Robotic Platforms for Therapeutic Flexible Endoscopy: A Literature Review
Abstract
1. Introduction
2. Technological Features
2.1. The Direct Drive Endoscopic System (DDES)
2.2. EndoSAMURAI
2.3. Master and Slave Transluminal Endoscopic Robot (MASTER)
2.4. Endoluminal Assistant for Surgical Endoscopy (EASE)
2.5. Endoscopic Therapeutic Robot System (ETRS)
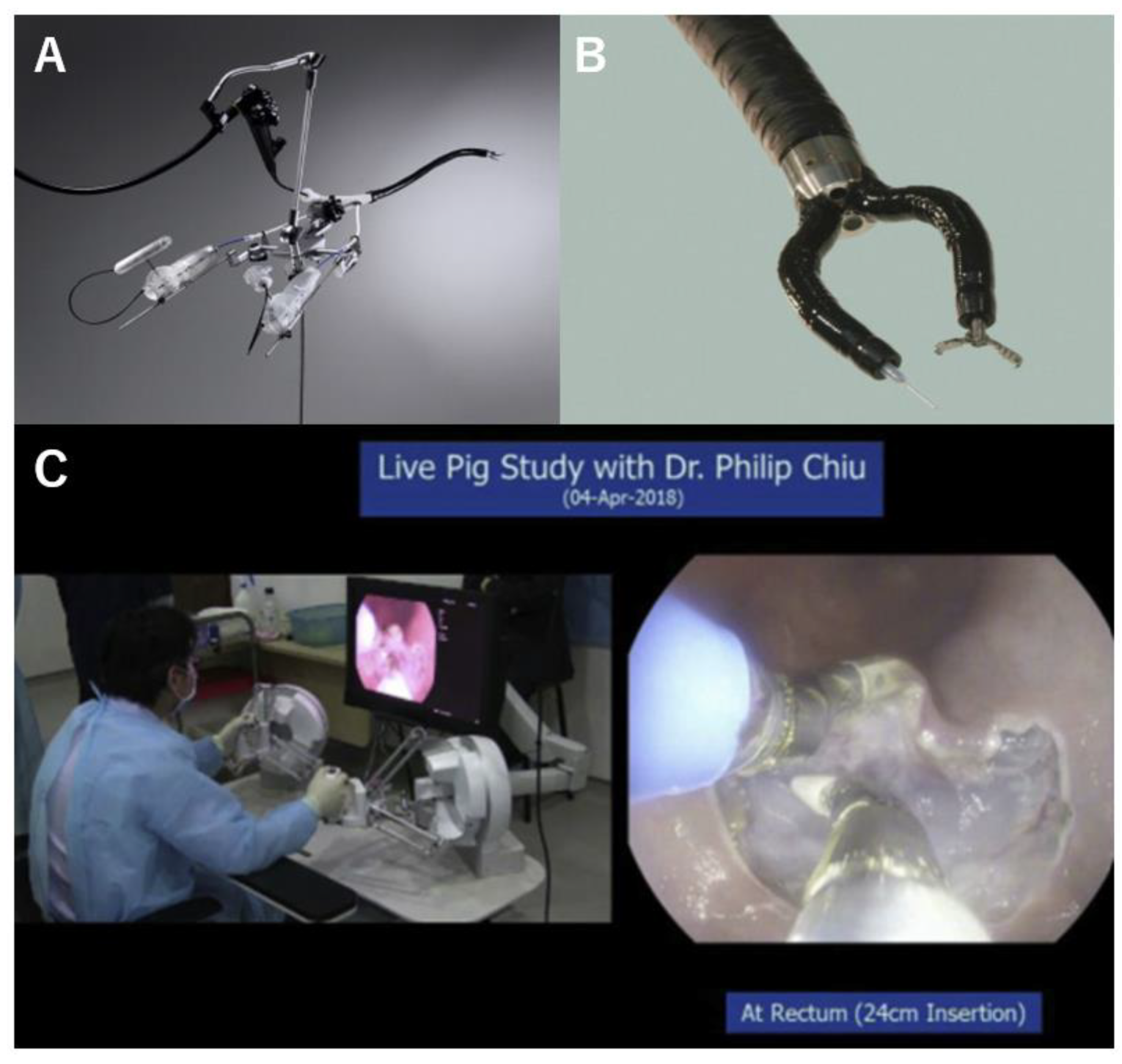
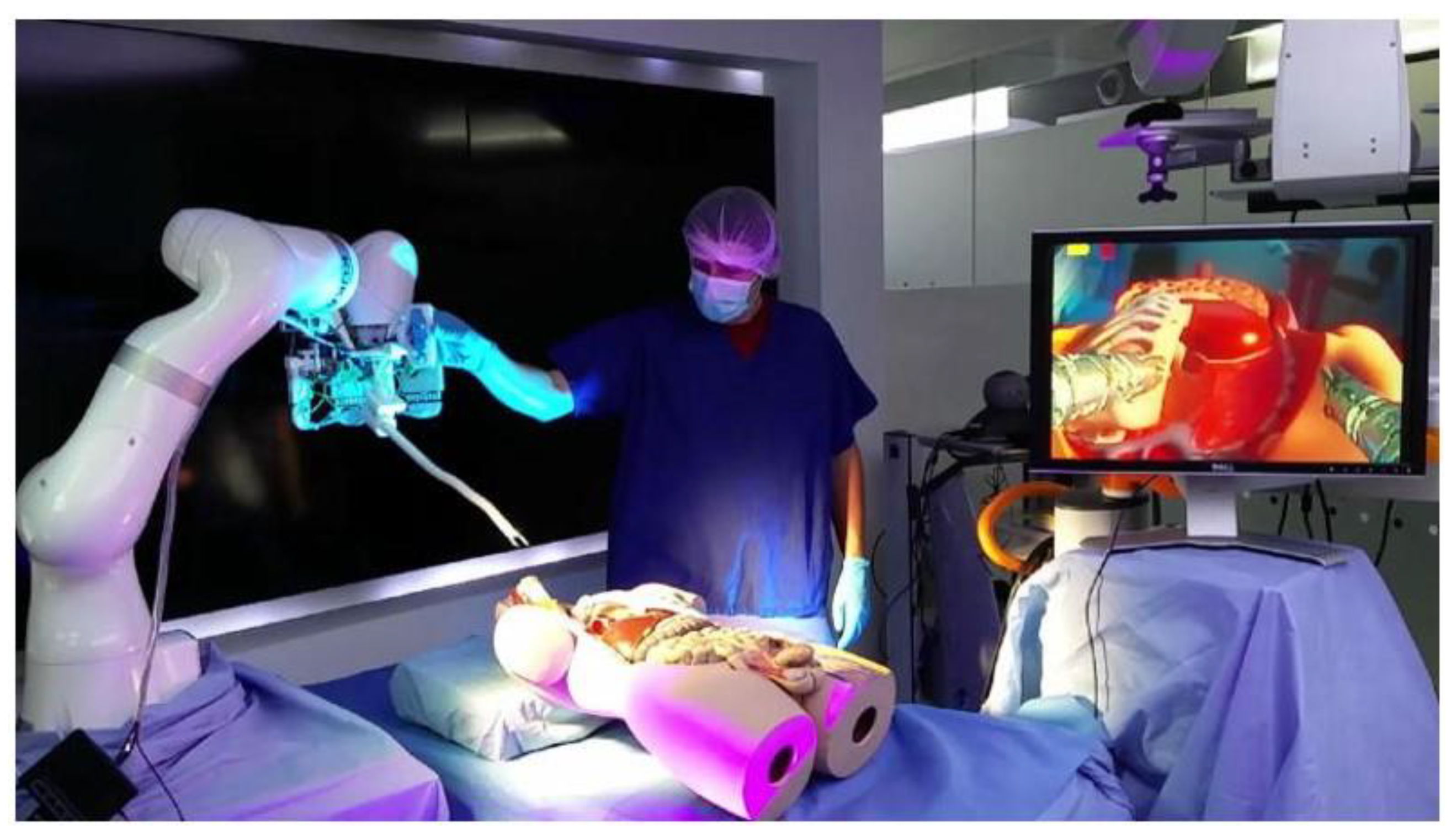
2.6. i2 Snake Robotic Platform
2.7. Flex Robotic System
2.8. Revolute Joint-Based Auxiliary Transluminal Endoscopic Robot (REXTER)
2.9. Portable Endoscopic Tool Handler (PETH)
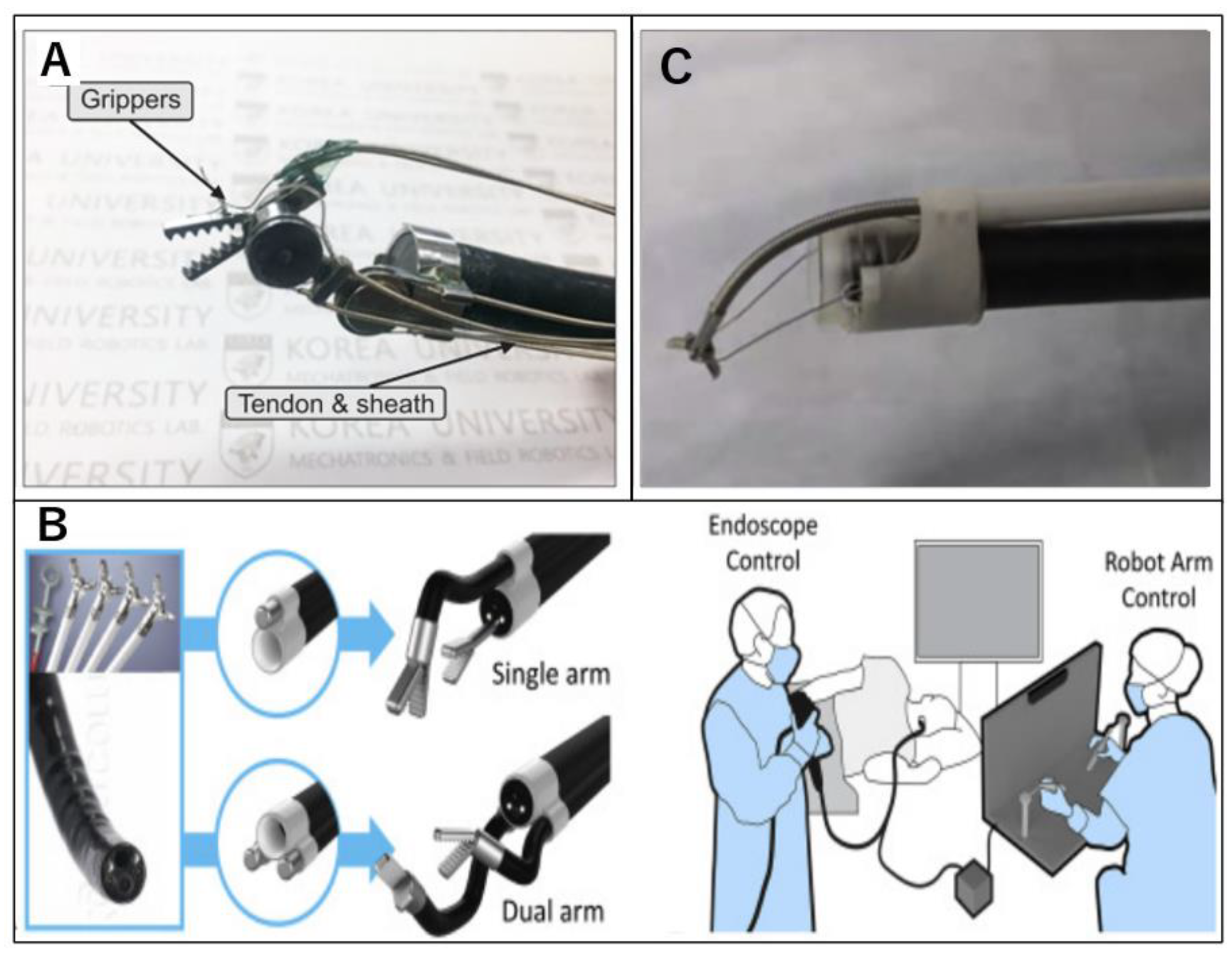
2.10. K-FLEX
2.11. Endoluminal Surgical (ELS) System
2.12. Flexible Auxiliary Single-Arm Transluminal Endoscopic Robot System (FASTER)
3. Discussion
3.1. Applications for ESD, EFTR, and NOTES
3.1.1. ESD
3.1.2. EFTR
3.1.3. NOTES
3.2. Considerations and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalloo, A.N.; Singh, V.K.; Jagannath, S.B.; Niiyama, H.; Hill, S.L.; Vaughn, C.A.; Magee, C.A.; Kantsevoy, S.V. Flexible transgastric peritoneoscopy: A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest. Endosc. 2004, 60, 114–117. [Google Scholar] [CrossRef] [PubMed]
- ASGE; SAGES. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery White Paper October 2005. Gastrointest. Endosc. 2006, 63, 199–203. [Google Scholar] [CrossRef]
- Park, P.O.; Bergström, M.; Ikeda, K.; Fritscher-Ravens, A.; Swain, P. Experimental studies of transgastric gallbladder surgery: Cholecystectomy and cholecystogastric anastomosis (videos). Gastrointest. Endosc. 2005, 61, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Fritscher-Ravens, A.; Mosse, C.A.; Mills, T.; Tajiri, H.; Swain, C.P. Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest. Endosc. 2005, 62, 122–129. [Google Scholar] [CrossRef]
- Ikeda, K.; Mosse, C.A.; Park, P.O.; Fritscher-Ravens, A.; Bergström, M.; Mills, T.; Tajiri, H.; Swain, C.P. Endoscopic full-thickness resection: Circumferential cutting method. Gastrointest. Endosc. 2006, 64, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, K.; Gostout, C.J.; Rajan, E.; Bakken, T.A.; Knipschield, M.A.; Chung, S.; Cotton, P.B.; Hawes, R.H.; Kalloo, A.N.; Kantsevoy, S.V.; et al. Transgastric cholecystectomy: Transgastric accessibility to the gallbladder improved with the SEMF method and a novel multibending therapeutic endoscope. Gastrointest. Endosc. 2007, 65, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, K.; Gostout, C.J.; Rajan, E.; Bakken, T.A.; Knipschield, M.A. Transesophageal mediastinoscopy by submucosal endoscopy with mucosal flap safety valve technique. Gastrointest. Endosc. 2007, 65, 679–683. [Google Scholar] [CrossRef]
