Analysis of IVIM Perfusion Fraction Improves Detection of Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Magnetic Resonance Imaging and Postprocessing
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.E.; Gupta, S.; Kang, J.Y.; Quinn, M.J.; Maxwell, J.D.; Mudan, S.; Majeed, A. Pancreatic Cancer in England and Wales 1975–2000: Patterns and Trends in Incidence, Survival and Mortality. Aliment. Pharmacol. Ther. 2006, 23, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and Non-Modifiable Risk Factors for Pancreatic Cancer: A Review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of Pancreatic Cancer. World J. Gastroenterol. 2016, 22, 9694. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Luo, J.; Xiao, L.; Wu, C.; Zheng, Y.; Zhao, N. The Incidence and Survival Rate of Population-Based Pancreatic Cancer Patients: Shanghai Cancer Registry 2004–2009. PLoS ONE 2013, 8, e76052. [Google Scholar] [CrossRef]
- Kim, S.S.; Choi, G.C.; Jou, S.S. Pancreas Ductal Adenocarcinoma and Its Mimics: Review of Cross-Sectional Imaging Findings for Differential Diagnosis. J. Belg. Soc. Radiol. 2018, 102, 71. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef]
- Burk, K.S.; Lo, G.C.; Gee, M.S.; Sahani, D.V. Imaging and Screening of Pancreatic Cancer. Radiol. Clin. N. Am. 2017, 55, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Feldman, M.K.; Le, O.; Morris-Stiff, G. Imaging Mimics of Pancreatic Ductal Adenocarcinoma. Abdom. Radiol. 2017, 43, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.H.; Rini, N.J.; Keppke, A.L. MRI of Adenocarcinoma of the Pancreas. AJR Am. J. Roentgenol. 2006, 187, W365–W374. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.M.; Hough, D.M.; Tolat, P.P.; Soloff, E.V.; Kambadakone, A.R. Pancreatic Adenocarcinoma: Cross-Sectional Imaging Techniques. Abdom. Radiol. 2017, 43, 253–263. [Google Scholar] [CrossRef]
- Igarashi, T.; Shiraishi, M.; Watanabe, K.; Ohki, K.; Takenaga, S.; Ashida, H.; Ojiri, H. 3d Quantitative Analysis of Diffusion-Weighted Imaging for Predicting the Malignant Potential of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pol. J. Radiol. 2021, 86, 298–308. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR Imaging of Intravoxel Incoherent Motions: Application to Diffusion and Perfusion in Neurologic Disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Le Bihan, D. What Can We See with IVIM MRI? Neuroimage 2019, 187, 56–67. [Google Scholar] [CrossRef]
- Federau, C.; Meuli, R.; O’Brien, K.; Maeder, P.; Hagmann, P. Perfusion Measurement in Brain Gliomas with Intravoxel Incoherent Motion MRI. Am. J. Neuroradiol. 2014, 35, 256–262. [Google Scholar] [CrossRef]
