Optimizing Palliative Pelvic Radiotherapy in Gynecological Cancers: A Systematic Review and Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Evaluation of Studies
2.4. Data Extraction and Management
3. Results
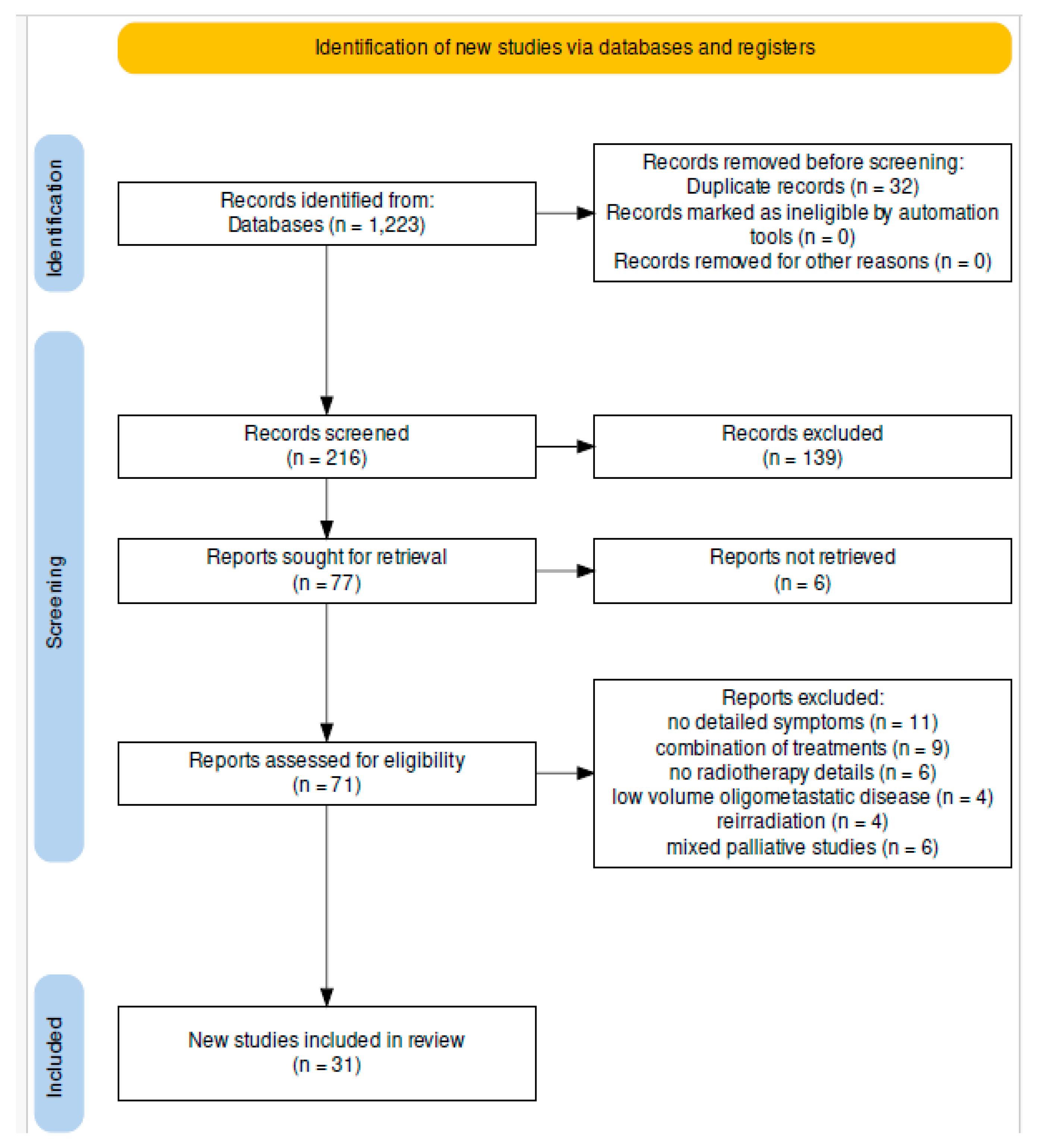
3.1. Study Selection
3.2. Study Characteristics
3.3. Patient Characteristics and Symptoms
3.4. Radiotherapy Dose and Fractionation
3.5. Treatment Response
3.6. Durability of Response
3.7. Dose Response
3.8. Toxicity
| First Author and Year of Publication | Study Design | Number of Patients | Gynecological Cancer | RT Dose | Number of Fractions | Dose Per Fraction | Outcome | Follow-Up | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Faul et al., 2000 [14] | Observational prospective | 2 | Ovarian | 7 | 1 | 7 | 100% complete response (bleeding control) at 1 month | n/a | n/a |
| Macchia et al., 2016 [15] | Observational prospective | 9 | Cervical and uterine | 30 | 3 | 10 | 89% CR; 11% marked improvement (bleeding control) | 20 months | Low |
| Georgina L Jones et al., 2006 [16] | Observational prospective | 16 | Ovarian | n/a | n/a | Single fraction of 7 Gy or 2 fractions of 3 Gy b.d. | Effective palliation, pain relief, and symptom relief | 3 months | n/a |
| F L Ampil et al., 2006 [17] | Retrospective observational | 79 | Cervical | n/a | n/a | n/a | Tumor control | 11 months | Moderate |
| Benoîte Méry et al., 2016 [10] | Retrospective observational | 19 | Uterine, cervical, vulvar, and vaginal | Median of 45 Gy (range: 6–76 Gy) | Median of 18 (range: 1–36 fractions) | Median of 3 Gy (range: 1.5–6 Gy) | Tumor control | 4.5 months | n/a |
| A Tinger et al., 2001 [18] | Retrospective observational | 80 | Ovarian | n/a | n/a | n/a | Partial response | 60 months | Moderate |
| Sri Harsha Kombathula et al., 2022 [19] | Retrospective observational | 184 | Cervical, vaginal, uterine, and ovarian | Median of 35 Gy (range: 10–50 Gy) | Median of 15 (range: 1–20) | Median of 2.33 Gy (range: 2.33–10) | Symptom relief | 36 months | n/a |
| Boulware et al., 1979 [20] | Retrospective observational | 86 | Cervical, vaginal, uterine, and ovarian | 10 Gy | 1 | 10 Gy | Bleeding control and pain relief | 6 months | Low |
| Boulware et al., 1979 [20] | Retrospective observational | 55 | Cervical, vaginal, uterine, and ovarian | 10 Gy at 3–4-week interval | 1 | 10 Gy | Bleeding control and pain relief | 6 months | Moderate |
| Boulware et al., 1979 [20] | Retrospective observational | 20 | Cervical, vaginal, uterine, and ovarian | 10 Gy at 3–4-week interval | 1 | 10 Gy | Bleeding control and pain relief | 6 months | n/a |
| Hodson et al., 1983 [21] | Retrospective observational | 27 | Cervical, vaginal, uterine, and ovarian | 10 Gy at 3–4-week interval | 1 | 10 Gy | Bleeding control, pain relief, and improved vaginal discharge | 7 months | Low |
| Halle et al., 1986 [22] | Retrospective observational | 42 | Cervical and uterine | 10 Gy at 3–4-week interval | 1 | 10 Gy | Bleeding control, pain relief, and improved vaginal discharge | 10 months | Low |
| Onsrud at al., 2001 [23] | Retrospective observational | 64 | Cervical and uterine | 10 Gy | 1 | 10 Gy | Bleeding control and improved vaginal discharge | 12 months | low |