- Kantsevoy, S.V.; Hu, B.; Jagannath, S.B.; Vaughn, C.A.; Beitler, D.M.; Chung, S.S.; Cotton, P.B.; Gostout, C.J.; Hawes, R.H.; Pasricha, P.J.; et al. Transgastric endoscopic splenectomy: Is it possible? Surg Endosc. 2006, 20, 522–525. [Google Scholar] [CrossRef]
- Ryou, M.; Fong, D.G.; Pai, R.D.; Tavakkolizadeh, A.; Rattner, D.W.; Thompson, C.C. Dual-port distal pancreatectomy using a prototype endoscope and endoscopic stapler: A natural orifice transluminal endoscopic surgery (NOTES) survival study in a porcine model. Endoscopy 2007, 39, 881–887. [Google Scholar] [CrossRef]
- Zhou, P.H.; Yao, L.Q.; Qin, X.Y.; Cai, M.Y.; Xu, M.D.; Zhong, Y.S.; Chen, W.F.; Zhang, Y.Q.; Qin, W.Z.; Hu, J.W.; et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg. Endosc. 2011, 25, 2926–2931. [Google Scholar] [CrossRef]
- Li, J.; Meng, Y.; Ye, S.; Wang, P.; Liu, F. Usefulness of the thread-traction method in endoscopic full-thickness resection for gastric submucosal tumor: A comparative study. Surg. Endosc. 2019, 33, 2880–2885. [Google Scholar] [CrossRef]
- Hu, J.; Ge, N.; Wang, S.; Guo, J.; Liu, X.; Wang, G.; Sun, S. Direct endoscopic full-thickness resection for submucosal tumors with an intraluminal growth pattern originating from the muscularis propria layer in the gastric fundus. BMC Gastroenterol. 2020, 20, 70. [Google Scholar] [CrossRef]
- Zhao, Y.; Pang, T.; Zhang, B.; Wang, L.; Lv, Y.; Ling, T.; Zhang, X.; Huang, Q.; Xu, G.; Zou, X. Retrospective Comparison of Endoscopic Full-Thickness Versus Laparoscopic or Surgical Resection of Small (≤ 5 cm) Gastric Gastrointestinal Stromal Tumors. J. Gastrointest. Surg. 2020, 24, 2714–2721. [Google Scholar] [CrossRef]
- Jung, A.L.; Park, S.W.; Hong, G.Y.; Moon, H.C.; Eun, S.J. Endoscopic Full-Thickness Resection for Gastric Subepithelial Lesions Arising from the Muscularis Propria. Clin. Endosc. 2021, 54, 131–135. [Google Scholar] [CrossRef]
- Wang, G.; Xiang, Y.; Miao, Y.; Wang, H.; Xu, M.; Yu, G. The application of endoscopic loop ligation in defect repair following endoscopic full-thickness resection of gastric submucosal tumors originating from the muscularis propria layer. Scand. J. Gastroenterol. 2022, 57, 119–123. [Google Scholar] [CrossRef]
- Shichijo, S.; Abe, N.; Takeuchi, H.; Ohata, K.; Minato, Y.; Hashiguchi, K.; Hirasawa, K.; Kayaba, S.; Shinkai, H.; Kobara, H.; et al. Endoscopic resection for gastric submucosal tumors: Japanese multicenter retrospective study. Dig. Endosc. 2023, 35, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, P.; Cai, M.; Song, B.; Zhou, P. Endoscopic full-thickness resection, indication, methods and perspectives. Dig. Endosc. 2023, 35, 195–205. [Google Scholar] [CrossRef]
