Artificial Intelligence in Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Data Analysis—Toward Retinal Metabolic Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Dataset
2.2. Data Acquisition for AI-Based Analysis
2.2.1. Data from Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO)
2.2.2. Data from OCT Angiography (OCT-A)
2.3. Data Analysis Using Different AI Methods
2.3.1. Preparation of FLIO Data
2.3.2. Initial AI Experiments with FLIO Data
2.3.3. Preparation of OCT-A Data and t-Distributed Stochastic Neighbor Embedding
2.3.4. Local Fractal Dimension of OCT-A Data
2.3.5. Support Vector Machine (SVM) for FLIO and OCT-A Data
2.4. Two-Sample T-Test
3. Results
3.1. FLIO
3.1.1. AI-Assessment with CNN and Encoder Networks
3.1.2. AI-Assessment with SVM
3.2. OCT-A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, D.; Schenke, S.; Hammer, M.; Schweitzer, F.; Jentsch, S.; Birckner, E.; Becker, W.; Bergmann, A. Towards metabolic mapping of the human retina. Microsc. Res. Tech. 2007, 70, 410–419. [Google Scholar] [CrossRef]
- Sauer, L.; Andersen, K.M.; Dysli, C.; Zinkernagel, M.S.; Bernstein, P.S.; Hammer, M. Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy. J. Biomed. Opt. 2018, 23, 091415. [Google Scholar] [CrossRef]
- Dysli, C.; Wolf, S.; Hatz, K.; Zinkernagel, M.S. Fluorescence Lifetime Imaging in Stargardt Disease: Potential Marker for Disease Progression. Investig. Ophthalmol. Vis. Sci. 2016, 57, 832–841. [Google Scholar] [CrossRef]
- Hutfilz, A.; Sonntag, S.R.; Lewke, B.; Theisen-Kunde, D.; Grisanti, S.; Brinkmann, R.; Miura, Y. Fluorescence Lifetime Imaging Ophthalmoscopy of the Retinal Pigment Epithelium during Wound Healing after Laser Irradiation. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Sonntag, S.R.; Seifert, E.; Hamann, M.; Lewke, B.; Theisen-Kunde, D.; Grisanti, S.; Brinkmann, R.; Miura, Y. Fluorescence Lifetime Changes Induced by Laser Irradiation: A Preclinical Study towards the Evaluation of Retinal Metabolic States. Life 2021, 11, 555. [Google Scholar] [CrossRef]
- Schweitzer, D.; Deutsch, L.; Klemm, M.; Jentsch, S.; Hammer, M.; Peters, S.; Haueisen, J.; Müller, U.A.; Dawczynski, J. Fluorescence lifetime imaging ophthalmoscopy in type 2 diabetic patients who have no signs of diabetic retinopathy. J. Biomed. Opt. 2015, 20, 61106. [Google Scholar] [CrossRef]
- Jentsch, S.; Schweitzer, D.; Schmidtke, K.U.; Peters, S.; Dawczynski, J.; Bär, K.J.; Hammer, M. Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer’s disease. Acta Ophthalmol. 2015, 93, e241–e247. [Google Scholar] [CrossRef]
- Dysli, C.; Zinkernagel, M.; Wolf, S. The Fluorescence Lifetime Imaging Ophthalmoscope. In Fluorescence Lifetime Imaging Ophthalmoscopy; Zinkernagel, M., Dysli, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 17–21. Available online: http://link.springer.com/10.1007/978-3-030-22878-1_5 (accessed on 30 October 2023).
