Experiences with Wearable Sensors in Oncology during Treatment: Lessons Learned from Feasibility Research Projects in Denmark
Abstract
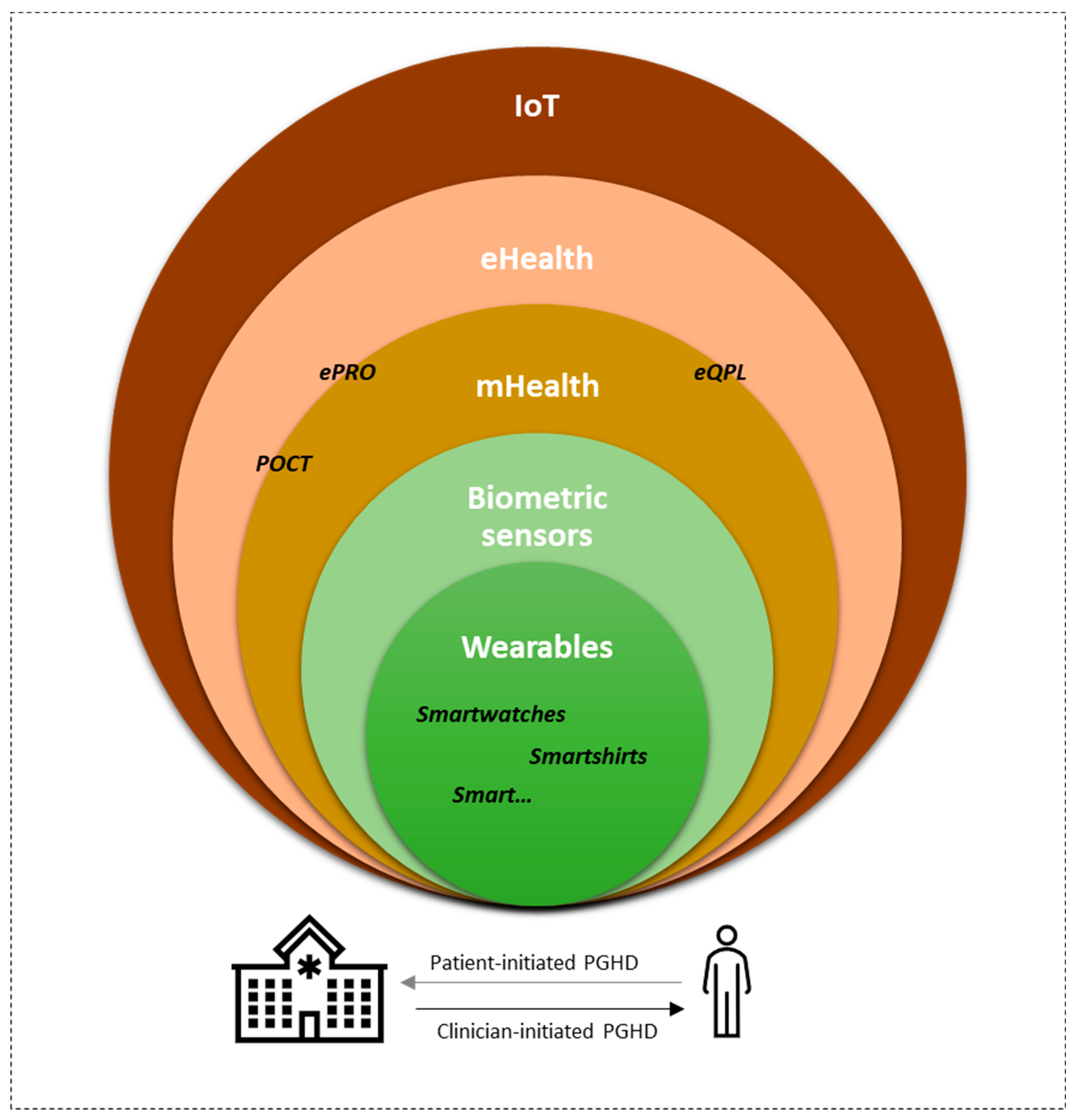
1. Introduction
2. Case Presentations
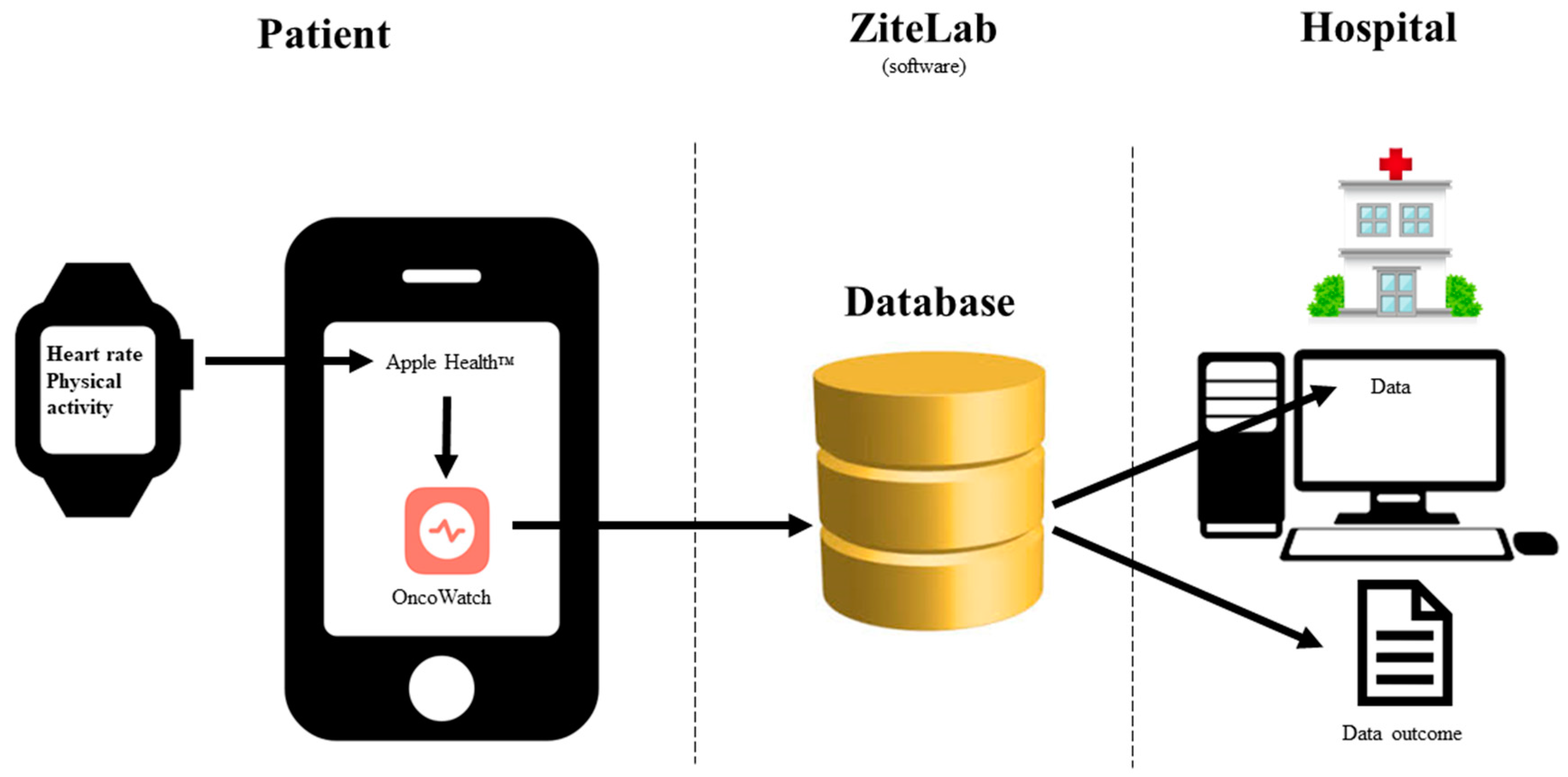
2.1. Case 1—OncoWatch1.0
2.1.1. Planned Study [48]
2.1.2. Actual Study Performed [49]