- Abdallah, M.; Suryawanshi, G.; McDonald, N.; Chandan, S.; Umar, S.; Azeem, N.; Bilal, M. Endoscopic full-thickness resection for upper gastrointestinal tract lesions: A systematic review and meta-analysis. Surg. Endosc. 2023, 37, 3293–3305. [Google Scholar] [CrossRef]
- Tada, N.; Kobara, H.; Nishiyama, N.; Fujihara, S.; Masaki, T.; Uedo, N. Current Status of Endoscopic Full-Thickness Resection for Gastric Subepithelial Tumors: A Literature Review Over Two Decades. Digestion 2023, 104, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Nabi, Z.; Samanta, J.; Dhar, J.; Mohan, B.P.; Facciorusso, A.; Reddy, D.N. Device-assisted endoscopic full-thickness resection in colorectum: Systematic review and meta-analysis. Dig. Endosc. 2023, 6, 116–128. [Google Scholar] [CrossRef]
- Thompson, C.C.; Ryou, M.; Soper, N.J.; Hungess, E.S.; Rothstein, R.I.; Swanstrom, L.L. Evaluation of a manually driven, multitasking platform for complex endoluminal and natural orifice transluminal endoscopic surgery applications (with video). Gastrointest. Endosc. 2009, 70, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Sumiyama, K.; Tajiri, H.; Yasuda, K.; Kitano, S. Evaluation of a new multitasking platform for endoscopic full-thickness resection. Gastrointest. Endosc. 2011, 73, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.W.Y.; Ho, K.Y.; Phee, S.J. Colonic endoscopic submucosal dissection using a novel robotic system (with video). Gastrointest. Endosc. 2021, 93, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Spaun, G.O.; Zheng, B.; Swanström, L.L. A multitasking platform for natural orifice translumenal endoscopic surgery (NOTES): A benchtop comparison of a new device for flexible endoscopic surgery and a standard dual-channel endoscope. Surg. Endosc. 2009, 23, 2720–2727. [Google Scholar] [CrossRef]
- Fuchs, K.H.; Breithaupt, W. Transgastric small bowel resection with the new multitasking platform EndoSAMURAI™ for natural orifice transluminal endoscopic surgery. Surg. Endosc. 2012, 26, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Phee, S.J.; Shabbir, A.; Low, S.C.; Huynh, V.A.; Kencana, A.P.; Yang, K.; Lomanto, D.; So, B.Y.; Wong, Y.Y.; et al. Endoscopic submucosal dissection of gastric lesions by using a Master and Slave Transluminal Endoscopic Robot (MASTER). Gastrointest. Endosc. 2010, 72, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Phee, S.J.; Lomanto, D.; Goel, R.; Rebala, P.; Sun, Z.L.; Trasti, S.; Reddy, N.; Wong, J.Y.; Ho, K.Y. Endoscopic submucosal dissection of gastric lesions by using a master and slave transluminal endoscopic robot: An animal survival study. Endoscopy 2012, 44, 690–694. [Google Scholar] [CrossRef]
