Thrombotic Disease in Hemophilic Patients: Is This a Paradox in a State of Hypocoagulability?
Abstract
1. Introduction
2. Genetic Mechanisms of Transmission
3. Severity of Bleeding in Hemophilia
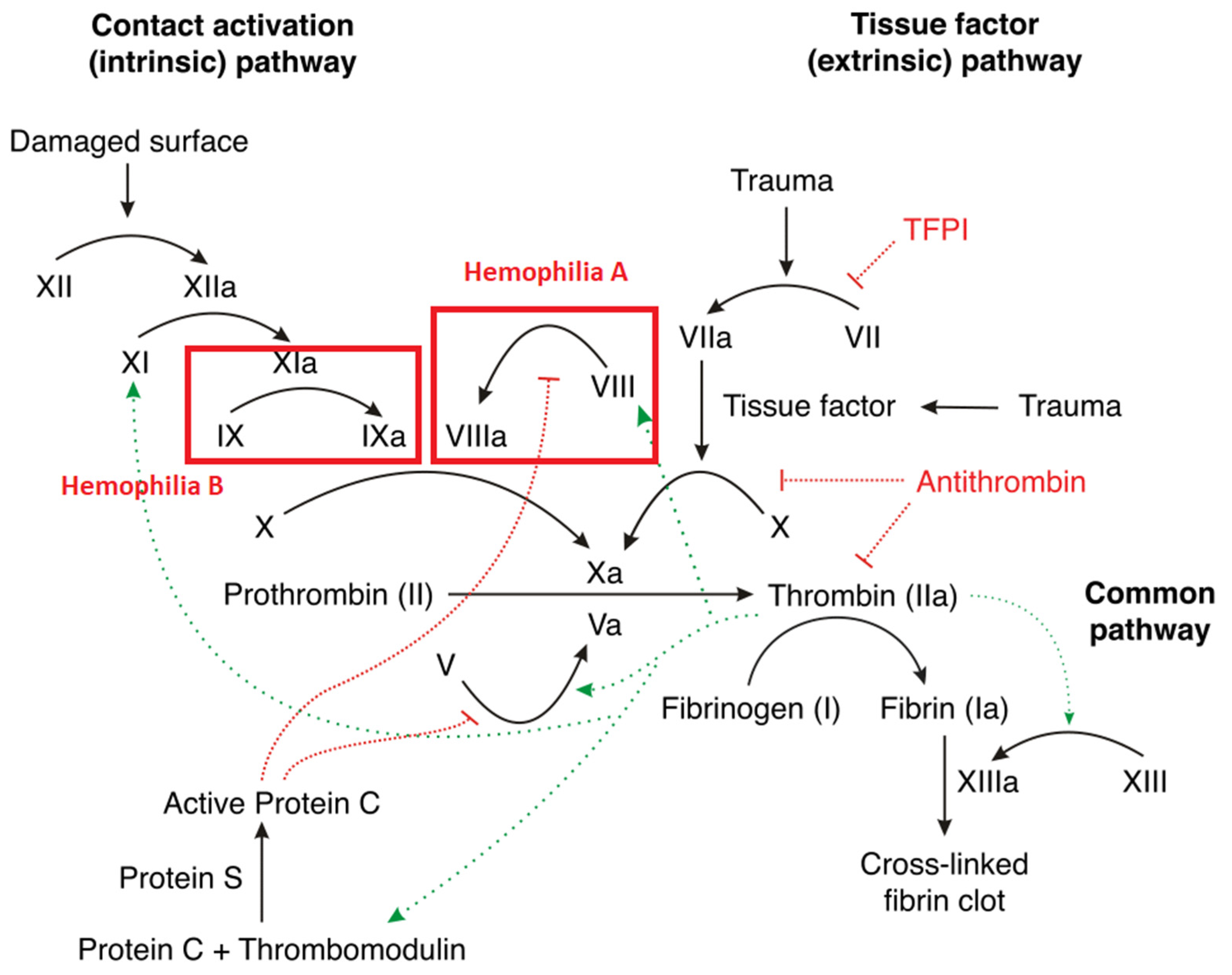
4. Pathophysiology of Hemophilia
5. Risk Factors for Thrombosis in Hemophilia
6. Potential Mechanisms of Thrombosis
6.1. Imbalance of Clotting Factors in Hemophilia
6.2. Platelet Activation
6.3. Endothelial Dysfunction
6.4. Genetic Factors
6.5. Viral Infections
6.6. Sepsis-Induced Coagulopathy
6.7. Therapy-Related Thrombotic Complications
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, P.; Reddivari, A.K.R. Hemophilia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Berntorp, E.; Shapiro, A.D. Modern haemophilia care. Lancet 2012, 379, 1447–1456. [Google Scholar] [CrossRef]
- Rogaev, E.I.; Grigorenko, A.P.; Faskhutdinova, G.; Kittler, E.L.W.; Moliaka, Y.K. Genotype Analysis Identifies the Cause of the “Royal Disease”. Science 2009, 326, 817. [Google Scholar] [CrossRef]
- Schramm, W. The history of haemophilia—A short review. Thromb. Res. 2014, 134, S4–S9. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Tuddenham, E.G. The Hemophilias—From Royal Genes to Gene Therapy. N. Engl. J. Med. 2001, 344, 1773–1779. [Google Scholar] [CrossRef]
- Salen, P.; Babiker, H.M. Hemophilia A. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kruse-Jarres, R.; Kempton, C.L.; Baudo, F.; Collins, P.W.; Knoebl, P.; Leissinger, C.A.; Tiede, A.; Kessler, C.M. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am. J. Hematol. 2017, 92, 695–705. [Google Scholar] [CrossRef]
- Bertamino, M.; Riccardi, F.; Banov, L.; Svahn, J.; Molinari, A.C. Hemophilia Care in the Pediatric Age. J. Clin. Med. 2017, 6, 54. [Google Scholar] [CrossRef]
- Dargaud, Y.; Meunier, S.; Negrier, C. Haemophilia and thrombophilia: An unexpected association! Haemophilia 2004, 10, 319–326. [Google Scholar] [CrossRef]
- Bicer, M.; Yanar, M.; Tuydes, O. Spontaneous deep vein thrombosis in hemophilia A: A case report. Cases J. 2009, 2, 6390. [Google Scholar] [CrossRef][Green Version]
- Chhabra, M.; Hii, Z.W.S.; Rajendran, J.; Ponnudurai, K.; Fan, B.E. Venous Thrombosis in Acquired Hemophilia: The Complex Management of Competing Pathologies. TH Open 2019, 3, e325–e330. [Google Scholar] [CrossRef]
- Sartori, M.T.; Bilora, F.; Zanon, E.; Varvarikis, C.; Saggiorato, G.; Campagnolo, E.; Pagnan, A.; Cella, G. Endothelial dysfunction in haemophilia patients. Haemophilia 2008, 14, 1055–1062. [Google Scholar] [CrossRef]
- Harper, P.S. Mary Lyon and the hypothesis of random X chromosome inactivation. Hum. Genet. 2011, 130, 169–174. [Google Scholar] [CrossRef]
- Pezeshkpoor, B.; Oldenburg, J.; Pavlova, A. Insights into the Molecular Genetic of Hemophilia A and Hemophilia B: The Relevance of Genetic Testing in Routine Clinical Practice. Hamostaseologie 2022, 42, 390–399. [Google Scholar] [CrossRef]