| Mishra et al., 2005 [24] | Retrospective observational | 100 | Cervical | 10 Gy at 4 weeks; brachytherapy 30 Gy at point A | 1–3 | 10 Gy | Bleeding control, pain relief, and improved vaginal discharge | 9 months | High |
| Patricio et al., 1987 [25] | Retrospective observational | 56 | Cervical | 13 Gy | 2 | 6.5 Gy | Bleeding control and pain relief | n/a | High |
| Spanos et al., 1996 [26] | Subgroup analysis of a prospective trial | 61 | Cervical | 14.8 Gy | 4 | 3.7 Gy b.d. | Bleeding control and pain relief | 12 months | Low |
| Grigsby et al., 2002 [27] | Retrospective observational | 15 | Cervical | 10 Gy | 2 | 5 Gy | Bleeding control | n/a | No toxicity |
| Choan E. et al., 2006 [28] | Retrospective observational | 53 | Ovarian | Median of 30 Gy (range: 5–52.5 Gy) | Median of 10 Gy (range: 1–20) | Median of 3 Gy (range: 2.62–5 Gy) | Bleeding control and pain relief | n/a | Low |
| M D Adelson et al., 1987 [29] | Retrospective observational | 42 | Ovarian | 10–30 Gy | 1 to 3 | 10 Gy | Bleeding control and pain relief | n/a | High |
| Corn et al., 2001 [30] | Retrospective observational | 33 | Ovarian | 35 Gy (range: 7.5–45 Gy) | n/a | Median of 2.5 Gy (range: 1–5 Gy) | Symptom relief, bleeding control, pain relief | n/a | Low |
4. Discussion
4.1. Symptom Control in Gynecological Cancers
| Symptom | Overall Response Rate |
|---|---|
| Bleeding | 45–100% [20,21,22,23,24,30,31,41,42,43,44] |
| Pain | 0–83% [20,21,22,23,24,45] |
| Discharge | 39–49% [20,23,24] |
| Obstruction | 19–100% [18,20,41,45] |
4.2. Toxicity Profiles and Technological Advancements
4.3. Clinical Applications and Challenges
4.4. Limitations
4.5. Future Research and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krakauer, E.L.; Kane, K.; Kwete, X.; Afshan, G.; Bazzett-Matabele, L.; Bien-Aimé, D.D.R.; Borges, L.F.; Byrne-Martelli, S.; Connor, S.; Correa, R.; et al. Essential Package of Palliative Care for Women with Cervical Cancer: Responding to the Suffering of a Highly Vulnerable Population. JCO Glob. Oncol. 2021, 7, 873–885. [Google Scholar] [CrossRef]
- Librach, S.L. The principles of palliative radiotherapy: A palliative care physician’s perspective. Can. J. Oncol. 1996, 6 (Suppl. 1), 2–4. [Google Scholar]
- Mackillop, W.J. The principles of palliative radiotherapy: A radiation oncologist’s perspective. Can. J. Oncol. 1996, 6 (Suppl. 1), 5–11. [Google Scholar]
- Preti, M.; Bucchi, L.; Micheletti, L.; Privitera, S.; Corazza, M.; Cosma, S.; Gallio, N.; Borghi, A.; Bevilacqua, F.; Benedetto, C. Four-decade trends in lymph node status of patients with vulvar squamous cell carcinoma in northern Italy. Sci. Rep. 2021, 11, 5661. [Google Scholar] [CrossRef]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the Systematic Review of Palliative Radiotherapy Trials for Bone Metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Lutz, S.T.; Chow, E.L.; Hartsell, W.F.; Konski, A.A. A review of hypofractionated palliative radiotherapy. Cancer 2007, 109, 1462–1470. [Google Scholar] [CrossRef]
