Differentiating Viral from Bacterial Pneumonia in Children: The Diagnostic Role of Lung Ultrasound—A Prospective Observational Study
Abstract
1. Introduction
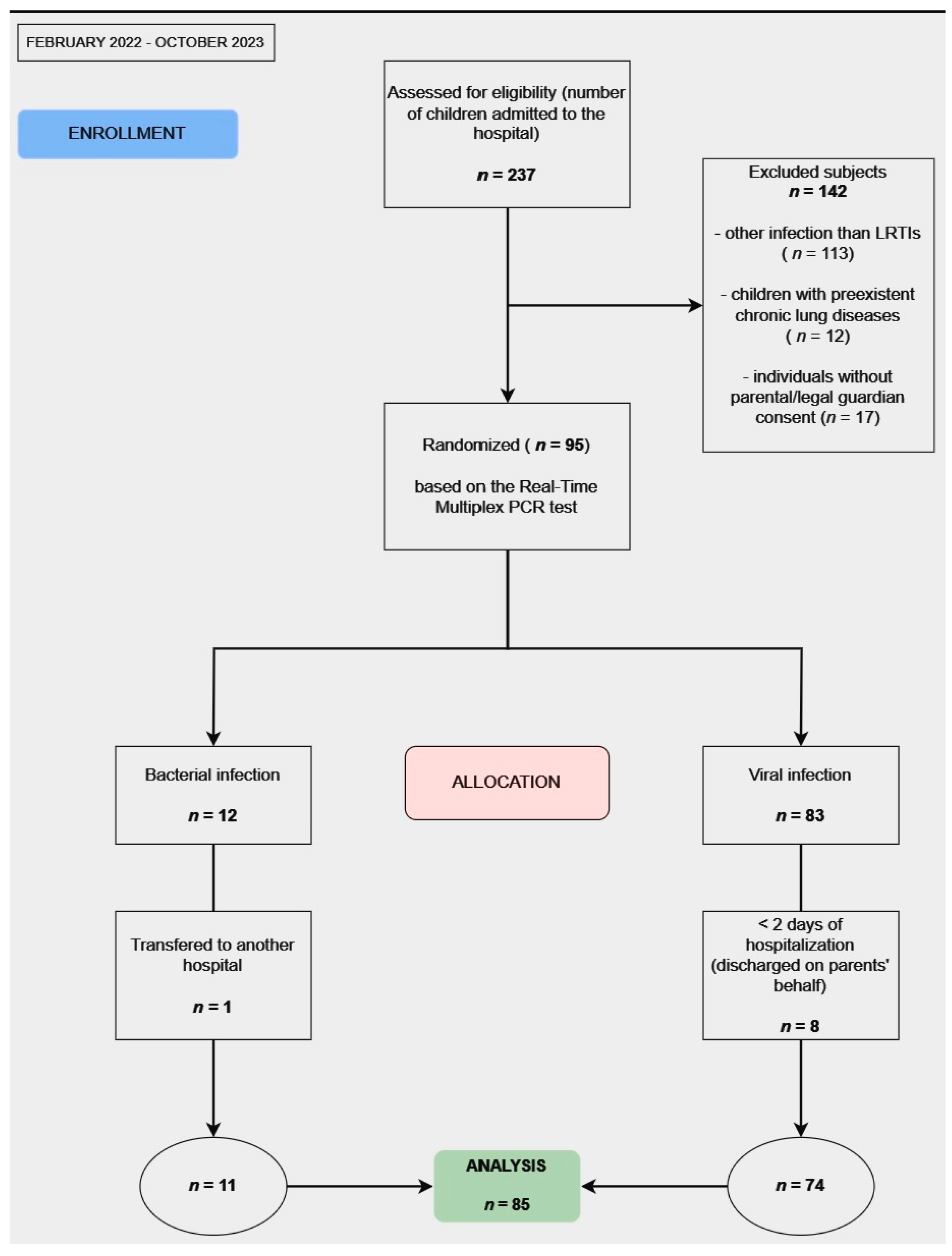
2. Materials and Methods
2.1. Study Design
2.2. Participant Selection
2.3. Lung Ultrasound Examination
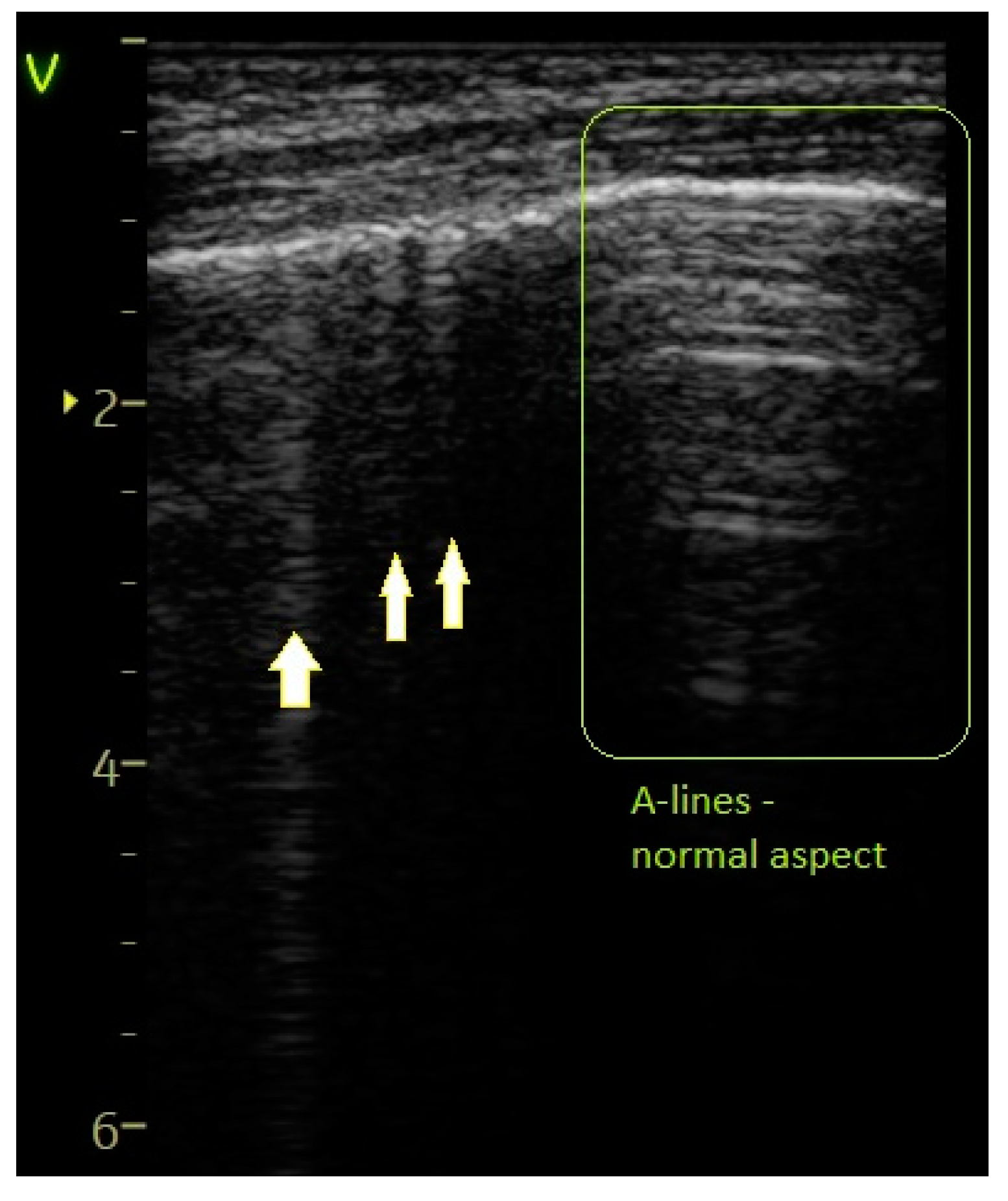
2.4. Lung Ultrasound Protocol
- ▪