- Fischer, K.; Ljung, R.; Platokouki, H.; Liesner, R.; Claeyssens, S.; Smink, E.; Berg, H.M.v.D. Prospective observational cohort studies for studying rare diseases: The European PedNet Haemophilia Registry. Haemophilia 2014, 20, e280–e286. [Google Scholar] [CrossRef]
- Radic, C.P.; Rossetti, L.C.; Abelleyro, M.M.; Tetzlaff, T.; Candela, M.; Neme, D.; Sciuccati, G.; Bonduel, M.; Medina-Acosta, E.; Larripa, I.B.; et al. Phenotype–genotype correlations in hemophilia A carriers are consistent with the binary role of the phase between F8 and X-chromosome inactivation. J. Thromb. Haemost. 2015, 13, 530–539. [Google Scholar] [CrossRef]
- Konkle, B.A.; Nakaya Fletcher, S.; Hemophilia, B.; Adam, M.P.; Mirzaa, G.M.; Pagon, R.A. (Eds.) GeneReviews [Internet]; University of Washington: Seattle, WA, USA, 2023. [Google Scholar]
- Gomez, K.; Laffan, M.; Keeney, S.; Sutherland, M.; Curry, N.; Lunt, P. Recommendations for the clinical interpretation of genetic variants and presentation of results to patients with inherited bleeding disorders. A UK Haemophilia Centre Doctors’ Organisation Good Practice Paper. Haemophilia 2019, 25, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.; Oldenburg, J. Defining Severity of Hemophilia: More than Factor Levels. Semin. Thromb. Hemost. 2013, 39, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. 6), 1–158, Erratum in: Haemophilia 2021, 27, 699. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, U.A.; Ahmed, S.G. Pathophysiology of bleeding diathesis in haemophilia-A: A sequential and critical appraisal of non-FVIII related haemostatic dysfunctions and their therapeutic implications. Egypt. J. Med. Hum. Genet. 2018, 19, 285–295. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Lisman, T.; van den Berg, H.M.; Nieuwenhuis, H.K.; Meijers, J.C.; Bouma, B.N. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb. Haemost. 2001, 86, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- van Bladel, E.R.; Roest, M.; de Groot, P.G.; Schutgens, R.E.G. Up-regulation of platelet activation in hemophilia A. Haematologica 2011, 96, 888–895. [Google Scholar] [CrossRef]
- Eyster, M.E.; Asaad, S.M.; Gold, B.D.; Cohn, S.E.; Goedert, J.J.; The Second Multicenter Hemophilia Study Group. Upper gastrointestinal bleeding in haemophiliacs: Incidence and relation to use of non-steroidal anti-inflammatory drugs. Haemophilia 2007, 13, 279–286. [Google Scholar] [CrossRef]
- Tantawy, A.A.G. Molecular genetics of hemophilia A: Clinical perspectives. Egypt. J. Med. Hum. Genet. 2010, 11, 105–114. [Google Scholar] [CrossRef]
- Sreejith, N. Coagulation. 2023. Available online: https://teachmephysiology.com/immune-system/haematology/coagulation/ (accessed on 17 April 2023).
- Larsen, J.B.; Nielsen, K.B.J.; Poulsen, L.H.; Bor, M.V. Arterial and Venous Thrombosis in Haemophilia Patients: Experiences from a Danish Haemophilia Centre. Acta Haematol. 2017, 138, 91–95. [Google Scholar] [CrossRef]
- Lim, M.Y.; Pruthi, R.K. Cardiovascular disease risk factors prevalence and management in adult hemophilia patients. Blood Coagul. Fibrinolysis 2011, 22, 402–406. [Google Scholar] [CrossRef]