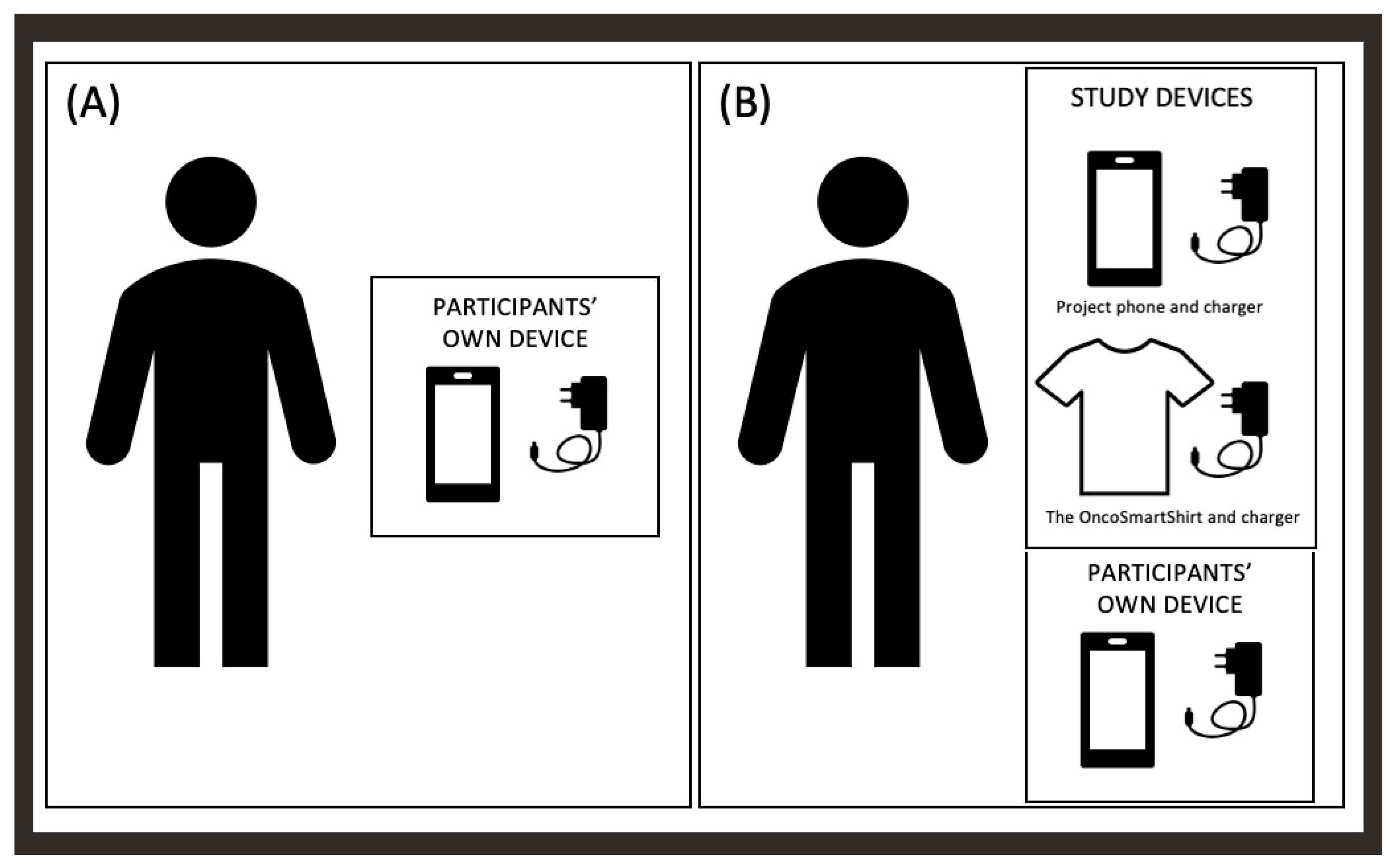
2.2. Case 2—OncoSmartShirt
2.2.1. Planned Study Design [50]
2.2.2. Actual Study Performed
2.3. Case 3—OncoWatch2.0
2.3.1. Planned Study Design
2.3.2. Actual Study Performed
3. Results from the Three Case Report Studies
- OncoWatch1.0Patients undergoing curatively intended RT were not able to wear a smartwatch during their treatment trajectory when successful adherence was described as wearing the device for a minimum 12 h/day during the study period [49].
- OncoSmartShirtAYA patients were not able to wear a smart t-shirt during cancer treatment when a successful completion rate was defined as using the smart t-shirt for at least 8 h per day during the 2-week study period [53].