- Phee, S.J.; Reddy, N.; Chiu, P.W.; Rebala, P.; Rao, G.V.; Wang, Z.; Sun, Z.; Wong, J.Y.; Ho, K.Y. Robot-assisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin. Gastroenterol. Hepatol. 2012, 10, 1117–1121. [Google Scholar] [CrossRef]
- Sun, Z.; Ang, R.Y.; Lim, E.W.; Wang, Z.; Ho, K.Y.; Phee, S.J. Enhancement of a master-slave robotic system for natural orifice transluminal endoscopic surgery. Ann. Acad. Med. Singap. 2011, 40, 223–230. [Google Scholar] [CrossRef]
- Chiu, P.W.; Phee, S.J.; Wang, Z.; Sun, Z.; Poon, C.C.; Yamamoto, T.; Penny, I.; Wong, J.Y.; Lau, J.Y.; Ho, K.Y. Feasibility of full-thickness gastric resection using master and slave transluminal endoscopic robot and closure by Overstitch: A preclinical study. Surg. Endosc. 2014, 28, 319–324. [Google Scholar] [CrossRef]
- Takeshita, N.; Ho, K.Y.; Phee, S.J.; Wong, J.; Chiu, P.W. Feasibility of performing esophageal endoscopic submucosal dissection using master and slave transluminal endoscopic robot. Endoscopy 2017, 49, E27–E28. [Google Scholar] [CrossRef] [PubMed]
- Phee, S.J.; Ho, K.Y.; Lomanto, D.; Low, S.C.; Huynh, V.A.; Kencana, A.P.; Yang, K.; Sun, Z.L.; Chung, S.C. Natural orifice transgastric endoscopic wedge hepatic resection in an experimental model using an intuitively controlled master and slave transluminal endoscopic robot (MASTER). Surg. Endosc. 2010, 24, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Dallemagne, B.; Marescaux, J. The ANUBIS™ project. Minim. Invasive Ther. Allied Technol. 2010, 19, 257–261. [Google Scholar] [CrossRef] [PubMed]
- De Donno, A.; Zorn, L.; Zanne, P. Introducing STRAS: A new flexible robotic system for minimally invasive surgery. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 1213–1220. [Google Scholar] [CrossRef]
- Diana, M.; Chung, H.; Liu, K.H.; Dallemagne, B.; Demartines, N.; Mutter, D.; Marescaux, J. Endoluminal surgical triangulation: Overcoming challenges of colonic endoscopic submucosal dissections using a novel flexible endoscopic surgical platform: Feasibility study in a porcine model. Surg. Endosc. 2013, 27, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Zorn, L.; Nageotte, F.; Zanne, P.; Legner, A.; Dallemagne, B.; Marescaux, J.; de Mathelin, M. A Novel Telemanipulated Robotic Assistant for Surgical Endoscopy: Preclinical Application to ESD. IEEE Trans. Biomed. Eng. 2018, 65, 797–808. [Google Scholar] [CrossRef]