- A score of 0 was assigned for a normal or physiological pattern displaying A-lines, along with one or two B-lines per intercostal space.
- ▪
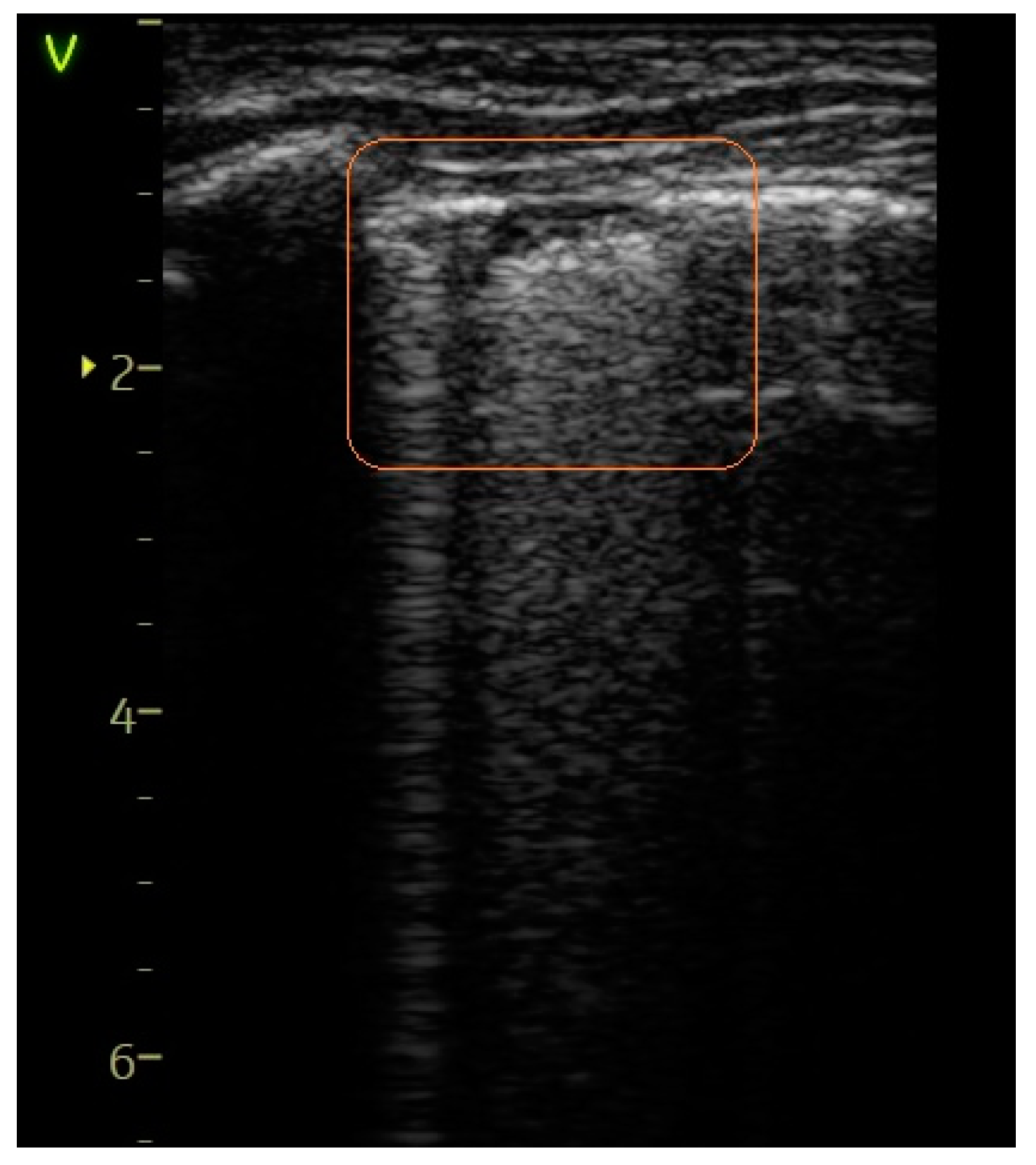
- A score of 1 indicated the observation of more than two B-lines (referred to as sparse B-lines) per intercostal space, accompanied by pleural abnormalities, such as irregularities or thickening.