- Shapiro, S.; Benson, G.; Evans, G.; Harrison, C.; Mangles, S.; Makris, M. Cardiovascular disease in hereditary haemophilia: The challenges of longevity. Br. J. Haematol. 2022, 197, 397–406. [Google Scholar] [CrossRef]
- Sharathkumar, A.A.; Soucie, J.M.; Trawinski, B.; Greist, A.; Shapiro, A.D. Prevalence and risk factors of cardiovascular disease (CVD) events among patients with haemophilia: Experience of a single haemophilia treatment centre in the United States (US). Haemophilia 2011, 17, 597–604. [Google Scholar] [CrossRef]
- Boylan, B.; Rice, A.S.; De Staercke, C.; Eyster, M.E.; Yaish, H.M.; Knoll, C.M.; Bean, C.J.; Miller, C.H.; Abshire, T.; Dunn, A.; et al. Evaluation of von Willebrand factor phenotypes and genotypes in Hemophilia A patients with and without identified F8 mutations. J. Thromb. Haemost. 2015, 13, 1036–1042. [Google Scholar] [CrossRef]
- Rosendaal, F. Venous thrombosis: A multicausal disease. Lancet 1999, 353, 1167–1173. [Google Scholar] [CrossRef]
- Rajpal, S.; Ahluwalia, J.; Kumar, N.; Malhotra, P.; Uppal, V. Elevated Von Willebrand Factor Antigen Levels are an Independent Risk Factor for Venous Thromboembolism: First Report from North India. Indian J. Hematol. Blood Transfus. 2019, 35, 489–495. [Google Scholar] [CrossRef]
- Lip, G.Y.; Blann, A. Von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Blann, A. Von Willebrand factor and the endothelium in vascular disease. Br. J. Biomed. Sci. 1993, 50, 125–134. [Google Scholar]
- Boneu, B.; Abbal, M.; Plante, J.; Bierme, R. Letter: Factor-VIII Complex and Endothelial Damage. Lancet 1975, 305, 1430. [Google Scholar] [CrossRef]
- Grünewald, M.; Siegemund, A.; Grünewald, A.; Konegen, A.; Koksch, M.; Griesshammer, M. Absence of compensatory platelet activation in patients with severe haemophilia, but evidence for a platelet collagen-activation defect. Platelets 2002, 13, 451–458. [Google Scholar] [CrossRef]
- Satchell, C.S.; O’halloran, J.A.; Cotter, A.G.; Peace, A.J.; O’Connor, E.F.; Tedesco, A.F.; Feeney, E.R.; Lambert, J.S.; Sheehan, G.J.; Kenny, D.; et al. Increased Platelet Reactivity in HIV-1–Infected Patients Receiving Abacavir-Containing Antiretroviral Therapy. J. Infect. Dis. 2011, 204, 1202–1210. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Kushwaha, R.; Yadav, G.; Tripathi, T.; Chaudhary, S.C.; Verma, S.P.; Singh, U.S. Hyperlipidemia and Platelet Parameters: Two Sides of the Same Coin. Cureus 2022, 14, e25884. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K. Endothelial Dysfunction and Venous Thrombosis. Angiology 2018, 69, 564–567. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor. Arter. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef]
- Bilora, F.; Zanon, E.; Petrobelli, F.; Cavraro, M.; Prandoni, P.; Pagnan, A.; Girolami, A. Does Hemophilia Protect Against Atherosclerosis? A Case-Control Study. Clin. Appl. Thromb. Hemost. 2006, 12, 193–198. [Google Scholar] [CrossRef]
- Franchini, M. Thrombotic complications in patients with hereditary bleeding disorders. Arthritis Res. Ther. 2004, 92, 298–304. [Google Scholar] [CrossRef]