- Gutt, R.; Dawson, G.; Cheuk, A.V.; Fosmire, H.; Moghanaki, D.; Kelly, M.; Jolly, S. Palliative Radiotherapy for the Management of Metastatic Cancer: Bone Metastases, Spinal Cord Compression, and Brain Metastases. Fed. Pract. 2015, 32 (Suppl. 4), 12S–16S. [Google Scholar]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Méry, B.; Ndong, S.M.; Guy, J.-B.; Assouline, A.; Falk, A.T.; Valeille, A.; Trone, J.-C.; Rivoirard, R.; Auberdiac, P.; Vallard, A.; et al. Radiotherapy for gynecologic cancer in nonagenarian patients: A framework for new paradigms. Chin. J. Cancer 2016, 35, 43. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, S.; Takahashi, R.; Isohashi, F.; Yokoi, T.; Okazawa, M.; Sasano, T.; Maruoka, S.; Anzai, M.; Yoshioka, Y.; Ogawa, K.; et al. Reirradiation Using High-Dose-Rate Interstitial Brachytherapy for Locally Recurrent Cervical Cancer: A Single Institutional Experience. Int. J. Gynecol. Cancer 2014, 24, 141–148. [Google Scholar] [CrossRef]
- Trippa, F.; Draghini, L.; di Marzo, A.; Anselmo, P.; Arcidiacono, F.; Terenzi, S.; Sivolella, S.; Bassetti, A.; Sdrobolini, A.; Maranzano, E. Long-term palliation of lymph node oligometastatic ovarian carcinoma after repeated stereotactic body radiotherapy: Case report. Tumori J. 2020, 106, NP63–NP66. [Google Scholar] [CrossRef] [PubMed]
- Aoshika, T.; Abe, T.; Iino, M.; Saito, S.; Ryuno, Y.; Ohta, T.; Igari, M.; Hirai, R.; Kumazaki, Y.; Noda, S.-E.; et al. Safety and Efficacy of Palliative Radiotherapy (25 Gy × 5 Fractions) for Symptomatic Pelvic Tumors. Anticancer Res. 2022, 42, 6099–6103. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Gerszten, K.; Edwards, R.; Land, S.; D’angelo, G.; Kelley, J.; Price, F. A phase I/II study of hypofractionated whole abdominal radiation therapy in patients with chemoresistant ovarian carcinoma: Karnofsky score determines treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Macchia, G.; Deodato, F.; Cilla, S.; Legge, F.; Carone, V.; Chiantera, V.; Valentini, V.; Morganti, A.G.; Ferrandina, G. Progestin-releasing intrauterine device insertion plus palliative radiotherapy in frail, elderly uterine cancer patients unfit for radical treatment. Oncol. Lett. 2016, 11, 3446–3450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, G.L.; Ledger, W.; Bonnett, T.J.; Radley, S.; Parkinson, N.; Kennedy, S.H. The impact of treatment for gynecological cancer on health-related quality of life (HRQoL): A systematic review. Am. J. Obstet. Gynecol. 2006, 194, 26–42. [Google Scholar] [CrossRef]
- Ampil, F.L.; Unger, J.B.; Caldito, G.; Charrier, A. Definitive and palliative radiotherapy for cervix cancer in the elderly. Eur. J. Gynaecol. Oncol. 2006, 27, 115–118. [Google Scholar]
- Tinger, A.; Waldron, T.; Peluso, N.; Katin, M.J.; Dosoretz, D.E.; Blitzer, P.H.; Rubenstein, J.H.; Garton, G.R.; Nakfoor, B.A.; Patrice, S.J.; et al. Effective palliative radiation therapy in advanced and recurrent ovarian carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 1256–1263. [Google Scholar] [CrossRef]
- Kombathula, S.H.; Cree, A.; Joshi, P.V.; Akturk, N.; Barraclough, L.H.; Haslett, K.; Choudhury, A.; Hoskin, P. Palliative radiotherapy in cancers of female genital tract: Outcomes and prognostic factors. Radiother. Oncol. 2022, 175, 42–46. [Google Scholar] [CrossRef]