- ▪
- A score of 2 was allocated for the presence of coalescent or merging B-lines, a ‘white-lung’ appearance, or small peripheral consolidations smaller than 1 cm.
- ▪
- A score of 3 was given for substantial peripheral consolidations wider than 1 cm, regardless of the presence of air bronchograms.
2.5. Data Collection and Analysis
2.6. Ethical Considerations
3. Results
3.1. Baseline Characteristics
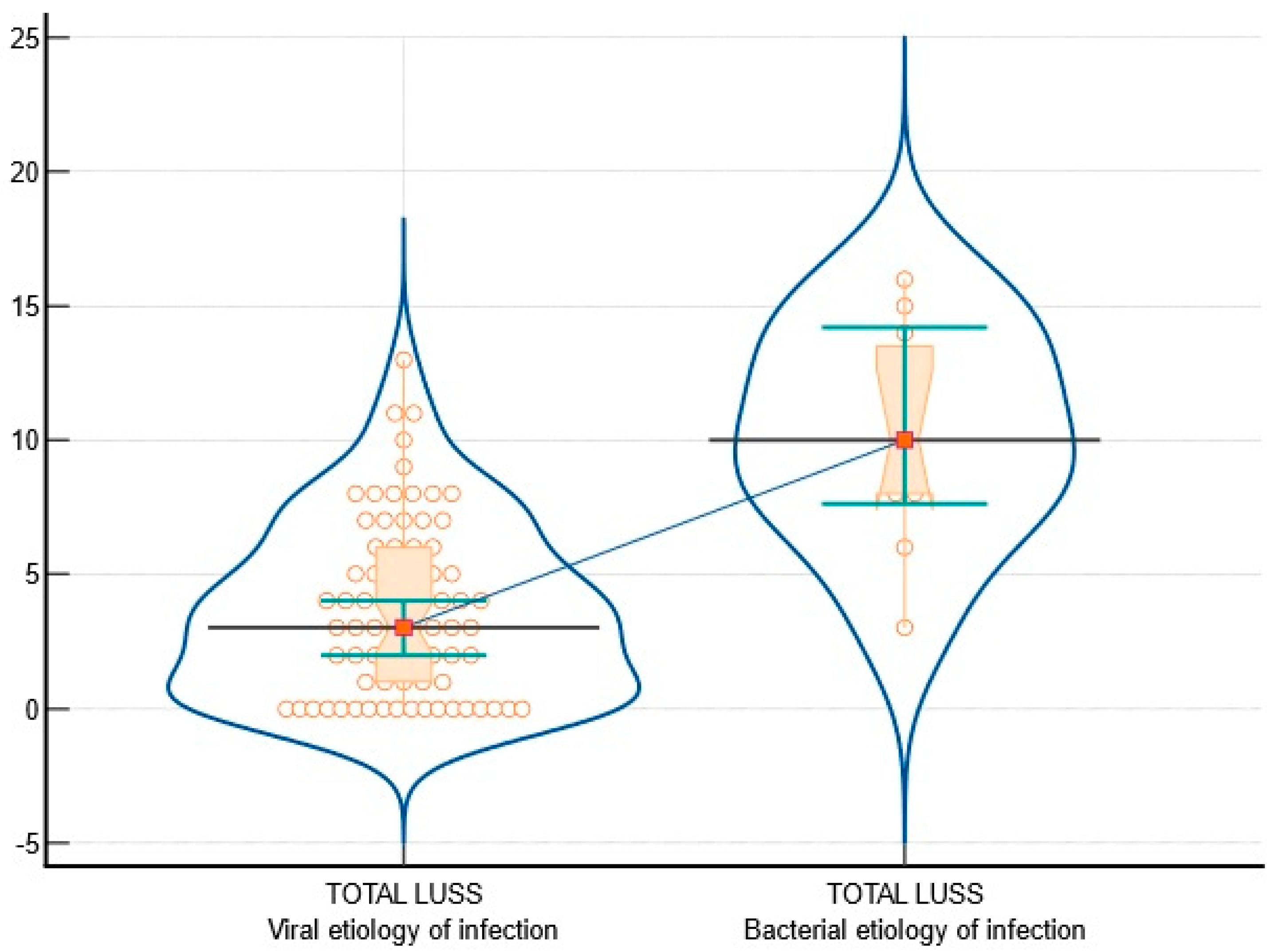
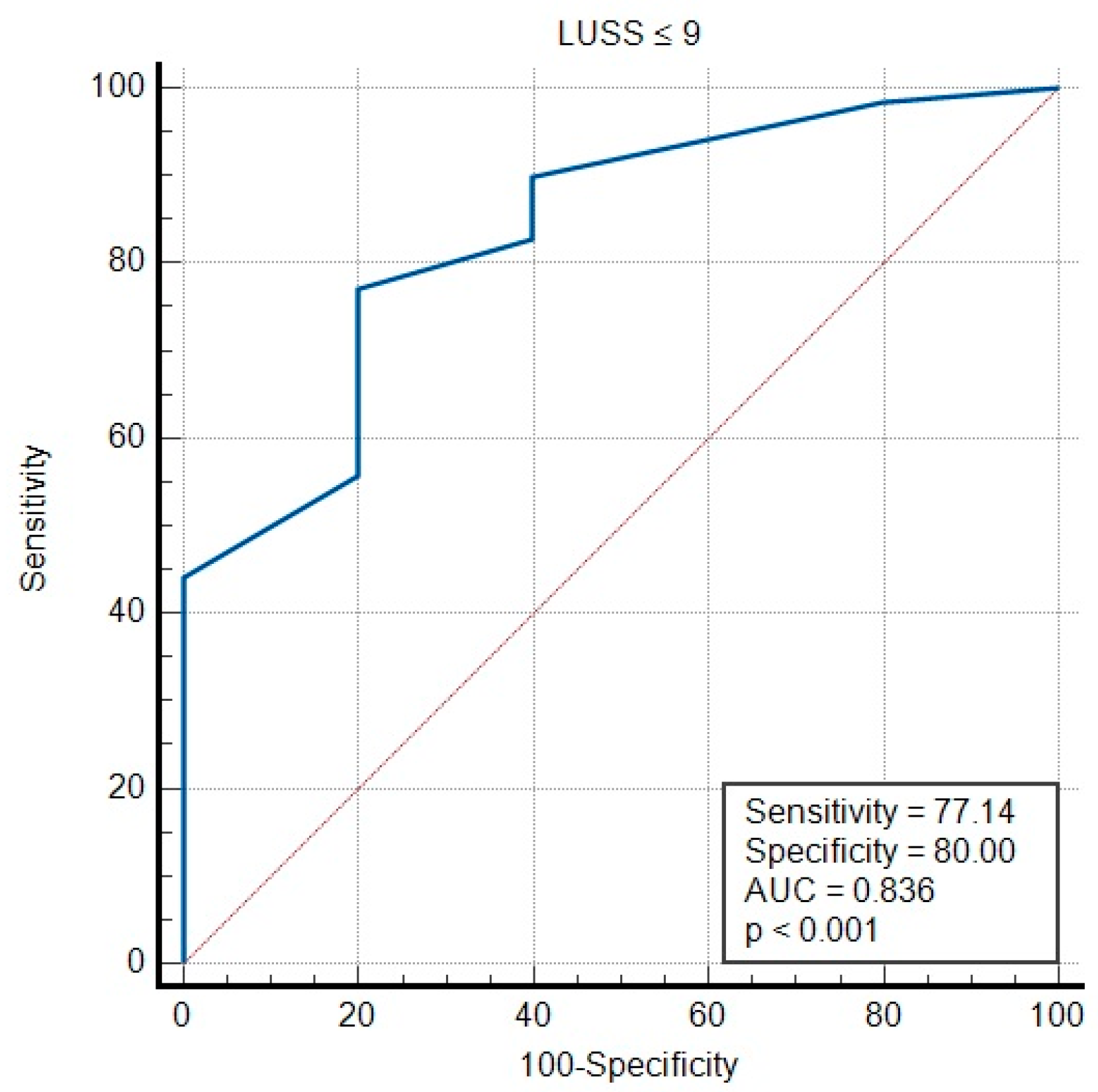
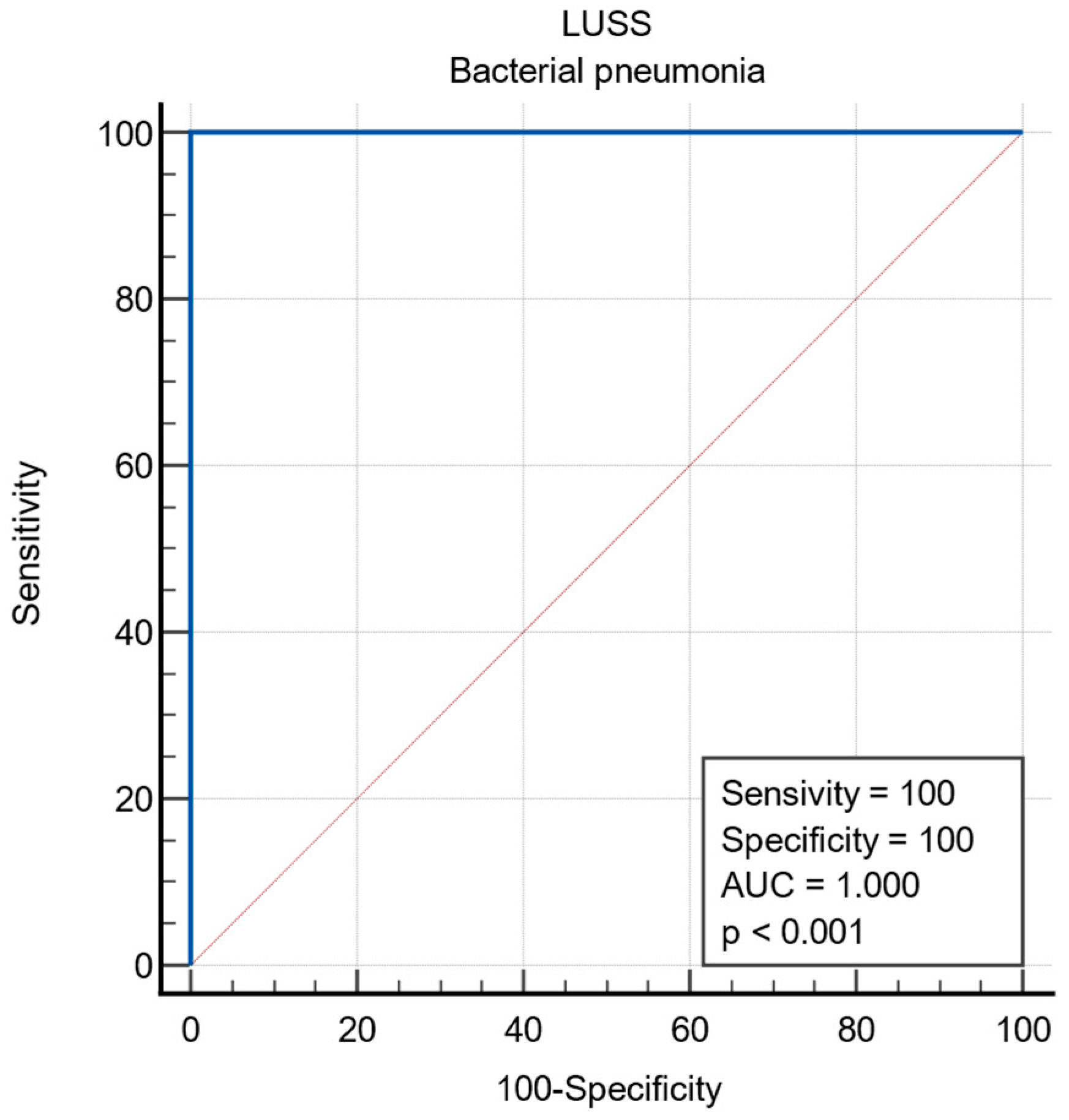
3.2. Viral vs. Bacterial Etiology of Infection
| LUS Findings | Number of Patients with Viral Pathologies (v = 74) | Number of Patients with Bacterial-Etiology Pathologies (b = 11) | Difference | Chi-Squared | Value |
|---|---|---|---|---|---|
| Sparse B-lines—Figure 3 | 55 (74.32%) | 11 (100%) | 25.68% | 3.59 | 0.0579 |
| Confluent B-lines—Figure 4, Figure 5 and Figure 6 | 23 (31.08%) | 10 (90.91%) | 59.83% | 14.26 | 0.0002 |
| Pleural abnormalities—Figure 4 and Figure 5 | 24 (32.43%) | 9 (81.82%) | 49.39% | 9.72 | 0.001 |
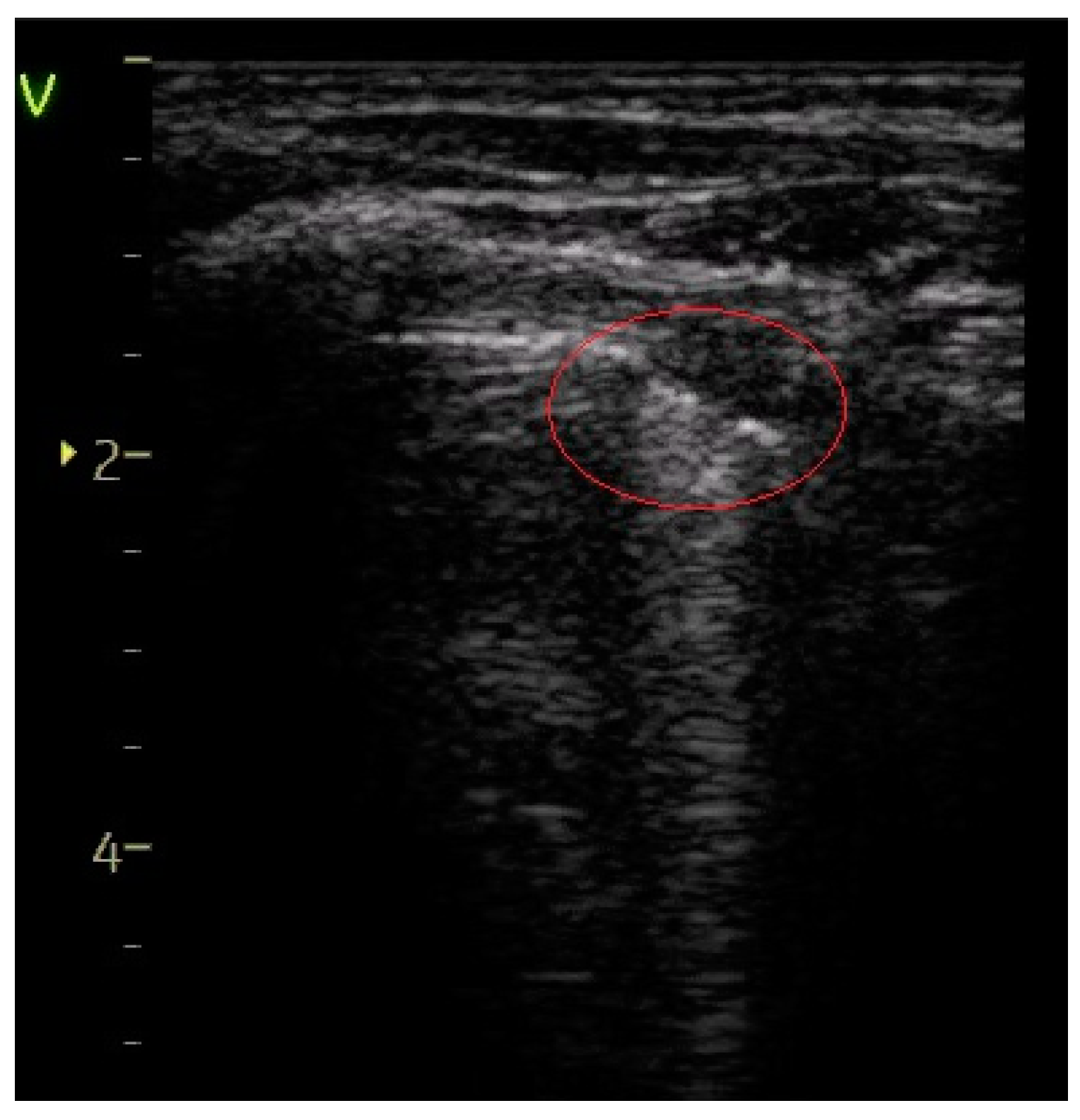
| Subpleural consolidation of < 1 cm—Figure 6 | 15 (20.27%) | 9 (81.82%) | 61.55% | 17.69 | <0.0001 |
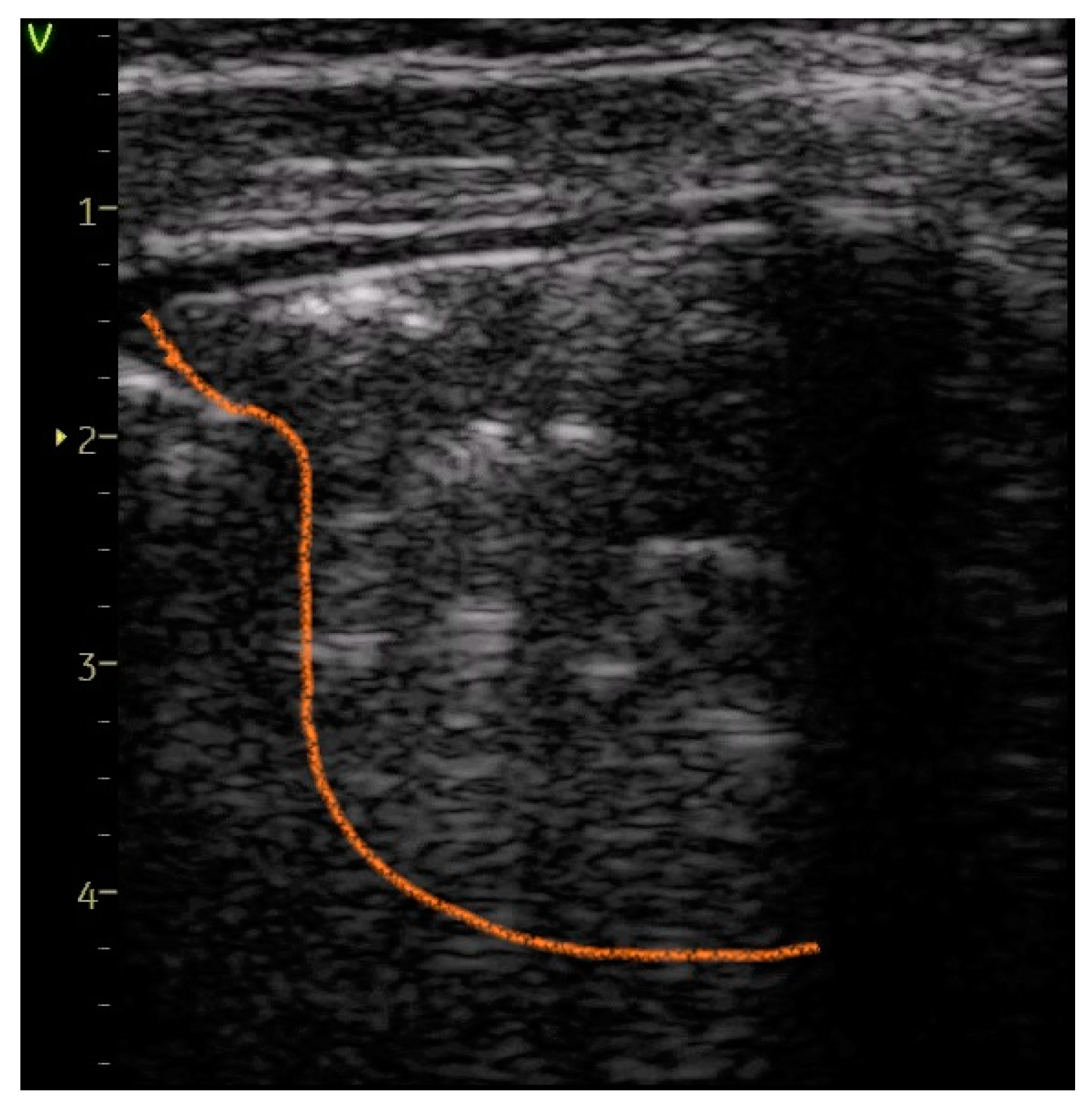
| Large consolidation of > 1 cm—Figure 7 | 0 | 5 (45.45%) | 45.45% | 35.31 | <0.0001 |
| Pleural effusion | 0 | 1 (9.09%) | 9.09% | 6.72 | 0.009 |
4. Discussion
4.1. Limitations
4.2. Further Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations in Alphabetical Order
| Abbreviation | Term |
| CT | Computed tomography |
| CXR | Chest radiography |
| LRTIs | Lower respiratory tract infections |
| LUS | Lung ultrasound |
| LUSS | Lung ultrasound score |
| PCR | Polymerase chain reaction |
| VIP | Viral interstitial pneumonia |
References
- Kevat, P.M.; Morpeth, M.; Graham, H.; Gray, A.Z. A Systematic Review of the Clinical Features of Pneumonia in Children Aged 5–9 Years: Implications for Guidelines and Research. J. Glob. Health 2022, 12, 10002. [Google Scholar] [CrossRef]
- Mathew, J.L. Etiology of Childhood Pneumonia: What We Know, and What We Need to Know: Based on 5th Dr. IC Verma Excellence Oration Award. Indian J. Pediatr. 2018, 85, 25–34. [Google Scholar] [CrossRef]
- Hansen, L.S.; Lykkegaard, J.; Thomsen, J.L.; Hansen, M.P. Acute Lower Respiratory Tract Infections: Symptoms, Findings and Management in Danish General Practice. Eur. J. Gen. Pract. 2020, 26, 14–20. [Google Scholar] [CrossRef]