- Sonntag, S.R.; Kreikenbohm, M.; Böhmerle, G.; Stagge, J.; Grisanti, S.; Miura, Y. Impact of cigarette smoking on fluorescence lifetime of ocular fundus. Sci. Rep. 2023, 13, 11484. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Dahrouj, M.; Miller, J.B. Artificial Intelligence (AI) and Retinal Optical Coherence Tomography (OCT). Semin. Ophthalmol. 2021, 36, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.; Sun, J.K. Artificial Intelligence in the assessment of diabetic retinopathy from fundus photographs. Semin. Ophthalmol. 2020, 35, 325–332. [Google Scholar] [CrossRef]
- Milea, D.; Najjar, R.P.; Zhubo, J.; Ting, D.; Vasseneix, C.; Xu, X.; Aghsaei Fard, M.; Fonseca, P.; Vanikieti, K.; Lagrèze, W.A.; et al. Artificial Intelligence to Detect Papilledema from Ocular Fundus Photographs. N. Engl. J. Med. 2020, 382, 1687–1695. [Google Scholar] [CrossRef]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.S.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018, 172, 1122–1131.e9. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402. [Google Scholar] [CrossRef]
- Colomer, A.; Igual, J.; Naranjo, V. Detection of Early Signs of Diabetic Retinopathy Based on Textural and Morphological Information in Fundus Images. Sensors 2020, 20, 1005. [Google Scholar] [CrossRef]
- Gallardo, M.; Munk, M.R.; Kurmann, T.; De Zanet, S.; Mosinska, A.; Karagoz, I.K.; Zinkernagel, M.S.; Wolf, S.; Sznitman, R. Machine Learning Can Predict Anti-VEGF Treatment Demand in a Treat-and-Extend Regimen for Patients with Neovascular, AMD, DME, and RVO Associated Macular Edema. Ophthalmol. Retin. 2021, 5, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Sauer, L.; Andersen, K.M.; Li, B.; Gensure, R.H.; Hammer, M.; Bernstein, P.S. Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) of Macular Pigment. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3094–3103. [Google Scholar] [CrossRef]
- Andersen, K.M.; Sauer, L.; Gensure, R.H.; Hammer, M.; Bernstein, P.S. Characterization of Retinitis Pigmentosa Using Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO). Transl. Vis. Sci. Technol. 2018, 7, 20. [Google Scholar] [CrossRef]
- Sauer, L.; Komanski, C.B.; Vitale, A.S.; Hansen, E.D.; Bernstein, P.S. Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) in Eyes with Pigment Epithelial Detachments Due to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3054–3063. [Google Scholar] [CrossRef]