- OncoWatch2.0The study was never performed due to technical challenges, and it was not possible to investigate if sensor data from smartwatches can reveal symptoms or signs of dehydration.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- World Health Organization (WHO). International Agency for Research on Cancer. World Health Organization. Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 23 September 2022).
- Van der Hout, A.; van Uden-Kraan, C.F.; Holtmaat, K.; Jansen, F.; Lissenberg-Witte, B.I.; Nieuwenhuijzen, G.A.P.; Hardillo, J.A.; Baatenburg de Jong, R.J.; Tiren-Verbeet, N.L.; Sommeijer, D.W.; et al. Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: A randomised, controlled trial. Lancet Oncol. 2019, 21, 80–94. [Google Scholar] [CrossRef]
- Izmailova, E.S.; Wood, W.A. Biometric Monitoring Technologies in Cancer: The Past, Present, and Future. JCO Clin. Cancer Inf. 2021, 5, 728–733. [Google Scholar] [CrossRef]
- Ashton, K. That ‘Internet of Things’ Thing. RFID J. 2009, 22, 97–114. Available online: https://www.rfidjournal.com/that-internet-of-things-thing (accessed on 1 October 2023).
- Eysenbach, G. What is e-health? J. Med. Internet Res. 2001, 3, E20. [Google Scholar] [CrossRef]
- Komarzynski, S.; Wreglesworth, N.I.; Griffiths, D.; Pecchia, L.; Subbe, C.P.; Hughes, S.F.; Davies, E.H.; Innominato, P.F. Embracing Change: Learnings from Implementing Multidimensional Digital Remote Monitoring in Oncology Patients at a District General Hospital During the COVID-19 Pandemic. JCO Clin. Cancer Inf. 2021, 5, 216–220. [Google Scholar] [CrossRef]
- Holländer-Mieritz, C.; Johansen, C.; Pappot, H. eHealth-mind the gap. Acta Oncol. 2020, 59, 877–878. [Google Scholar] [CrossRef]
- Shaverdian, N.; Gillespie, E.F.; Cha, E.; Kim, S.Y.; Benvengo, S.; Chino, F.; Kang, J.J.; Li, Y.; Atkinson, T.M.; Lee, N.; et al. Impact of Telemedicine on Patient Satisfaction and Perceptions of Care Quality in Radiation Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- González-Revuelta, M.E.; Novas, N.; Gázquez, J.A.; Rodríguez-Maresca, M.; García-Torrecillas, J.M. User Perception of New E-Health Challenges: Implications for the Care Process. Int. J. Environ. Res. Public Health 2022, 19, 3875. [Google Scholar] [CrossRef] [PubMed]
- Mottet, T.; Hémar, V.; Enfedaque, S.; Mathoulin-Pélissier, S.; Charitansky, H.; Godbert, Y.; Roubaud, G.; Cabart, M.; Chakiba, C.; Chomy, F.; et al. Evaluating video-based consultations in routine clinical practice at a comprehensive cancer center. Acta Oncol. 2023, 62, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Danske Regioner. Sundhed for Dig-Regionernes Samarbejde om Digitalisering. 2020. Available online: https://www.regioner.dk/media/13626/sundhed-for-dig-regionernes-samarbejde-om-digitalisering.pdf (accessed on 1 October 2023).
- Region Hovedstaden Centerfor Sundhed. Alle skal Med Region Hovedstadens Arbejde Med det Nære og Sammenhængende Sundhedsvæsen. 2021. Available online: https://www.regionh.dk/Sundhed/Politikker-Planer-Strategier/Documents/alle%20skal%20med-det%20naere-sammenhaengende-sundhedsvaesen.pdf (accessed on 1 October 2023).
- Danish Life Science Cluster (VIVE). Styrkelse af Digitale Kompetencer Inden for data Drevne Teknologier til Brug for Automatisering, Prædiktion og Beslutningsstøtte hos Sundhedspersoner. 2022. Available online: https://www.danishlifesciencecluster.dk/wp-content/uploads/2022/02/Styrkede-digitale-kompetencer-i-sundhedsvaesenet-perspektiverende-rapport-FINAL.pdf (accessed on 1 October 2023).