- Chee, E.; Huang, K.; Haggie, S.; Britton, P.N. Systematic Review of Clinical Practice Guidelines on the Management of Community Acquired Pneumonia in Children. Paediatr. Respir. Rev. 2022, 42, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, I.M.; Dediu, M.; Marc, M.S.; Lukic, M.; Horhat, D.I.; Pop, L.L. Lung Ultrasound Is More Sensitive for Hospitalized Consolidated Pneumonia Diagnosis Compared to CXR in Children. Children 2021, 8, 659. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Dediu, M.; Pop, L.L. Pediatric Pneumonia (PedPne) Lung Ultrasound Score and Inflammatory Markers: A Pilot Study. Pediatr. Pulmonol. 2022, 57, 576–582. [Google Scholar] [CrossRef]
- Bolintineanu Ghenciu, L.A.; Bolintineanu, S.L.; Iacob, R.; Stoicescu, E.R.; Zahoi, D.E. Hepatic Arterial Variations Detected at Multidetector Computer Tomography Angiography in the Romanian Population. Folia Morphol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lovrenski, J. Pediatric Lung Ultrasound—Pros and Potentials. Pediatr. Radiol. 2020, 50, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lovrenski, J. Pediatric Lung Ultrasound Cons—Are They Really Strong Enough? Pediatr. Radiol. 2020, 50, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Berce, V.; Tomazin, M.; Gorenjak, M.; Berce, T.; Lovrenčič, B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci. Rep. 2019, 9, 17957. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Pop, L.L.; Dediu, M.; Stoicescu, E.R.; Marc, M.S.; Manea, A.M.; Manolescu, D.L. Lung Ultrasound in Children with Cystic Fibrosis in Comparison with Chest Computed Tomography: A Feasibility Study. Diagnostics 2022, 12, 376. [Google Scholar] [CrossRef]
- Buonsenso, D.; Brancato, F.; Valentini, P.; Curatola, A.; Supino, M.; Musolino, A.M. The Use of Lung Ultrasound to Monitor the Antibiotic Response of Community-Acquired Pneumonia in Children: A Preliminary Hypothesis. J. Ultrasound Med. 2020, 39, 817–826. [Google Scholar] [CrossRef]