- Mascagni, P.; Lim, S.G.; Fiorillo, C.; Zanne, P.; Nageotte, F.; Zorn, L.; Perretta, S.; de Mathelin, M.; Marescaux, J.; Dallemagne, B. Democratizing Endoscopic Submucosal Dissection: Single-Operator Fully Robotic Colorectal Endoscopic Submucosal Dissection in a Pig Model. Gastroenterology. 2019, 156, 1569–1571.e2. [Google Scholar] [CrossRef]
- Perretta, S.; Dallemagne, B.; Barry, B.; Marescaux, J. The ANUBISCOPE® flexible platform ready for prime time: Description of the first clinical case. Surg. Endosc. 2013, 27, 2630. [Google Scholar] [CrossRef]
- Légner, A.; Diana, M.; Halvax, P.; Liu, Y.Y.; Zorn, L.; Zanne, P.; Nageotte, F.; De Mathelin, M.; Dallemagne, B.; Marescaux, J. Endoluminal surgical triangulation 2.0: A new flexible surgical robot. Preliminary pre-clinical results with colonic submucosal dissection. Int. J. Med. Robot. 2017, 13, e1819. [Google Scholar] [CrossRef]
- Kume, K.; Sakai, N.; Ueda, T. Development of a Novel Gastrointestinal Endoscopic Robot Enabling Complete Remote Control of All Operations: Endoscopic Therapeutic Robot System (ETRS). Gastroenterol. Res. Pract. 2019, 2019, 6909547, Erratum in Gastroenterol. Res. Pract. 2020, 2020, 6173978. [Google Scholar] [CrossRef]
- Kume, K. Flexible robotic endoscopy: Current and original devices. Comput. Assist. Surg. 2016, 21, 150–159. [Google Scholar] [CrossRef]
- Kume, K.; Kuroki, T.; Sugihara, T.; Shinngai, M. Development of a novel endoscopic manipulation system: The Endoscopic operation robot. World J. Gastrointest. Endosc. 2011, 3, 145–150. [Google Scholar] [CrossRef]
- Kume, K.; Kuroki, T.; Shingai, M.; Harada, M. Endoscopic submucosal dissection using the endoscopic operation robot. Endoscopy 2012, 44 (Suppl. S2), E399–E400. [Google Scholar] [CrossRef] [PubMed]
- Berthet-Rayne, P.; Gras, G.; Leibrandt, K.; Wisanuvej, P.; Schmitz, A.; Seneci, C.A.; Yang, G.Z. The i2 Snake Robotic Platform for Endoscopic Surgery. Ann. Biomed. Eng. 2018, 46, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.T.H.; Aihara, H.; Thompson, C.C. Robotic-assisted surgical endoscopy: A new era for endoluminal therapies. VideoGIE 2019, 4, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Turiani Hourneaux de Moura, D.; Aihara, H.; Jirapinyo, P.; Farias, G.; Hathorn, K.E.; Bazarbashi, A.; Sachdev, A.; Thompson, C.C. Robot-assisted endoscopic submucosal dissection versus conventional ESD for colorectal lesions: Outcomes of a randomized pilot study in endoscopists without prior ESD experience (with video). Gastrointest. Endosc. 2019, 90, 290–298. [Google Scholar] [CrossRef]