- Lincke, J.B.; Dysli, C.; Jaggi, D.; Fink, R.; Wolf, S.; Zinkernagel, M.S. The Influence of Cataract on Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO). Transl. Vis. Sci. Technol. 2021, 10, 33. [Google Scholar] [CrossRef]
- Vitale, A.S.; Sauer, L.; Modersitzki, N.K.; Bernstein, P.S. Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) in Patients with Choroideremia. Transl. Vis. Sci. Technol. 2020, 9, 33. [Google Scholar] [CrossRef]
- Sadda, S.R.; Borrelli, E.; Fan, W.; Ebraheem, A.; Marion, K.M.; Kwon, S. Impact of mydriasis in fluorescence lifetime imaging ophthalmoscopy. PLoS ONE 2018, 13, e0209194. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Suport-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Erfani, S.M.; Rajasegarar, S.; Karunasekera, S.; Leckie, C. High-dimensional and large-scale anomaly detection using a linear one-class SVM with deep learning. Pattern Recognit. 2016, 58, 121–134. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Du, S.; Yang, J.; Liu, B.; Gorriz, J.M.; Ramírez, J.; Yuan, T.F.; Zhang, Y. Wavelet Entropy and Directed Acyclic Graph Support Vector Machine for Detection of Patients with Unilateral Hearing Loss in MRI Scanning. Front. Comput. Neurosci. 2016, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Xie, G.; Li, Z.; Li, X.; Zhang, Y.; Wu, K.; Shao, G.; Lv, B.; Jing, H.; Zhang, C.; et al. Automatic depression diagnosis through hybrid EEG and near-infrared spectroscopy features using support vector machine. Front. Neurosci. 2023, 17, 1205931. [Google Scholar] [CrossRef]
- Panesar, S.S.; D’Souza, R.N.; Yeh, F.C.; Fernandez-Miranda, J.C. Machine Learning Versus Logistic Regression Methods for 2-Year Mortality Prognostication in a Small, Heterogeneous Glioma Database. World Neurosurg. X 2019, 2, 100012. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.F.; Hei, H.; Lim, S.Z.; Ng, E.Y.K. Applied machine learning for blood pressure estimation using a small, real-world electrocardiogram and photoplethysmogram dataset. Math. Biosci. Eng. 2022, 20, 975–997. [Google Scholar] [CrossRef] [PubMed]