- Meli, M.; Spicuzza, L.; Comella, M.; La Spina, M.; Trobia, G.L.; Parisi, G.F.; Di Cataldo, A.; Russo, G. The Role of Ultrasound in the Diagnosis of Pulmonary Infection Caused by Intracellular, Fungal Pathogens and Mycobacteria: A Systematic Review. Diagnostics 2023, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Cocco, G.; D’Ardes, D.; Delli Pizzi, A.; Vidili, G.; De Molo, C.; Vicari, S.; Serra, C.; Cipollone, F.; Schiavone, C.; et al. Infectious Pneumonia and Lung Ultrasound: A Review. J. Clin. Med. 2023, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; De Sanctis, R.; Chiaretti, A.; Biasucci, D.G.; et al. Role of Lung Ultrasound for the Etiological Diagnosis of Acute Lower Respiratory Tract Infection (ALRTI) in Children: A Prospective Study. J. Ultrasound 2022, 25, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Vardhelli, V.; Pandita, A.; Pillai, A.; Badatya, S.K. Perinatal COVID-19: Review of Current Evidence and Practical Approach towards Prevention and Management. Eur. J. Pediatr. 2021, 180, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, S.; Duggan, N.M.; Cohen, A.R.; Shokoohi, H. Lung Ultrasound in Children with Respiratory Tract Infections: Viral, Bacterial or COVID-19? A Narrative Review. Open Access Emerg. Med. 2020, 12, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, E.R.; Ciuca, I.M.; Iacob, R.; Iacob, E.R.; Marc, M.S.; Birsasteanu, F.; Manolescu, D.L.; Iacob, D. Is Lung Ultrasound Helpful in COVID-19 Neonates?—A Systematic Review. Diagnostics 2021, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Mearelli, F.; Casarsa, C.; Trapani, A.; D’agaro, P.; Moras, C.; Spagnol, F.; Pellicori, F.; Nunnari, A.; Massolin, A.; Barbati, G.; et al. Lung Ultrasound May Support Internal Medicine Physicians in Predicting the Diagnosis, Bacterial Etiology and Favorable Outcome of Community-Acquired Pneumonia. Sci. Rep. 2021, 11, 17016. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Pierantoni, L.; Baldazzi, M.; Greco, L.; Dormi, A.; Dondi, A.; Faldella, G.; Lanari, M. Lung Ultrasound for the Diagnosis of Pneumonia in Children with Acute Bronchiolitis. BMC Pulm. Med. 2018, 18, 191. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Buda, N.; Ciuca, I.M.; Dong, Y.; Fang, C.; Feldkamp, A.; Jüngert, J.; Kosiak, W.; Mentzel, H.J.; Pienar, C.; et al. Lung Ultrasound in Children, WFUMB Review Paper (Part 2). Med. Ultrason. 2021, 23, 443. [Google Scholar] [CrossRef]
- Iacob, R.; Stoicescu, E.R.; Cerbu, S.; Iacob, D.; Amaricai, E.; Catan, L.; Belei, O.; Iacob, E.R. Could Ultrasound Be Used as a Triage Tool in Diagnosing Fractures in Children? A Literature Review. Healthcare 2022, 10, 823. [Google Scholar] [CrossRef]