- Atallah, S.; Hodges, A.; Larach, S.W. Direct target NOTES: Prospective applications for next generation robotic platforms. Tech. Coloproctol. 2018, 22, 363–371. [Google Scholar] [CrossRef]
- Kim, B.G.; Choi, H.S.; Park, S.H.; Hong, J.H.; Lee, J.M.; Kim, S.H.; Chun, H.J.; Hong, D.; Keum, B. A Pilot Study of Endoscopic Submucosal Dissection Using an Endoscopic Assistive Robot in a Porcine Stomach Model. Gut Liver. 2019, 13, 402–408. [Google Scholar] [CrossRef]
- Hwang, M.; Lee, S.W.; Park, K.C.; Sul, H.J.; Kwon, D.S. Evaluation of a robotic arm-assisted endoscope to facilitate endoscopic submucosal dissection (with video). Gastrointest. Endosc. 2020, 91, 699–706. [Google Scholar] [CrossRef]
- Hwang, M.; Kwon, D.S. K-FLEX: A flexible robotic platform for scar-free endoscopic surgery. Int. J. Med. Robot. 2020, 16, e2078. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Sanchez, A.; Bianchi, E.; Larach, S.W. Envisioning the future of colorectal surgery: Preclinical assessment and detailed description of an endoluminal robotic system (ColubrisMX ELS). Tech. Coloproctol. 2021, 25, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Fu, S.C.; Ji, R.; Li, L.X.; Li, Y.Q.; Zuo, X.L. A novel flexible auxiliary single-arm transluminal endoscopic robot facilitates endoscopic submucosal dissection of gastric lesions (with video). Surg. Endosc. 2022, 36, 5510–5517. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Yang, J.L.; Yang, X.X.; Fu, S.C.; Li, L.X.; Li, Y.Q.; Zuo, X.L. Simplified robot-assisted endoscopic submucosal dissection for esophageal and gastric lesions: A randomized controlled porcine study (with videos). Gastrointest. Endosc. 2022, 96, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sumiyama, K.; Ban, Y.; Dobashi, A.; Ohya, T.R.; Aizawa, D.; Hirooka, S.; Nakajima, K.; Tajiri, H. Closure of iatrogenic large mucosal and full-thickness defects of the stomach with endoscopic interrupted sutures in in vivo porcine models: Are they durable enough? BMC Gastroenterol. 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Halvax, P.; Diana, M.; Lègner, A.; Lindner, V.; Liu, Y.Y.; Nagao, Y.; Cho, S.; Marescaux, J.; Swanström, L.L. Endoluminal full-thickness suture repair of gastrotomy: A survival study. Surg. Endosc. 2015, 29, 3404–3408. [Google Scholar] [CrossRef]
- Mori, H.; Kobara, H.; Fujihara, S.; Nishiyama, N.; Ayaki, M.; Chiyo, T.; Kobayashi, N.; Yachida, T.; Masaki, T. Innovative pure non-exposed endoscopic full-thickness resection using an endoscopic suturing device. Gastrointest. Endosc. 2016, 84, 178–179. [Google Scholar] [CrossRef]