- Boulware, R.J.; Caderao, J.B.; Delclos, L.; Wharton, J.T.; Peters, L.J. Whole pelvis megavoltage irradiation with single doses of 1000 rad to palliate advanced gynecologic cancers. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 333–338. [Google Scholar] [CrossRef]
- Hodson, D.; Krepart, G. Once-monthly radiotherapy for the palliation of pelvic gynecological malignancy. Gynecol. Oncol. 1983, 16, 112–116. [Google Scholar] [CrossRef]
- Halle, J.S.; Rosenman, J.G.; Varia, M.A.; Fowler, W.C.; Walton, L.A.; Currie, J.L. 1000 CGY single dose palliation for advanced carcinoma of the cervix or endometrium. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Onsrud, M.; Hagen, B.; Strickert, T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol. Oncol. 2001, 82, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Laskar, S.; Mishra, S.; Muckaden, M.A.; Mohindra, P.; Shrivastava, S.; Dinshaw, K. Monthly palliative pelvic radiotherapy in advanced carcinoma of uterine cervix. J. Cancer Res. Ther. 2005, 1, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Patrício, M.B.; Tavares, M.A.; Guimarães, M.F.; Belo, M.C.; Vilhena, M. Haemostatic and antialgic effects of the 25 mv photon beam concentrated dose in the treatment of carcinoma of the cervix. J. Surg. Oncol. 1987, 34, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Spanos, W.J.; Pajak, T.J.; Emami, B.; Rubin, P.; Cooper, J.S.; Russell, A.H.; Cox, J.D. Radiation palliation of cervical cancer. J. Natl. Cancer Inst. Monogr. 1996, 21, 127–130. [Google Scholar]
- Grigsby, P.W.; Portelance, L.; Williamson, J.F. High dose rate (HDR) cervical ring applicator to control bleeding from cervical carcinoma. Int. J. Gynecol. Cancer 2002, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Choan, E.; Quon, M.; Gallant, V.; Samant, R. Effective palliative radiotherapy for symptomatic recurrent or residual ovarian cancer. Gynecol. Oncol. 2006, 102, 204–209. [Google Scholar] [CrossRef]
- Adelson, M.D.; Wharton, J.T.; Delclos, L.; Copeland, L.; Gershenson, D. Palliative radiotherapy for ovarian cancer. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 17–21. [Google Scholar] [CrossRef]
- Corn, B.W.; Lanciano, R.M.; Boente, M.; Hunter, W.M.; Ladazack, J.; Ozols, R.F. Recurrent ovarian cancer. Effective radiotherapeutic palliation after chemotherapy failure. Cancer 1994, 74, 2979–2983. [Google Scholar] [CrossRef]
- Yan, J.; Milosevic, M.; Fyles, A.; Manchul, L.; Kelly, V.; Levin, W. A Hypofractionated Radiotherapy Regimen (0-7-21) for Advanced Gynaecological Cancer Patients. Clin. Oncol. 2011, 23, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Rai, B.; Kumar, S.; Suri, V.; Ghoshal, S. Fractionated Palliative Pelvic Radiotherapy as an Effective Modality in the Management of Recurrent/Refractory Epithelial Ovarian Cancers: An Institutional Experience. J. Obstet. Gynecol. India 2017, 67, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.H.; Ki, Y.K.; Nam, J.H.; Kim, W.T.; Jeon, H.S.; Park, D. Short-course palliative radiotherapy for uterine cervical cancer. Radiat. Oncol. J. 2013, 31, 216–221. [Google Scholar] [CrossRef]
- Tan, H.S. Use of high dose rate gammamed brachytherapy in the palliative treatment of gynecological cancer. Ann. Acad. Med. Singap. 1994, 23, 231–234. [Google Scholar]