- Kutanzi, K.R.; Lumen, A.; Koturbash, I.; Miousse, I.R. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public. Health 2016, 13, 1057. [Google Scholar] [CrossRef]
- Oakley, P.A.; Harrison, D.E. Death of the ALARA Radiation Protection Principle as Used in the Medical Sector. Dose Response 2020, 18, 1559325820921641. [Google Scholar] [CrossRef]
- Stoicescu, E.R.; Manolescu, D.L.; Iacob, R.; Cerbu, S.; Dima, M.; Iacob, E.R.; Ciuca, I.M.; Oancea, C.; Iacob, D. The Assessment of COVID-19 Pneumonia in Neonates: Observed by Lung Ultrasound Technique and Correlated with Biomarkers and Symptoms. JCM 2022, 11, 3555. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Dransart-Rayé, O.; Mojoli, F.; Mongodi, S. Lung Ultrasound for Diagnosis and Monitoring of Ventilator-Associated Pneumonia. Ann. Transl. Med. 2018, 6, 418. [Google Scholar] [CrossRef]
- Haggag, Y.I.; Mashhour, K.; Ahmed, K.; Samir, N.; Radwan, W. Effectiveness of Lung Ultrasound in Comparison with Chest X-Ray in Diagnosis of Lung Consolidation. Open Access Maced. J. Med. Sci. 2019, 7, 2457–2461. [Google Scholar] [CrossRef]
- Lentz, B.; Fong, T.; Rhyne, R.; Risko, N. A Systematic Review of the Cost-Effectiveness of Ultrasound in Emergency Care Settings. Ultrasound J. 2021, 13, 16. [Google Scholar] [CrossRef]
- Liu, X.; Si, S.; Guo, Y.; Wu, H. Limitations of Bedside Lung Ultrasound in Neonatal Lung Diseases. Front. Pediatr. 2022, 10, 855958. [Google Scholar] [CrossRef] [PubMed]
- Marini, T.J.; Rubens, D.J.; Zhao, Y.T.; Weis, J.; O’Connor, T.P.; Novak, W.H.; Kaproth-Joslin, K.A. Lung Ultrasound: The Essentials. Radiol. Cardiothorac. Imaging 2021, 3, e200564. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; Bouhemad, B.; Orlando, A.; Stella, A.; Tavazzi, G.; Via, G.; Iotti, G.A.; Braschi, A.; Mojoli, F. Modified Lung Ultrasound Score for Assessing and Monitoring Pulmonary Aeration. Ultraschall Med. 2017, 38, 530–537. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive Statistics and Normality Tests for Statistical Data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Hart, A. Mann-Whitney Test Is Not Just a Test of Medians: Differences in Spread Can Be Important. BMJ 2001, 323, 391–393. [Google Scholar] [CrossRef]
- Annamalay, A.; Le Souëf, P. Viral-Bacterial Interactions in Childhood Respiratory Tract Infections. In Viral Infections in Children, Volume I; Green, R.J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 193–214. ISBN 978-3-319-54032-0. [Google Scholar]