- Yang, F.; Wang, S.; Sun, S.; Liu, X.; Ge, N.; Wang, G.; Guo, J.; Liu, W.; Feng, L.; Ma, W. Factors associated with endoscopic full-thickness resection of gastric submucosal tumors. Surg. Endosc. 2015, 29, 3588–3593. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Sun, S.; Liu, X.; Wang, S.; Ge, N.; Wang, G.; Qi, Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg. Endosc. 2015, 29, 3356–3362. [Google Scholar] [CrossRef]
- Draganov, P.V.; Wang, A.Y.; Othman, M.O.; Fukami, N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 16–25.e1. [Google Scholar] [CrossRef]
- Keihanian, T.; Othman, M.O. Colorectal Endoscopic Submucosal Dissection: An Update on Best Practice. Clin. Exp. Gastroenterol. 2021, 14, 317–330. [Google Scholar] [CrossRef]
- Mann, R.; Gajendran, M.; Umapathy, C.; Perisetti, A.; Goyal, H.; Saligram, S.; Echavarria, J. Endoscopic Management of Complex Colorectal Polyps: Current Insights and Future Trends. Front. Med. 2022, 8, 728704. [Google Scholar] [CrossRef]
- Tanaka, S.; Toyonaga, T.; Kaku, H.; Sakaguchi, H.; Baba, S.; Takao, T.; Kodama, Y. A novel traction device (EndoTrac) for use during endoscopic submucosal dissection. Endoscopy 2019, 51, E90–E91. [Google Scholar] [CrossRef]
- Bordillon, P.; Pioche, M.; Wallenhorst, T.; Rivory, J.; Legros, R.; Albouys, J.; Lepetit, H.; Rostain, F.; Dahan, M.; Ponchon, T.; et al. Double-clip traction for colonic endoscopic submucosal dissection: A multicenter study of 599 consecutive cases (with video). Gastrointest. Endosc. 2021, 94, 333–343. [Google Scholar] [CrossRef]
- Grimaldi, J.; Masgnaux, L.J.; Rivory, J.; Legros, R.; Wallenhorst, T.; Jacques, J.; Pioche, M. Multipolar traction with adjustable force increases procedure speed during endoscopic submucosal dissection: The A-TRACT-4 traction device. Endoscopy 2022, 54, E1013–E1014. [Google Scholar] [CrossRef]
- Libânio, D.; Pimentel-Nunes, P.; Bastiaansen, B.; Bisschops, R.; Bourke, M.J.; Deprez, P.H.; Esposito, G.; Lemmers, A.; Leclercq, P.; Maselli, R.; et al. Endoscopic submucosal dissection techniques and technology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2023, 55, 361–389. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, J.; Vallabhajosyula, S.; E-Xin, B.; Napel, M.; Okolo, P.I., 3rd. The Impact of Traction Methods on Endoscopic Submucosal Dissection Efficacy for Gastric Neoplasia: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2023. [Google Scholar] [CrossRef]
- Kamigaichi, Y.; Oka, S.; Tanaka, S.; Nagata, S.; Kunihiro, M.; Kuwai, T.; Hiraga, Y.; Furudoi, A.; Onogawa, S.; Okanobu, H.; et al. Factors for conversion risk of colorectal endoscopic submucosal dissection: A multicenter study. Surg. Endosc. 2022, 36, 5698–5709. [Google Scholar] [CrossRef] [PubMed]