- Biswal, B.; Lal, P.; Rath, G.; Mohanti, B. Hemostatic radiotherapy in carcinoma of the uterine cervix. Int. J. Gynecol. Obstet. 1995, 50, 281–285. [Google Scholar] [CrossRef]
- Firat, S.; Erickson, B. Selective Irradiation for the Treatment of Recurrent Ovarian Carcinoma Involving the Vagina or Rectum. Gynecol. Oncol. 2001, 80, 213–220. [Google Scholar] [CrossRef]
- Pirtoli, L.; Ciatto, S.; Cionini, L.; Taddei, G.; Colafranceschi, M. Salvage with Radiotherapy of Postsurgical Relapses of Endometrial Cancer. Tumori J. 1980, 66, 475–480. [Google Scholar] [CrossRef]
- Chung, J.Y.; Roberts, K.; Peschel, R.E.; Nath, R.; Pourang, R.; Kacinski, B.; Wilson, L. Treatment of recurrent pelvic and selected primary gynecologic malignancies with 241Am. Radiat. Oncol. Investig. 1997, 5, 227–234. [Google Scholar] [CrossRef]
- Gelblum, D.; Mychalczak, B.; Almadrones, L.; Spriggs, D.; Barakat, R. Palliative benefit of external-beam radiation in the management of platinum refractory epithelial ovarian carcinoma. Gynecol. Oncol. 1998, 69, 36–41. [Google Scholar] [CrossRef]
- Chafe, W.; Fowler, W.C.; Currie, J.L.; Davis, M.L.; Walton, L.A.; Montana, G. Single-fraction palliative pelvic radiation therapy in gynecologic oncology: 1,000 rads. Am. J. Obstet. Gynecol. 1984, 148, 701–705. [Google Scholar] [CrossRef]
- May, L.F.; Belinson, J.L.; Roland, T.A. Palliative benefit of radiation therapy in advanced ovarian cancer. Gynecol. Oncol. 1990, 37, 408–411. [Google Scholar] [CrossRef]
- Caravatta, L.; Padula, G.D.; Macchia, G.; Ferrandina, G.; Bonomo, P.; Deodato, F.; Massaccesi, M.; Mignogna, S.; Tambaro, R.; Rossi, M.; et al. Short-course accelerated radiotherapy in palliative treatment of advanced pelvic malignancies: A phase I study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e627–e631. [Google Scholar] [CrossRef]
- De Meerleer, G.; Vandecasteele, K.; Ost, P.; Delrue, L.; Denys, H.; Makar, A.; Speleers, B.; Van Belle, S.; Broecke, R.V.D.; Fonteyne, V.; et al. Whole abdominopelvic radiotherapy using intensity-modulated arc therapy in the palliative treatment of chemothera-py-resistant ovarian cancer with bulky peritoneal disease: A single-institution experience. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Butala, A.A.; Lee, D.Y.; Patel, R.R.; Latif, N.A.; Haggerty, A.F.; Paydar, I.; Jones, J.A.; Taunk, N.K. A Retrospective Study of Rapid Symptom Response in Bleeding Gynecologic Malignancies with Short Course Palliative Radiation Therapy: Less is More. J. Pain Symptom Manag. 2021, 61, 377–383.e2. [Google Scholar] [CrossRef] [PubMed]
- Spanos, W.J.; Perez, C.A.; Marcus, S.; Poulter, C.A.; Doggett, R.; Steinfeld, A.D.; Grigsby, P.W. Effect of rest interval on tumor and normal tissue response—A report of phase III study of accelerated split course palliative radiation for advanced pelvic malignancies (RTOG-8502). Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Hoskin, P.; Mitera, G.; Zeng, L.; Lutz, S.; Roos, D.; Hahn, C.; van der Linden, Y.; Hartsell, W.; Kumar, E.; et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Hopkins, K.; Misra, V.; Holt, T.; McMenemin, R.; Dubois, D. Effect of single-fraction vs. multifraction radiotherapy on ambulatory status among patients with spinal canal compression from metastatic cancer: The SCORAD randomized clinical trial. JAMA 2019, 322, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO evidence-based guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef]