- Rapport, F.; Clay-Williams, R.; Churruca, K.; Shih, P.; Hogden, A.; Braithwaite, J. The struggle of translating science into action: Foundational concepts of implementation science. J. Eval. Clin. Pr. 2018, 24, 117–126. [Google Scholar] [CrossRef]
- LeRouge, C.; Durneva, P.; Lyon, V.; Thompson, M. Health Consumer Engagement, Enablement, and Empowerment in Smartphone-Enabled Home-Based Diagnostic Testing for Viral Infections: Mixed Methods Study. JMIR Mhealth Uhealth 2022, 10, e34685. [Google Scholar] [CrossRef]
- Stover, A.M.; Tompkins Stricker, C.; Hammelef, K.; Henson, S.; Carr, P.; Jansen, J.; Deal, A.M.; Bennett, A.V.; Basch, E.M. Using Stakeholder Engagement to Overcome Barriers to Implementing Patient-reported Outcomes (PROs) in Cancer Care Delivery: Approaches from 3 Prospective Studies. Med. Care 2019, 57 (Suppl. S5, Suppl. S1), S92–S99. [Google Scholar] [CrossRef]
- Baines, R.; Bradwell, H.; Edwards, K.; Stevens, S.; Prime, S.; Tredinnick-Rowe, J.; Sibley, M.; Chatterjee, A. Meaningful patient and public involvement in digital health innovation, implementation and evaluation: A systematic review. Health Expect. 2022, 25, 1232–1245. [Google Scholar] [CrossRef]
- The Office of the National Coordinator for Health Information Technology (ONC). Patient-Generated Health Data. Available online: https://www.healthit.gov/topic/otherhot-topics/what-are-patient-generated-health-data (accessed on 19 September 2022).
- Shapiro, M.J.D.; Wald, J.; Mon, D. Patient-Generated Health Data. White Paper. Prepared for Office of Policy and Planning Office of the National Coordinator for Health Information Technology. 2012. Available online: https://www.healthit.gov/sites/default/files/rti_pghd_whitepaper_april_2012.pdf (accessed on 1 October 2023).
- Jim, H.S.L.; Hoogland, A.I.; Brownstein, N.C.; Barata, A.; Dicker, A.P.; Knoop, H.; Gonzalez, B.D.; Perkins, R.; Rollison, D.; Gilbert, S.M.; et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J. Clin. 2020, 70, 182–199. [Google Scholar] [CrossRef]
- Mars, M.; Scott, R.E. Electronic Patient-Generated Health Data for Healthcare. In Digital Health; Linwood, S.L., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- General Data Protection Regulation (GDPR): Programme of the European Union. Available online: https://gdpr.eu/ (accessed on 1 October 2023).
- Lawlor, R.T. The impact of GDPR on data sharing for European cancer research. Lancet Oncol. 2023, 24, 6–8. [Google Scholar] [CrossRef] [PubMed]
- The Danish Ministry of Health. The Danish Ministry of Health. 2015. Available online: https://sum.dk/ (accessed on 7 October 2022).
- Ammitzbøll, G.; Levinsen, A.K.G.; Kjær, T.K.; Ebbestad, F.E.; Horsbøl, T.A.; Saltbæk, L.; Badre-Esfahani, S.K.; Joensen, A.; Kjeldsted, E.; Halgren Olsen, M.; et al. Socioeconomic inequality in cancer in the Nordic countries. A systematic review. Acta Oncol. 2022, 61, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.; la Cour, K.; Pilegaard, M.S.; Dalton, S.O.; Bidstrup, P.E.; Möller, S.; Jarlbaek, L. Social vulnerability among cancer patients and changes in vulnerability during their trajectories—A longitudinal population-based study. Cancer Epidemiol. 2023, 85, 102401. [Google Scholar] [CrossRef] [PubMed]
- Sollie, M.; Hansen, M.; Thomsen, J.B. Health Technology Readiness amongst Patients with Suspected Breast Cancer Using the READHY-tool—A Cross-sectional Study. J. Med. Syst. 2023, 47, 118. [Google Scholar] [CrossRef]
- Brundage, M.; Blazeby, J.; Revicki, D.; Bass, B.; de Vet, H.; Duffy, H.; Efficace, F.; King, M.; Lam, C.L.; Moher, D.; et al. Patient-reported outcomes in randomized clinical trials: Development of ISOQOL reporting standards. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2013, 22, 1161–1175. [Google Scholar] [CrossRef]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, U.L.; Pappot, H.; Holländer-Mieritz, C. The Use of Wearables in Clinical Trials during Cancer Treatment: Systematic Review. JMIR mHealth uHealth 2020, 8, e22006. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.K.; Garden, A.S.; Shinn, E.H.; Shete, S.; Martch, S.L.; Camero, M.; Baum, G.; Farcas, E.; Lin, K.; Raab, F.; et al. Using mobile and sensor technology to identify early dehydration risk in head and neck cancer patients undergoing radiation treatment: Impact on symptoms. J. Clin. Oncol. 2018, 36, 6063. [Google Scholar] [CrossRef]