- Mohidin, N.; Jaafar, A. Effect of smoking on tear stability and corneal surface. J. Curr. Ophthalmol. 2020, 32, 232. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Fluorescence lifetime imaging—Techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. arXiv 2015, arXiv:1512.03385. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv 2015, arXiv:1505.04597. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F. ImageNet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar] [CrossRef]
- Pearson, K. LIII. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar]
- Maaten, L.; Hinton, G. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Gadde, S.G.K.; Anegondi, N.; Bhanushali, D.; Chidambara, L.; Yadav, N.K.; Khurana, A.; Sinha Roy, A. Quantification of Vessel Density in Retinal Optical Coherence Tomography Angiography Images Using Local Fractal Dimension. Investig. Ophthalmol. Vis. Sci. 2016, 57, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Koutroumbas, K.; Theodoridis, S. Pattern Recognition, 4th ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. arXiv 2012, arXiv:1201.0490. [Google Scholar] [CrossRef]
- Yadav, S.S.; Jadhav, S.M. Deep convolutional neural network based medical image classification for disease diagnosis. J. Big Data 2019, 6, 113. [Google Scholar] [CrossRef]
- Cen, L.P.; Ji, J.; Lin, J.W.; Ju, S.T.; Lin, H.J.; Li, T.P.; Wang, Y.; Yang, J.F.; Liu, T.F.; Tang, S. Automatic detection of 39 fundus diseases and conditions in retinal photographs using deep neural networks. Nat. Commun. 2021, 12, 4828. [Google Scholar] [CrossRef]
- Bian, Y.; Zheng, Z.; Fang, X.; Jiang, H.; Zhu, M.; Yu, J.; Zhao, H.; Zhang, L.; Yao, J.; Lu, L.; et al. Artificial Intelligence to Predict Lymph Node Metastasis at CT in Pancreatic Ductal Adenocarcinoma. Radiology 2023, 306, 160–169. [Google Scholar] [CrossRef]
- Chinn, E.; Arora, R.; Arnaout, R.; Arnaout, R. ENRICHing medical imaging training sets enables more efficient machine learning. J. Am. Med. Inform. Assoc. 2023, 30, 1079–1090. [Google Scholar] [CrossRef]
- Geman, S.; Bienenstock, E.; Doursat, R. Neural Networks and the Bias/Variance Dilemma. Neural Comput. 1992, 4, 1–58. [Google Scholar] [CrossRef]
- Sauer, L.; Calvo, C.M.; Vitale, A.S.; Henrie, N.; Milliken, C.M.; Bernstein, P.S. Imaging of Hydroxychloroquine Toxicity with Fluorescence Lifetime Imaging Ophthalmoscopy. Ophthalmol. Retin. 2019, 3, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Akdogan, M.; Gulyesil, F.F.; Sabaner, M.C.; Gobeka, H.H. Cigarette smoking reduces deep retinal vascular density. Clin. Exp. Optom. 2020, 103, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.B.; Chen, H.C.; Patel, V.; Herbert, L.; Kohner, E.M. The acute effect of smoking on retinal blood flow in subjects with and without diabetes. Ophthalmology 1994, 101, 1220–1226. [Google Scholar] [CrossRef]