- Guckenberger, M.; Baus, W.W.; Blanck, O.; Combs, S.E.; Debus, J.; Engenhart-Cabillic, R.; Gauer, T.; Grosu, A.L.; Schmitt, D.; Tanadini-Lang, S.; et al. Definition and quality requirements for stereotactic radiotherapy: Consensus statement from the DEGRO/DGMP Working Group Stereotactic Radiotherapy and Radiosurgery. Strahlenther. Onkol. 2020, 196, 417–420. [Google Scholar] [CrossRef]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: An open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2022, 23, 397–407. [Google Scholar] [CrossRef]
- Ahmed, K.A.; Caudell, J.J.; El-Haddad, G.; Berglund, A.E.; Welsh, E.A. Stereotactic body radiotherapy for liver metastases. Lancet Oncol. 2019, 20, e364. [Google Scholar]
- Tsai, C.J.; Hong, J.C.; Alektiar, K.M. Cost-effectiveness analysis of conventional and hypofractionated radiation therapy for localized prostate cancer from a randomized trial. JAMA Oncol. 2021, 7, 1049–1056. [Google Scholar]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W.; Thompson, S.; et al. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology–RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.; Pugh, S.; Klopp, A.; Gil, K.; Wenzel, L.; Westin, S.; Konski, A.; Thompson, J.; Doncals, D.; Cantuaria, G.; et al. IMRT Improves Late Toxicity Compared to Conventional RT: An Update on NRG Oncology-RTOG 1203. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S50. [Google Scholar] [CrossRef]
- Rezk, Y.; Timmins, P.F.; Smith, H.S. Review Article: Palliative Care in Gynecologic Oncology. Am. J. Hosp. Palliat. Med. 2010, 28, 356–374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, B.; Georgescu, M.-T.; Serboiu, C.S.; Marinescu, A.N.; Aliuș, C.; Georgescu, D.-E.; Mocanu, B.; Sucuri, S.; Stanescu, A.D. Optimizing Palliative Pelvic Radiotherapy in Gynecological Cancers: A Systematic Review and Analysis. Diagnostics 2024, 14, 547. https://doi.org/10.3390/diagnostics14050547
Anghel B, Georgescu M-T, Serboiu CS, Marinescu AN, Aliuș C, Georgescu D-E, Mocanu B, Sucuri S, Stanescu AD. Optimizing Palliative Pelvic Radiotherapy in Gynecological Cancers: A Systematic Review and Analysis. Diagnostics. 2024; 14(5):547. https://doi.org/10.3390/diagnostics14050547
Chicago/Turabian StyleAnghel, Beatrice, Mihai-Teodor Georgescu, Crenguta Sorina Serboiu, Andreea Nicoleta Marinescu, Cătălin Aliuș, Dragoș-Eugen Georgescu, Bogdan Mocanu, Sabina Sucuri, and Anca Daniela Stanescu. 2024. "Optimizing Palliative Pelvic Radiotherapy in Gynecological Cancers: A Systematic Review and Analysis" Diagnostics 14, no. 5: 547. https://doi.org/10.3390/diagnostics14050547
APA StyleAnghel, B., Georgescu, M.-T., Serboiu, C. S., Marinescu, A. N., Aliuș, C., Georgescu, D.-E., Mocanu, B., Sucuri, S., & Stanescu, A. D. (2024). Optimizing Palliative Pelvic Radiotherapy in Gynecological Cancers: A Systematic Review and Analysis. Diagnostics, 14(5), 547. https://doi.org/10.3390/diagnostics14050547