- Malla, D.; Rathi, V.; Gomber, S.; Upreti, L. Can Lung Ultrasound Differentiate between Bacterial and Viral Pneumonia in Children? J. Clin. Ultrasound 2021, 49, 91–100. [Google Scholar] [CrossRef]
- Stoicescu, E.R.; Lovrenski, J.; Iacob, R.; Cerbu, S.; Iacob, D.; Iacob, E.R.; Susa, S.R.; Ciuca, I.M.; Bolintineanu, L.A.; Ciornei-Hoffman, A.; et al. COVID-19 in Infants and Children under 2 Years—Could Lung Ultrasound Score Be Correlated with Biomarkers and Symptoms? Biomedicines 2023, 11, 2620. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Licari, A.; Montagna, L.; Votto, M.; Leonardi, S.; Brambilla, I.; Castagnoli, R.; Foiadelli, T.; Marseglia, G.L.; Cardinale, F.; et al. SARS-CoV-2 Infection in Pediatric Population. Acta Biomed. Atenei Parm. 2020, 91, e2020003. [Google Scholar] [CrossRef]
- Goldsmith, A.J.; Al Saud, A.; Duggan, N.M.; Ma, I.W.; Huang, C.K.; Eke, O.; Kapur, T.; Kharasch, S.; Liteplo, A.; Shokoohi, H. Point-of-Care Lung Ultrasound for Differentiating COVID-19 From Influenza. Cureus 2022, 14, e21116. [Google Scholar] [CrossRef] [PubMed]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E.; Mele, G. Lung Ultrasound Characteristics of Community-Acquired Pneumonia in Hospitalized Children. Pediatr. Pulmonol. 2013, 48, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Caroselli, C.; Blaivas, M.; Falzetti, S. Diagnostic Imaging in Newborns, Children and Adolescents Infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Is There a Realistic Alternative to Lung High-Resolution Computed Tomography (HRCT) and Chest X-Rays? A Systematic Review of the Literature. Ultrasound Med. Biol. 2021, 47, 3034–3040. [Google Scholar] [CrossRef]
- Maggi, L.; Biava, A.M.; Fiorelli, S.; Coluzzi, F.; Ricci, A.; Rocco, M. Lung Ultrasound: A Diagnostic Leading Tool for SARS-CoV-2 Pneumonia: A Narrative Review. Diagnostics 2021, 11, 2381. [Google Scholar] [CrossRef]

| Characteristic | Entire Lot (n = 85) | Viral Infection (v = 74) | Bacterial Infection (b = 11) | p-Value |
|---|---|---|---|---|
| Female gender | 37 (43.52%) | 35 (47.29%) | 2 (18.18%) | 0.0709 |
| Rural areas | 29 (34.11%) | 24 (32.43%) | 5 (45.45%) | 0.5845 |
| Weight (kg) | 10.8; (7, 15) | 9.8; (7, 15) | 14; (10.05, 21.25) | 0.0379 |