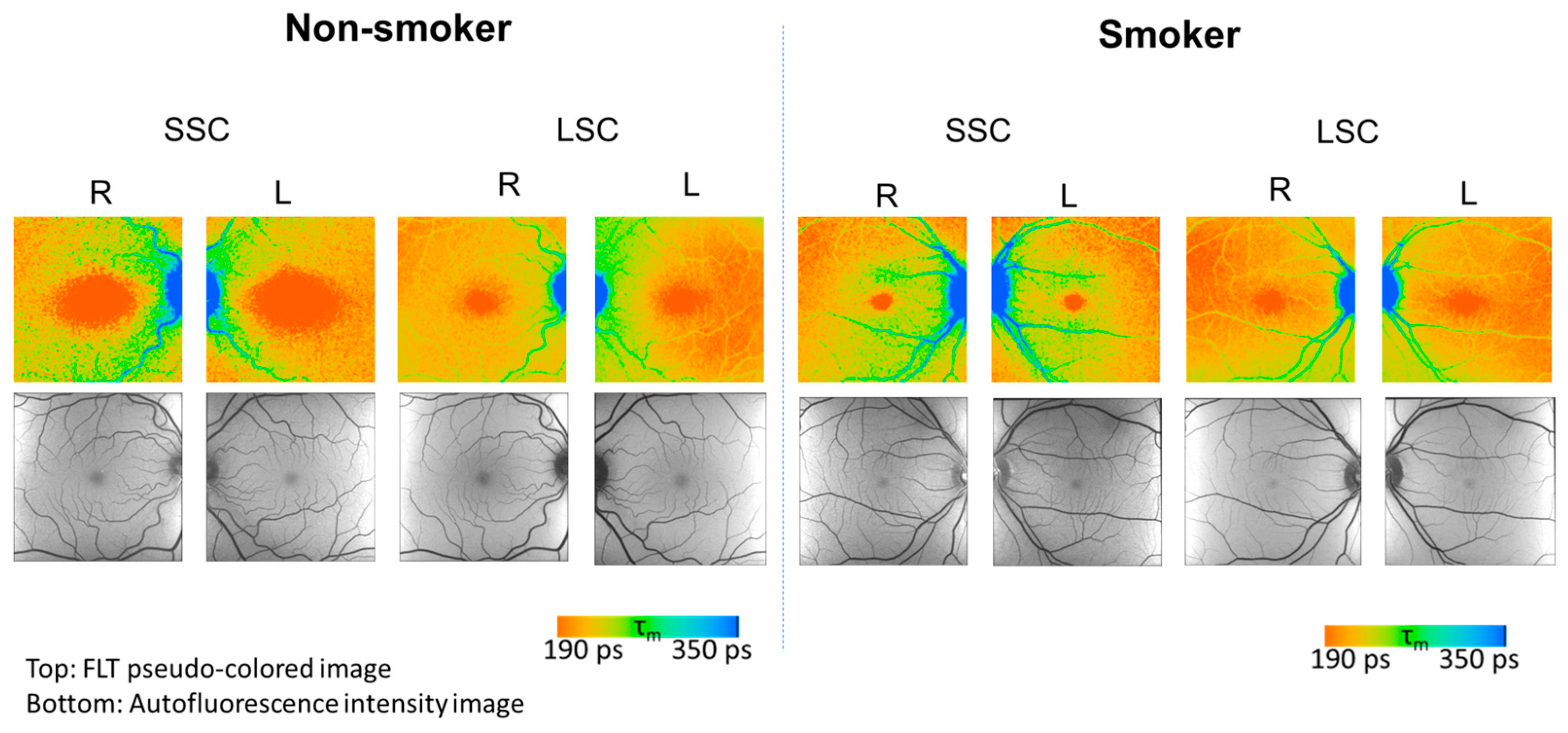


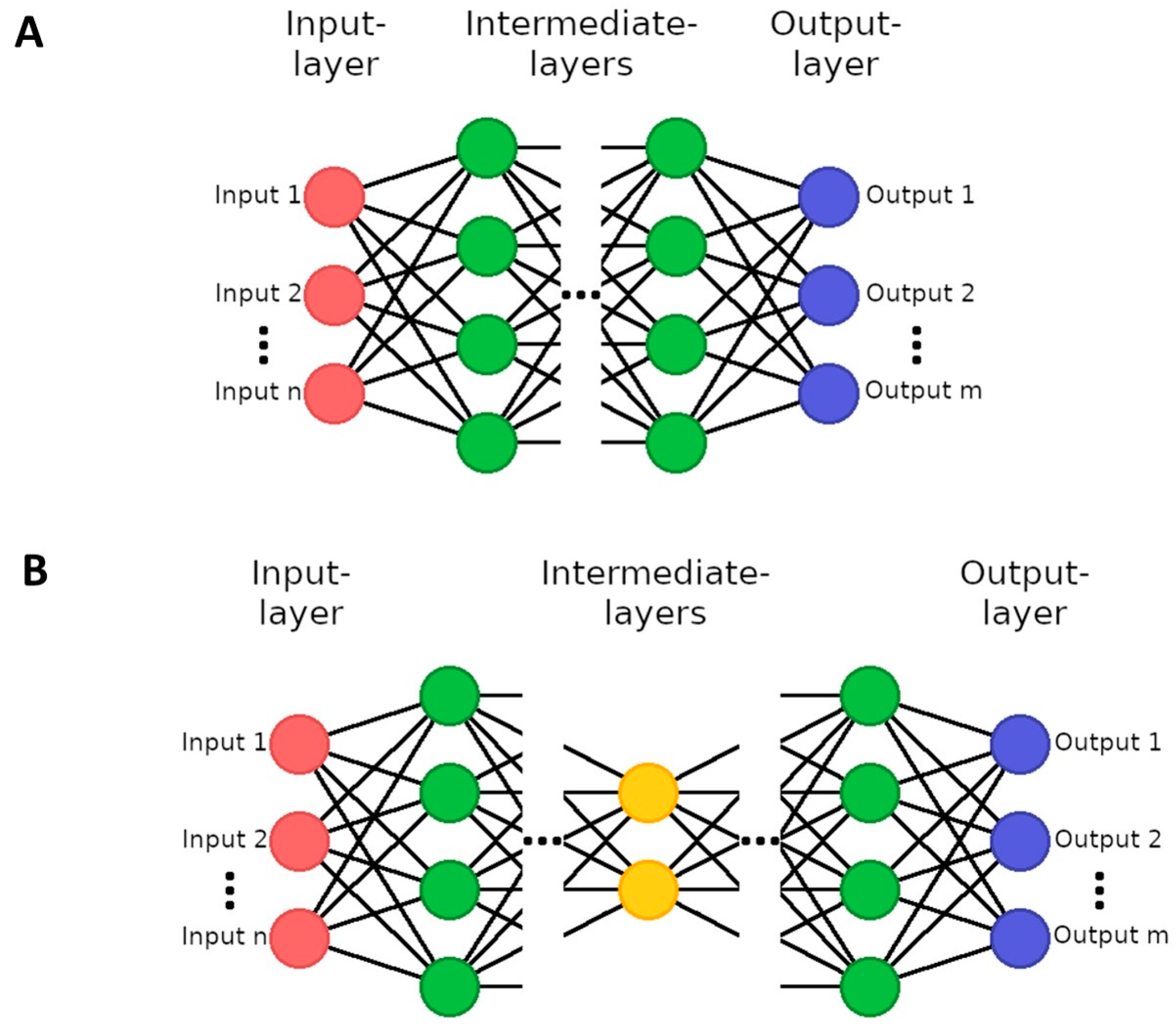


| Parameter | Unit | Non-Smokers (n = 26) | Smokers (n = 28) | ||||
|---|---|---|---|---|---|---|---|
| Male | (No. of subjects) | 13 | 15 | ||||
| Female | (No. of subjects) | 13 | 15 | ||||
| Mean (SD) | Median | IQR | Mean (SD) | Median | IQR | ||
| Age | years old | 26.7 (4.1) | 26.5 | 23.0 to 30.3 | 28.5 (4.7) | 28 | 25.0 to 32.0 |
| Years smoked | years | 0 | 0 | 0 | 12.0 (4.9) | 10.8 | 9.0 to 15.8 |
| Cumulative packs | 0 | 0 | 0 | 2915 (2224) | 2594 | 883 to 4280 | |
| Feature Set | n | Mean TP | Mean FN | Mean FP | Mean TN | Mean TPR | Mean FPR | Mean Accuracy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All features | 36 | 32.70 | 23.30 | 21.35 | 30.65 | 58.39% | ±6.44% | 41.06% | ±4.44% | 58.66% | ±4.63% |
| FLIO intensity | 18 | 27.30 | 28.70 | 27.90 | 24.10 | 48.75% | ±5.18% | 53.65% | ±6.20% | 47.59% | ±3.30% |
| FLIO τm only | 18 | 34.90 | 21.10 | 21.15 | 30.85 | 62.32% | ±4.85% | 40.67% | ±4.48% | 60.88% | ±3.14% |
| FLIO τm; SSC | 9 | 33.50 | 22.50 | 33.40 | 18.60 | 59.82% | ±3.50% | 64.23% | ±5.35% | 48.24% | ±3.26% |
| FLIO τm; LSC | 9 | 33.25 | 22.75 | 18.90 | 33.10 | 59.38% | ±6.00% | 36.35% | ±4.75% | 61.44% | ±3.91% |
| FLIO τm; IR | 8 | 38.80 | 17.20 | 18.05 | 33.95 | 69.29% | ±4.78% | 34.71% | ±5.91% | 67.36% | ±3.04% |
| FLIO τm; OR | 8 | 27.25 | 28.75 | 19.05 | 32.95 | 48.66% | ±3.33% | 36.63% | ±4.73% | 55.74% | ±2.68% |
| FLIO τm; IR- SSC, OR- LSC | 8 | 35.00 | 21.00 | 17.05 | 34.95 | 62.50% | ±3.66% | 32.79% | ±5.62% | 64.77% | ±3.54% |
| FLIO τm; OR- SSC, IR- LSC | 8 | 26.80 | 29.20 | 27.45 | 24.55 | 47.86% | ±5.43% | 52.79% | ±7.36% | 47.55% | ±3.24% |
| FLIO τm; T1-SSC, S2-LSC, S1-SSC | 3 | 40.65 | 15.35 | 16.40 | 35.60 | 72.59% | ±3.88% | 31.54% | ±4.49% | 70.60% | ±2.36% |
| Feature Set | n | Mean TP | Mean FN | Mean FP | Mean TN | Mean TPR | Mean FPR | Mean Accuracy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All features | 36 | 11.85 | 16.15 | 9.50 | 42.50 | 42.32% | ±7.34% | 18.27% | ±2.75% | 67.94% | ±3.02% |