- Vaughan, D.E. PAI-1 and atherothrombosis. J. Thromb. Haemost. 2005, 3, 1879–1883. [Google Scholar] [CrossRef]
- Sjögren, L.S.; Doroudi, R.; Gan, L.-M.; Jungersten, L.; Hrafnkelsdóttir, T.; Jern, S. Elevated Intraluminal Pressure Inhibits Vascular Tissue Plasminogen Activator Secretion and Downregulates Its Gene Expression. Hypertension 2000, 35, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592. [Google Scholar] [CrossRef]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martin, C.; Daley, M.; Miller, M.R.; Patel, M.A.; dos Santos, C.C.; Bosma, K.J.; et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef]
- Hauw, W.W.S.; Chia, J.S.J.; Nandurkar, H.H.; Sashindranath, M. The potential role of protease systems in hemophilic arthropathy. Blood Adv. 2022, 6, 5505–5515. [Google Scholar] [CrossRef] [PubMed]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.; Martins, R.B.; Benatti, M.N.; Almado, C.E.; de Sá, K.S.; Bonato, V.L.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; A Perez-Romero, B.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. Factor V Leiden and hemophilia. Thromb. Res. 2010, 125, 119–123. [Google Scholar] [CrossRef]
- Kenet, G.; Bidlingmaier, C.; Bogdanova, N.; Ettingshausen, C.E.; Goldenberg, N.; Gutsche, S.; Halimeh, S.; Holzhauer, S.; Kurnik, K.; Limperger, V.; et al. Influence of factor 5 rs6025 and factor 2 rs1799963 mutation on inhibitor development in patients with hemophilia A—An Israeli-German multicenter database study. Thromb. Res. 2014, 133, 544–549. [Google Scholar] [CrossRef]
- Lee, D.H.; Walker, I.; Teitel, J.; Poon, M.-C.; Ritchie, B.; Akabutu, J.; Sinclair, G.D.; Pai, M.; Wu, J.W.Y.; Reddy, S.; et al. Effect of the Factor V Leiden Mutation on the Clinical Expression of Severe Hemophilia A. Arthritis Res. Ther. 2000, 83, 387–391. [Google Scholar] [CrossRef]
- Vezendi, K.; Tápai, K.; Erdödi, E.; Szabó, I.; Tomek, A.; Oszlács, J.; Scheibl, E. Thrombophilic markers in patients with congenital bleeding disorders. Haematologia 2002, 32, 467–473. [Google Scholar]
- Mukhopadhyay, S.; Johnson, T.A.; Duru, N.; Buzza, M.S.; Pawar, N.R.; Sarkar, R.; Antalis, T.M. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front. Immunol. 2019, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.B. Clinical disorders of fibrinolysis: A critical review. Blut 1989, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saes, J.; Schols, S.; van Heerde, W.; Nijziel, M. Hemorrhagic disorders of fibrinolysis: A clinical review. J. Thromb. Haemost. 2018, 16, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Malkhassian, D.; Sabir, S.; Sharma, S. Physiology, Factor XIII. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ząbczyk, M.; Natorska, J.; Undas, A. Factor XIII and Fibrin Clot Properties in Acute Venous Thromboembolism. Int. J. Mol. Sci. 2021, 22, 1607. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Sang, Y. Fibrinogen and Factor XIII in Venous Thrombosis and Thrombus Stability. Arter. Thromb. Vasc. Biol. 2022, 42, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Newly-Recognized Roles of Factor XIII in Thrombosis. Semin. Thromb. Hemost. 2016, 42, 445–454. [Google Scholar] [CrossRef]