| Age (months) | 14; (5, 39) | 12; (4, 24) | 36; (23.25, 81.75) | 0.0123 |
| Days of hospitalization | 5; (4, 7) | 5; (4, 6) | 7; (6.25, 8.75) | 0.0007 |
| Days of convalescence | 9; (7, 11.25) | 8; (6, 11) | 13; (9, 14.75) | 0.0070 |
| Finding | Bacterial | Viral | SARS-CoV-2 |
|---|---|---|---|
| Distribution of involvement | Lateral and posterior areas | More diffuse | Posterior/lateral subpleural |
| Pleural line | Irregular near consolidation | Irregular and thickened | Irregular and thickened |
| Lung parenchyma | B-lines ↓ Focal consolidations with confluent B-lines nearby Air bronchogram | Sparse B-lines ↑ Confluent B-lines ↓ | Sparse B-lines Confluent B-lines |
| Consolidations | Focal consolidations (> 1 cm) and hepatization | < 1 cm | < 1 cm subpleural consolidations |
| Pleural effusion | +/− | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoicescu, E.R.; Iacob, R.; Ilie, A.C.; Iacob, E.R.; Susa, S.R.; Ghenciu, L.A.; Constantinescu, A.; Cocolea, D.M.; Oancea, C.; Manolescu, D.L. Differentiating Viral from Bacterial Pneumonia in Children: The Diagnostic Role of Lung Ultrasound—A Prospective Observational Study. Diagnostics 2024, 14, 480. https://doi.org/10.3390/diagnostics14050480
Stoicescu ER, Iacob R, Ilie AC, Iacob ER, Susa SR, Ghenciu LA, Constantinescu A, Cocolea DM, Oancea C, Manolescu DL. Differentiating Viral from Bacterial Pneumonia in Children: The Diagnostic Role of Lung Ultrasound—A Prospective Observational Study. Diagnostics. 2024; 14(5):480. https://doi.org/10.3390/diagnostics14050480
Chicago/Turabian StyleStoicescu, Emil Robert, Roxana Iacob, Adrian Cosmin Ilie, Emil Radu Iacob, Septimiu Radu Susa, Laura Andreea Ghenciu, Amalia Constantinescu, Daiana Marina Cocolea, Cristian Oancea, and Diana Luminita Manolescu. 2024. "Differentiating Viral from Bacterial Pneumonia in Children: The Diagnostic Role of Lung Ultrasound—A Prospective Observational Study" Diagnostics 14, no. 5: 480. https://doi.org/10.3390/diagnostics14050480
APA StyleStoicescu, E. R., Iacob, R., Ilie, A. C., Iacob, E. R., Susa, S. R., Ghenciu, L. A., Constantinescu, A., Cocolea, D. M., Oancea, C., & Manolescu, D. L. (2024). Differentiating Viral from Bacterial Pneumonia in Children: The Diagnostic Role of Lung Ultrasound—A Prospective Observational Study. Diagnostics, 14(5), 480. https://doi.org/10.3390/diagnostics14050480