- Aggio, D.; Smith, L.; Fisher, A.; Hamer, M. Context-Specific Associations of Physical Activity and Sedentary Behavior with Cognition in Children. Am. J. Epidemiol. 2016, 183, 1075–1082. [Google Scholar] [CrossRef]
- Ohri, N.; Halmos, B.; Bodner, W.R.; Cheng, H.; Guha, C.; Kalnicki, S.; Garg, M. Daily step counts: A new prognostic factor in locally advanced non-small cell lung cancer? Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 745–751. [Google Scholar] [CrossRef]
- Ward, W.H.; Meeker, C.R.; Handorf, E.; Hill, M.V.; Einarson, M.; Alpaugh, R.K.; Holden, T.L.; Astsaturov, I.; Denlinger, C.S.; Hall, M.J.; et al. Feasibility of Fitness Tracker Usage to Assess Activity Level and Toxicities in Patients with Colorectal Cancer. JCO Clin. Cancer Inf. 2021, 5, 125–133. [Google Scholar] [CrossRef]
- Beg, M.S.; Gupta, A.; Stewart, T.; Rethorst, C.D. Promise of Wearable Physical Activity Monitors in Oncology Practice. J. Oncol. Pract. 2017, 13, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Goldsack, J.C.; Coravos, A.; Bakker, J.P.; Bent, B.; Dowling, A.V.; Fitzer-Attas, C.; Godfrey, A.; Godino, J.G.; Gujar, N.; Izmailova, E.; et al. Verification, analytical validation, and clinical validation (V3): The foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit. Med. 2020, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Groarke, J.D.; Gallagher, J.; Stack, J.; Aftab, A.; Dwyer, C.; McGovern, R.; Courtney, G. Use of an admission early warning score to predict patient morbidity and mortality and treatment success. Emerg. Med. J. 2008, 25, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, M.G.; Connolly, S.; Martin, S.S.; Parakh, K. Grains of sand to clinical pearls: Realizing the potential of wearable data. Am. J. Med. 2022, 136, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.A.; Dilip, D.; Derkach, A.; Grover, N.S.; Elemento, O.; Levine, R.; Thanarajasingam, G.; Batsis, J.A.; Bailey, C.; Kannappan, A.; et al. Wearable sensor-based performance status assessment in cancer: A pilot multicenter study from the Alliance for Clinical Trials in Oncology (A19_Pilot2). PLOS Digit. Health 2023, 2, e0000178. [Google Scholar] [CrossRef]
- Haemmerli, M.; Ammann, R.A.; Roessler, J.; Koenig, C.; Brack, E. Vital signs in pediatric oncology patients assessed by continuous recording with a wearable device, NCT04134429. Sci. Data 2022, 9, 89. [Google Scholar] [CrossRef]
- Low, C.A. Harnessing consumer smartphone and wearable sensors for clinical cancer research. NPJ Digit. Med. 2020, 3, 140. [Google Scholar] [CrossRef]
- Gresham, G.; Schrack, J.; Gresham, L.M.; Shinde, A.M.; Hendifar, A.E.; Tuli, R.; Rimel, B.J.; Figlin, R.; Meinert, C.L.; Piantadosi, S. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp. Clin. Trials 2018, 64, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.J.; Chen, R.; Tam, R.M.; Narayan, H.K.; Natarajan, L.; Liu, L. Fitbit Use and Activity Levels From Intervention to 2 Years after: Secondary Analysis of a Randomized Controlled Trial. JMIR mHealth uHealth 2022, 10, e37086. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, S.; Spruijt-Metz, D.; Farias, A.J. Associations Among Wearable Activity Tracker Use, Exercise Motivation, and Physical Activity in a Cohort of Cancer Survivors: Secondary Data Analysis of the Health Information National Trends Survey. JMIR Cancer 2021, 7, e24828. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Upadhyay, U.; Dhar, E.; Kuo, L.J.; Syed-Abdul, S. A Scoping Review to Assess Adherence to and Clinical Outcomes of Wearable Devices in the Cancer Population. Cancers 2022, 14, 4437. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Transformation Initiative (CTTI). Developing Novel Endpoints Generated by Mobile Technologies for Use in Clinical Trials. 2017. Available online: https://www.ctti-clinicaltrials.org/files/novelendpoints-recs.pdf (accessed on 1 October 2023).