- Hiki, N.; Yamamoto, Y.; Fukunaga, T.; Yamaguchi, T.; Nunobe, S.; Tokunaga, M.; Miki, A.; Ohyama, S.; Seto, Y.; Fukunaga, T. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg. Endosc. 2008, 22, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, K.; Futakuchi, T.; Kamba, S.; Matsui, H.; Tamai, N. Artificial intelligence in endoscopy: Present and future perspectives. Dig. Endosc. 2021, 33, 218–230. [Google Scholar] [CrossRef] [PubMed]


| Device | Operator | Degree of Freedom | Outer Diameter (mm) | Clinical Trial | Approval | |
|---|---|---|---|---|---|---|
| The direct drive endoscopic system (DDES) | Double | 7 | 22 | EMR NOTES | Animal in vivo | None |
| EndoSAMURAI | Double | 5 | 15 | EFTR NOTES | Animal in vivo | None |
| Master and slave transluminal endoscopic robot (EndoMaster EASE) | Double | 10 | N/A | ESD EFTR NOTES | Animal in vivo Human | None |
| Endoluminal Assistant for Surgical Endoscopy (EASE) | Double | 10 | 18 | ESD | Animal in vivo | None |
| Endoscopic therapeutic robot system (ETRS) | Single | N/A | N/A | ESD | Animal ex vivo | None |
| i2 snake robotic platform | Single | 7 | 16 | None | None | None |
| Flex robotic system | Single | N/A | 18 | ESD EFTR NOTES | Animal in vivo Human | FDA and CE |
| Revolute joint-based auxiliary transluminal endoscopic robot (REXTER) | Double | 4 | N/A | ESD | Animal ex vivo | None |
| Portable endoscopic tool handler (PETH) | Double | 2 | N/A | ESD | Animal ex vivo | None |
| K-FLEX | Single | 14 | 17 | ESD | Animal ex vivo | None |
| Endoluminal surgical system (ELS) | Single | 7 | 22 | ESD | Animal ex vivo | None |
| Flexible Auxiliary Single-arm Transluminal Endoscopic Robot system (FASTER) | Double | 3 | N/A | ESD | Animal in vivo | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, N.; Sumiyama, K. Robotic Platforms for Therapeutic Flexible Endoscopy: A Literature Review. Diagnostics 2024, 14, 595. https://doi.org/10.3390/diagnostics14060595
Tada N, Sumiyama K. Robotic Platforms for Therapeutic Flexible Endoscopy: A Literature Review. Diagnostics. 2024; 14(6):595. https://doi.org/10.3390/diagnostics14060595
Chicago/Turabian StyleTada, Naoya, and Kazuki Sumiyama. 2024. "Robotic Platforms for Therapeutic Flexible Endoscopy: A Literature Review" Diagnostics 14, no. 6: 595. https://doi.org/10.3390/diagnostics14060595
APA StyleTada, N., & Sumiyama, K. (2024). Robotic Platforms for Therapeutic Flexible Endoscopy: A Literature Review. Diagnostics, 14(6), 595. https://doi.org/10.3390/diagnostics14060595