- Kim, H.S.; Suh, C.H.; Kim, N.; Choi, C.G.; Kim, S.J. Histogram Analysis of Intravoxel Incoherent Motion for Differentiating Recurrent Tumor from Treatment Effect in Patients with Glioblastoma: Initial Clinical Experience. Am. J. Neuroradiol. 2014, 35, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Bisdas, S.; Braun, C.; Skardelly, M.; Schittenhelm, J.; Teo, T.H.; Thng, C.H.; Klose, U.; Koh, T.S. Correlative Assessment of Tumor Microcirculation Using Contrast-Enhanced Perfusion MRI and Intravoxel Incoherent Motion Diffusion-Weighted MRI: Is There a Link between Them? NMR Biomed. 2014, 27, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Fujima, N.; Yoshida, D.; Sakashita, T.; Homma, A.; Tsukahara, A.; Tha, K.K.; Kudo, K.; Shirato, H. Intravoxel Incoherent Motion Diffusion-Weighted Imaging in Head and Neck Squamous Cell Carcinoma: Assessment of Perfusion-Related Parameters Compared to Dynamic Contrast-Enhanced MRI. Magn. Reson. Imaging 2014, 32, 1206–1213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szubert-Franczak, A.E.; Naduk-Ostrowska, M.; Pasicz, K.; Podgórska, J.; Skrzyński, W.; Cieszanowski, A. Intravoxel Incoherent Motion Magnetic Resonance Imaging: Basic Principles and Clinical Applications. Pol. J. Radiol. 2020, 85, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Hejduk, B.; Bobek-Billewicz, B.; Rutkowski, T.; Hebda, A.; Zawadzka, A.; Jurkowski, M.K. Application of Intravoxel Incoherent Motion (IVIM) Model for Differentiation between Metastatic and Non-Metastatic Head and Neck Lymph Nodes. Pol. J. Radiol. 2017, 82, 506–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Jia, H.; Fang, X.; Lin, T.; Wei, C.; Qian, L.; Dong, J. Feasibility of Predicting Pelvic Lymph Node Metastasis Based on IVIM-DWI and Texture Parameters of the Primary Lesion and Lymph Nodes in Patients with Cervical Cancer. Acad. Radiol. 2021, 29, 1048–1057. [Google Scholar] [CrossRef]
- Klau, M.; Lemke, A.; Grünberg, K.; Simon, D.; Re, T.J.; Wente, M.N.; Laun, F.B.; Kauczor, H.U.; Delorme, S.; Grenacher, L.; et al. Intravoxel Incoherent Motion MRI for the Differentiation between Mass Forming Chronic Pancreatitis and Pancreatic Carcinoma. Investig. Radiol. 2011, 46, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Klauß, M.; Maier-Hein, K.; Tjaden, C.; Hackert, T.; Grenacher, L.; Stieltjes, B. IVIM DW-MRI of Autoimmune Pancreatitis: Therapy Monitoring and Differentiation from Pancreatic Cancer. Eur. Radiol. 2016, 26, 2099–2106. [Google Scholar] [CrossRef]
