNNs | 98.66 | 1.0 | 99 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, W.; Koyuncu, H. Merging Two Models of One-Dimensional Convolutional Neural Networks to Improve the Differential Diagnosis between Acute Asthma and Bronchitis in Preschool Children. Diagnostics 2024, 14, 599. https://doi.org/10.3390/diagnostics14060599
Salih W, Koyuncu H. Merging Two Models of One-Dimensional Convolutional Neural Networks to Improve the Differential Diagnosis between Acute Asthma and Bronchitis in Preschool Children. Diagnostics. 2024; 14(6):599. https://doi.org/10.3390/diagnostics14060599
Chicago/Turabian StyleSalih, Waleed, and Hakan Koyuncu. 2024. "Merging Two Models of One-Dimensional Convolutional Neural Networks to Improve the Differential Diagnosis between Acute Asthma and Bronchitis in Preschool Children" Diagnostics 14, no. 6: 599. https://doi.org/10.3390/diagnostics14060599
APA StyleSalih, W., & Koyuncu, H. (2024). Merging Two Models of One-Dimensional Convolutional Neural Networks to Improve the Differential Diagnosis between Acute Asthma and Bronchitis in Preschool Children. Diagnostics, 14(6), 599. https://doi.org/10.3390/diagnostics14060599