- Holländer-Mieritz, C.; Vogelius, I.R.; Kristensen, C.A.; Green, A.; Rindum, J.L.; Pappot, H. Using Biometric Sensor Data to Monitor Cancer Patients during Radiotherapy: Protocol for the OncoWatch Feasibility Study. JMIR Res. Protoc. 2021, 10, e26096. [Google Scholar] [CrossRef] [PubMed]
- Holländer-Mieritz, C.; Steen-Olsen, E.B.; Kristensen, C.A.; Johansen, C.; Vogelius, I.R.; Pappot, H. Feasibility of Using Wearables for Home Monitoring during Radiotherapy for Head and Neck Cancer-Results from the OncoWatch 1.0 Study. Cancers 2023, 15, 422. [Google Scholar] [CrossRef] [PubMed]
- Steen-Olsen, E.B.; Pappot, H.; Green, A.; Langberg, H.; Holländer-Mieritz, C. Feasibility of Monitoring Patients Who Have Cancer with a Smart T-shirt: Protocol for the OncoSmartShirt Study. JMIR Res. Protoc. 2022, 11, e37626. [Google Scholar] [CrossRef] [PubMed]
- Eliasen, A.; Abildtoft, M.K.; Krogh, N.S.; Rechnitzer, C.; Brok, J.S.; Mathiasen, R.; Schmiegelow, K.; Dalhoff, K.P. Smartphone App to Self-Monitor Nausea During Pediatric Chemotherapy Treatment: User-Centered Design Process. JMIR mHealth uHealth 2020, 8, e18564. [Google Scholar] [CrossRef]
- Chronolife. Chronolife Product Description. 2021. Available online: https://www.chronolife.net/product/keesense/ (accessed on 1 October 2023).
- Steen-Olsen, E.B.; Pappot, H.; Hjerming, M.; Hanghøj, S.; Holländer-Mieritz, C. Feasibility of monitoring AYA cancer patients with a smart t-shirt: Results from the OncoSmartShirt study. 2024; Accepted for publication in JMIR Mhealth Uhealth, February 2024. [Google Scholar]
- Hollander-Mieritz, C.; Johansen, J.; Taarnhoj, G.A.; Johansen, C.; Vogelius, I.R.; Kristensen, C.A.; Pappot, H. Systematic use of patient reported outcome during radiotherapy for head and neck cancer: Study protocol for the national DAHANCA 38 trial. Acta Oncol. 2020, 59, 603–607. [Google Scholar] [CrossRef]
- Yu, A.; Zhu, M.; Chen, C.; Li, Y.; Cui, H.; Liu, S.; Zhao, Q. Implantable Flexible Sensors for Health Monitoring. Adv. Health Mater. 2023, 13, 2302460. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Drkulec, H.; Im, J.H.B.; Tsai, J.; Nafees, A.; Kumar, S.; Hou, T.; Fazelzad, R.; Leighl, N.B.; Krzyzanowska, M.; et al. The Use of Wearable Devices in Oncology Patients: A Systematic Review. Oncologist 2023, XX, 1–12. [Google Scholar] [CrossRef]
- Flaucher, M.; Zakreuskaya, A.; Nissen, M.; Mocker, A.; Fasching, P.A.; Beckmann, M.W.; Eskofier, B.M.; Leutheuser, H. Evaluating the Effectiveness of Mobile Health in Breast Cancer Care: A Systematic Review. Oncologist 2023, 28, e847–e858. [Google Scholar] [CrossRef]
- Kos, M.; Brouwer, C.G.; van Laarhoven, H.W.M.; Hopman, M.T.E.; van Oijen, M.G.H.; Buffart, L.M. The association between wearable device metrics and clinical outcomes in oncology: A systematic review with evidence synthesis and meta-analysis. Crit. Rev. Oncol. Hematol. 2023, 185, 103979. [Google Scholar] [CrossRef] [PubMed]