- Nogueira, L.; Brandão, S.; Matos, E.; Nunes, R.G.; Loureiro, J.; Ramos, I.; Ferreira, H.A. Application of the Diffusion Kurtosis Model for the Study of Breast Lesions. Eur. Radiol. 2014, 24, 1197–1203. [Google Scholar] [CrossRef]
- De Robertis, R.; Cardobi, N.; Ortolani, S.; Tinazzi Martini, P.; Stemmer, A.; Grimm, R.; Gobbo, S.; Butturini, G.; D’Onofrio, M. Intravoxel Incoherent Motion Diffusion-Weighted MR Imaging of Solid Pancreatic Masses: Reliability and Usefulness for Characterization. Abdom. Radiol. 2019, 44, 131–139. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.S.; Sung, Y.S.; Cheong, H.; Byun, J.H.; Kim, H.J.; Kim, J.H. Intravoxel Incoherent Motion Diffusion-Weighted Imaging of the Pancreas: Characterization of Benign and Malignant Pancreatic Pathologies. J. Magn. Reson. Imaging 2017, 45, 260–269. [Google Scholar] [CrossRef]
- Barral, M.; Taouli, B.; Guiu, B.; Koh, D.M.; Luciani, A.; Manfredi, R.; Vilgrain, V.; Hoeffel, C.; Kanematsu, M.; Soyer, P. Diffusion-Weighted MR Imaging of the Pancreas: Current Status and Recommendations. Radiology 2015, 274, 45–63. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Wang, L.; Wang, Y.; Zhang, Y.; Wang, H.; Chen, S.; Lu, J. Intravoxel Incoherent Motion DWI of the Pancreatic Adenocarcinomas: Monoexponential and Biexponential Apparent Diffusion Parameters and Histopathological Correlations. Cancer Imaging 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.L.; Zhang, C.D.; Yan, J.X.; Sun, J.; Zhao, X.Y.; Zhang, L.S.; Yin, L.L. Accuracy of Quantitative Diffusion-Weighted Imaging for Differentiating Benign and Malignant Pancreatic Lesions: A Systematic Review and Meta-Analysis. Eur. Radiol. 2021, 31, 7746–7759. [Google Scholar] [CrossRef] [PubMed]

| Set | Parameters | Applied b-Values [s/mm2] |
|---|---|---|
| 1. | ADC1, D*1, D1, f1 | all: 0, 10, 20, 50, 100, 200, 400, 600, 1000 |
| 2. | ADC2, D*2, D2, f2 | low: 0, 10, 20, 50, 100, 200 |
| 3. | ADC3, D*3, D3, f3 | high: 400, 600, 1000 |
| 4. | ADC4, D*4, D4, f4 | 0 and high: 0, 400, 600, 1000 |
| Parameter | Control Group Mean (95% CI) | Study Group Mean (95% CI) | Mean Difference | p-Value |
|---|---|---|---|---|
| ADC1 | 1.86 (1.66–2.06) | 1.24 (1.15–1.33) | 0.62 | <0.0001 |
| ADC2 | 2.03 (1.69–2.36) | 1.34 (1.25–1.42) | 0.52 | <0.0001 |
| ADC3 | 1.96 (1.71–2.20) | 1.41 (1.34–1.48) | 0.55 | <0.0001 |
| ADC4 | 2.51 (1.47–3.54) | 1.60 (1.40–1.79) | 0.91 | 0.0054 |
| D*1 | 18.5 (15.1–21.8) | 12.4 (9.4–15.4) | 6.04 | 0.0080 |
| D*2 | 17.7 (14.0–21.5) | 9.16 (7.78–10.54) | 8.56 | 0.0001 |
| D*3 | 55.7 (49.5–61.8) | 30.9 (25.7–36.1) | 24.79 | 0.0001 |
| D*4 | 19.2 (15.8–23.0) | 8.08 (6.78–9.39) | 11.11 | 0.0001 |
| D1 | 1.19 (1.08–1.30) | 1.01 (0.94–1.08) | 0.18 | 0.0054 |