| FLIO intensity | 18 | 8.15 | 19.85 | 13.45 | 38.55 | 29.11% | ±8.70% | 25.87% | ±3.67% | 58.38% | ±3.97% |
| FLIO τm only | 18 | 14.30 | 13.70 | 8.45 | 43.55 | 51.07% | ±3.41% | 16.25% | ±3.25% | 72.31% | ±2.72% |
| FLIO τm; SSC | 9 | 6.05 | 21.95 | 14.50 | 37.50 | 21.61% | ±7.78% | 27.88% | ±3.96% | 54.44% | ±3.43% |
| FLIO τm; LSC | 9 | 11.75 | 16.25 | 10.15 | 41.85 | 41.96% | ±5.16% | 19.52% | ±4.00% | 67.00% | ±2.60% |
| FLIO τm; IR | 8 | 15.30 | 12.70 | 10.70 | 41.30 | 54.64% | ±6.40% | 20.58% | ±2.28% | 70.75% | ±2.72% |
| FLIO τm; OR | 8 | 11.95 | 16.05 | 10.95 | 41.05 | 42.68% | ±6.03% | 21.06% | ±5.52% | 66.25% | ±3.49% |
| FLIO τm; IR- SSC, OR- LSC | 8 | 18.05 | 9.95 | 6.40 | 45.60 | 64.46% | ±5.23% | 12.31% | ±4.19% | 79.56% | ±3.31% |
| FLIO τm; OR- SSC, IR- LSC | 8 | 9.15 | 18.85 | 11.90 | 40.10 | 32.68% | ±5.90% | 22.88% | ±5.47% | 61.56% | ±3.75% |
| FLIO τm; T1-SSC, S2-LSC, S1-SSC | 3 | 19.10 | 8.90 | 7.10 | 44.90 | 68.21% | ±6.07% | 13.65% | ±2.91% | 80.00% | ±2.98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiemann, N.; Sonntag, S.R.; Kreikenbohm, M.; Böhmerle, G.; Stagge, J.; Grisanti, S.; Martinetz, T.; Miura, Y. Artificial Intelligence in Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Data Analysis—Toward Retinal Metabolic Diagnostics. Diagnostics 2024, 14, 431. https://doi.org/10.3390/diagnostics14040431
Thiemann N, Sonntag SR, Kreikenbohm M, Böhmerle G, Stagge J, Grisanti S, Martinetz T, Miura Y. Artificial Intelligence in Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Data Analysis—Toward Retinal Metabolic Diagnostics. Diagnostics. 2024; 14(4):431. https://doi.org/10.3390/diagnostics14040431
Chicago/Turabian StyleThiemann, Natalie, Svenja Rebecca Sonntag, Marie Kreikenbohm, Giulia Böhmerle, Jessica Stagge, Salvatore Grisanti, Thomas Martinetz, and Yoko Miura. 2024. "Artificial Intelligence in Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Data Analysis—Toward Retinal Metabolic Diagnostics" Diagnostics 14, no. 4: 431. https://doi.org/10.3390/diagnostics14040431
APA StyleThiemann, N., Sonntag, S. R., Kreikenbohm, M., Böhmerle, G., Stagge, J., Grisanti, S., Martinetz, T., & Miura, Y. (2024). Artificial Intelligence in Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Data Analysis—Toward Retinal Metabolic Diagnostics. Diagnostics, 14(4), 431. https://doi.org/10.3390/diagnostics14040431