- Oakley-Girvan, I.; Yunis, R.; Fonda, S.J.; Neeman, E.; Liu, R.; Aghaee, S.; Ramsey, M.E.; Kubo, A.; Davis, S.W. Usability evaluation of mobile phone technologies for capturing cancer patient-reported outcomes and physical functions. Digit. Health 2023, 9, 20552076231186515. [Google Scholar] [CrossRef]
- Cho, P.J.; Yi, J.; Ho, E.; Shandhi, M.M.H.; Dinh, Y.; Patil, A.; Martin, L.; Singh, G.; Bent, B.; Ginsburg, G.; et al. Demographic Imbalances Resulting from the Bring-Your-Own-Device Study Design. JMIR mHealth uHealth 2022, 10, e29510. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Ensari, I.; Weng, C.; Kahn, M.G.; Natarajan, K. Factors Affecting the Quality of Person-Generated Wearable Device Data and Associated Challenges: Rapid Systematic Review. JMIR mHealth uHealth 2021, 9, e20738. [Google Scholar] [CrossRef]
- Olsen, M.H.; Boje, C.R.; Kjaer, T.K.; Steding-Jessen, M.; Johansen, C.; Overgaard, J.; Dalton, S.O. Socioeconomic position and stage at diagnosis of head and neck cancer—A nationwide study from DAHANCA. Acta Oncol. 2015, 54, 759–766. [Google Scholar] [CrossRef]
- Sher, D.J.; Radpour, S.; Shah, J.L.; Pham, N.L.; Jiang, S.; Vo, D.; Sumer, B.D.; Day, A.T. Pilot Study of a Wearable Activity Monitor During Head and Neck Radiotherapy to Predict Clinical Outcomes. JCO Clin. Cancer Inf. 2022, 6, e2100179. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Exworthy, M. Wearing the Future-Wearables to Empower Users to Take Greater Responsibility for Their Health and Care: Scoping Review. JMIR mHealth uHealth 2022, 10, e35684. [Google Scholar] [CrossRef] [PubMed]
- Hofer-Schmitz, K.; Stojanović, B. Towards formal verification of IoT protocols: A Review. Comput. Netw. 2020, 174, 107233. [Google Scholar] [CrossRef]

| Device | PRO | User Interviews | Own Device | Feed-Back to Patient | Surveillance in Study | |
|---|---|---|---|---|---|---|
| OncoSmartWatch1.0 | Apple Watch | - | - | - | - | - |
| OncoSmartShirt | Chronolife t-shirt | - | + | - | - | - |
| OncoSmartWatch2.0 | Apple Watch + ePRO | + | + | - | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappot, H.; Steen-Olsen, E.B.; Holländer-Mieritz, C. Experiences with Wearable Sensors in Oncology during Treatment: Lessons Learned from Feasibility Research Projects in Denmark. Diagnostics 2024, 14, 405. https://doi.org/10.3390/diagnostics14040405
Pappot H, Steen-Olsen EB, Holländer-Mieritz C. Experiences with Wearable Sensors in Oncology during Treatment: Lessons Learned from Feasibility Research Projects in Denmark. Diagnostics. 2024; 14(4):405. https://doi.org/10.3390/diagnostics14040405
Chicago/Turabian StylePappot, Helle, Emma Balch Steen-Olsen, and Cecilie Holländer-Mieritz. 2024. "Experiences with Wearable Sensors in Oncology during Treatment: Lessons Learned from Feasibility Research Projects in Denmark" Diagnostics 14, no. 4: 405. https://doi.org/10.3390/diagnostics14040405
APA StylePappot, H., Steen-Olsen, E. B., & Holländer-Mieritz, C. (2024). Experiences with Wearable Sensors in Oncology during Treatment: Lessons Learned from Feasibility Research Projects in Denmark. Diagnostics, 14(4), 405. https://doi.org/10.3390/diagnostics14040405