| D2 | 2.88 (2.56–3.20) | 1.64 (1.49–1.79) | 1.24 | 0.0001 |
| D3 | 1.78 (1.46–2.10) | 1.10 (1.02–1.18) | 0.67 | 0.0001 |
| D4 | 1.18 (1.10–1.27) | 1.17 (1.09–1.25) | 0.01 | 0.8118 |
| f1 | 0.40 (0.35–0.45) | 0.27 (0.23–0.31) | 0.13 | 0.0001 |
| f2 | 0.23 (0.19–0.28) | 0.10 (0.09–0.12) | 0.13 | 0.0001 |
| f3 | 0.97 (0.96–0.99) | 0.95 (0.90–0.99) | 0.03 | 0.3072 |
| f4 | 0.37 (0.33–0.41) | 0.20 (0.16–0.23) | 0.17 | 0.0001 |
| T2 | 676 (627–724) | 640 (609–672) | 36.5 | 0.1981 |
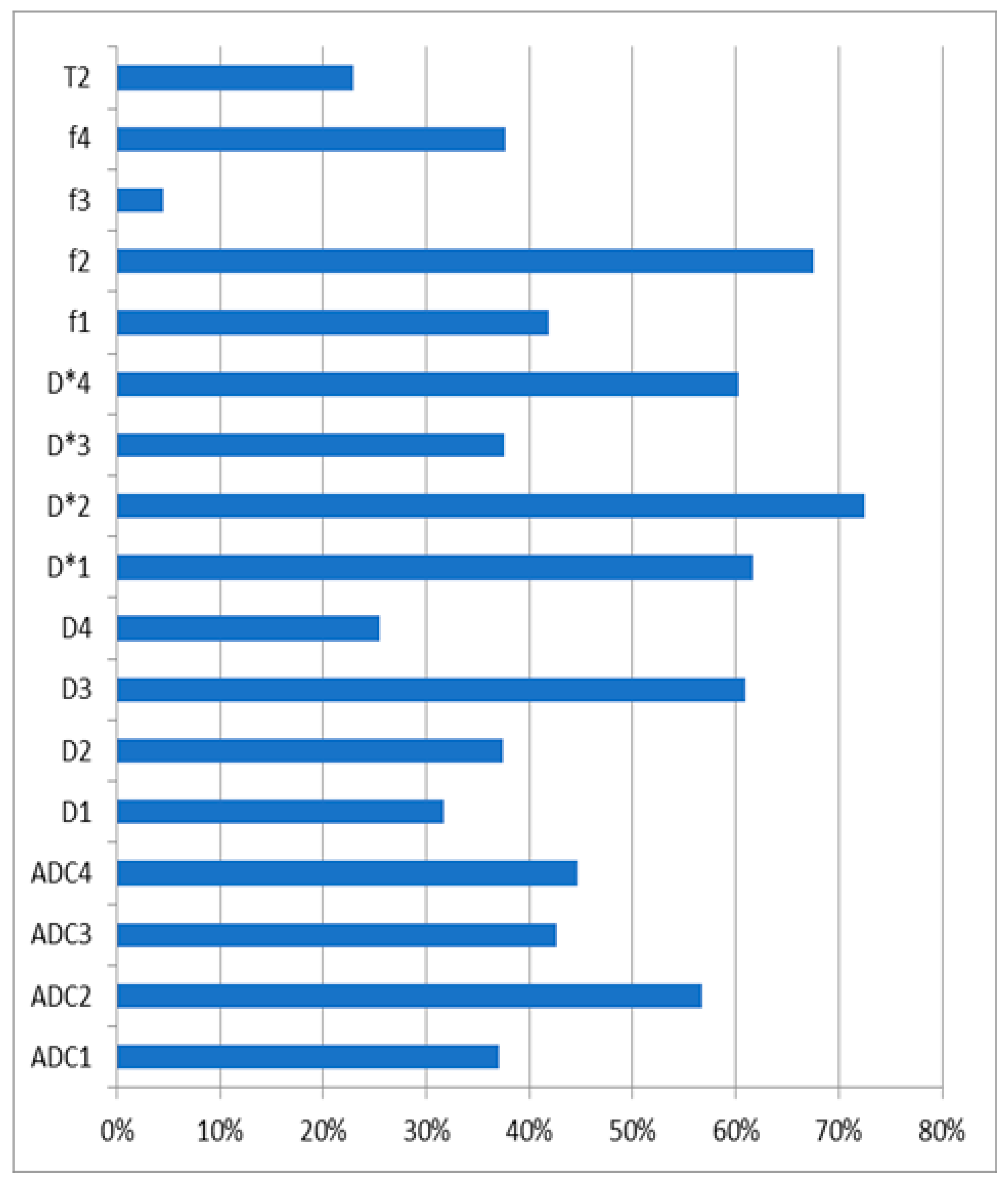
| Parameter | AUC (95% CI) | Sensitivity | Specificity | Threshold | p-Value |
|---|---|---|---|---|---|
| ADC1 | 0.78 (0.69–0.86) | 93% | 53% | ≤1.65 | <0.0001 |
| ADC2 | 0.76 (0.66–0.84) | 84% | 66% | ≤1.48 | <0.0001 |
| ADC3 | 0.79 (0.70–0.87) | 71% | 81% | ≤1.46 | <0.0001 |
| ADC4 | 0.75 (0.53–0.90) | 89% | 71% | ≤1.86 | 0.0711 |
| D*1 | 1.00 (0.97–1.00) | 100% | 100% | ≤1.57 | <0.0001 |
| D*2 | 1.00 (0.97–1.00) | 100% | 100% | ≤3.22 | <0.0001 |
| D*3 | 1.00 (0.97–1.00) | 100% | 100% | ≤1.84 | <0.0001 |
| D*4 | 1.00 (0.97–1.00) | 100% | 100% | ≤1.67 | <0.0001 |
| D1 | 0.64 (0.53–0.73) | 82% | 51% | ≤1.19 | 0.0163 |
| D2 | 0.86 (0.78–0.92) | 89% | 72% | ≤2.21 | <0.0001 |
| D3 | 0.76 (0.66–0.84) | 71% | 77% | ≤1.18 | <0.0001 |
| D4 | 0.51 (0.41–0.61) | 70% | 38% | ≤1.3 | 0.8777 |
| f1 | 0.71 (0.61–0.79) | 84% | 51% | ≤0.41 | <0.0001 |
| f2 | 0.84 (0.75–0.90) | 63% | 89% | ≤0.10 | <0.0001 |
| f3 | 0.51 (0.41–0.61) | 93% | 2% | >0.95 | 0.931 |
| f4 | 0.82 (0.73–0.89) | 70% | 83% | ≤0.24 | <0.0001 |
| T2 | 0.59 (0.49–0.69) | 43% | 81% | ≤592 | 0.1347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadolska, K.; Białecka, A.; Zawada, E.; Kazimierczak, W.; Serafin, Z. Analysis of IVIM Perfusion Fraction Improves Detection of Pancreatic Ductal Adenocarcinoma. Diagnostics 2024, 14, 571. https://doi.org/10.3390/diagnostics14060571
Nadolska K, Białecka A, Zawada E, Kazimierczak W, Serafin Z. Analysis of IVIM Perfusion Fraction Improves Detection of Pancreatic Ductal Adenocarcinoma. Diagnostics. 2024; 14(6):571. https://doi.org/10.3390/diagnostics14060571
Chicago/Turabian StyleNadolska, Katarzyna, Agnieszka Białecka, Elżbieta Zawada, Wojciech Kazimierczak, and Zbigniew Serafin. 2024. "Analysis of IVIM Perfusion Fraction Improves Detection of Pancreatic Ductal Adenocarcinoma" Diagnostics 14, no. 6: 571. https://doi.org/10.3390/diagnostics14060571
APA StyleNadolska, K., Białecka, A., Zawada, E., Kazimierczak, W., & Serafin, Z. (2024). Analysis of IVIM Perfusion Fraction Improves Detection of Pancreatic Ductal Adenocarcinoma. Diagnostics, 14(6), 571. https://doi.org/10.3390/diagnostics14060571