- Saif, M.W.; Bona, R.; Greenberg, B. AIDS and Thrombosis: Retrospective Study of 131 HIV-Infected Patients. AIDS Patient Care STDs 2001, 15, 311–320. [Google Scholar] [CrossRef]
- Estival, J.L.; Debourdeau, P.; Zammit, C.; Teixeira, L.; Guérard, S.; Colle, B. Spontaneous portal vein thrombosis associated with acute cy-tomegalovirus infection in an immunocompetent patient. Presse Med. 2001, 30, 1876–1878. (In French) [Google Scholar]
- Sherman, S.; Eytan, O.; Justo, D. State of the art paper Thrombosis associated with acute cytomegalovirus infection: A narrative review. Arch. Med. Sci. 2014, 6, 1186–1190. [Google Scholar] [CrossRef]
- Violi, F.; Ferro, D.; Basili, S.; Artini, M.; Valesini, G.; Levrero, M.; Cordova, C. Increased rate of thrombin generation in hepatitis C virus cirrhotic patients. Relationship to venous thrombosis. J. Investig. Med. 1995, 43, 550–554. [Google Scholar]
- Coppola, A.; Franchini, M.; Makris, M.; Santagostino, E.; DI Minno, G.; Mannucci, P.M. Thrombotic adverse events to coagulation factor concentrates for treatment of patients with haemophilia and von Willebrand disease: A systematic review of prospective studies. Haemophilia 2012, 18, e173–e187. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Parastatidou, S.; Tsantes, E.A.; Bonova, E.; Tsante, K.A.; Mantzios, P.G.; Vaiopoulos, A.G.; Tsalas, S.; Konstantinidi, A.; Houhoula, D.; et al. Sepsis-Induced Coagulopathy: An Update on Pathophysiology, Biomarkers, and Current Guidelines. Life 2023, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.; Pittet, J.-F. The coagulopathy of acute sepsis. Curr. Opin. Anaesthesiol. 2015, 28, 227–236. [Google Scholar] [CrossRef]
- Jha, N.K.; Shestopal, S.A.; Gourley, M.J.; Woodle, S.A.; Liang, Y.; Sarafanov, A.G.; Weinstein, M.; Ovanesov, M.V. Optimization of the thrombin generation test components to measure potency of factor VIII concentrates. Haemophilia 2016, 22, 780–789. [Google Scholar] [CrossRef]
- Manco-Johnson, M.J. Advances in the Care and Treatment of Children with Hemophilia. Adv. Pediatr. 2010, 57, 287–294. [Google Scholar] [CrossRef]
- Ragni, M.V. Thrombosis Complicating Non-Factor Therapy for Hemophilia. Med. Res. Arch. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Non-factor replacement therapy for haemophilia: A current update. Blood Transfus. 2018, 16, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, R.; Herzog, R.W. Treatment-induced hemophilic thrombosis? Mol. Ther. 2022, 30, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Study to Evaluate the Efficacy and Safety of PF-07055480/Giroctocogene Fitelparvovec Gene Therapy in Moderately Severe to Severe Hemophilia A Adults (AFFINE). ClinicalTrials.gov Identifier: NCT04370054. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04370054 (accessed on 17 April 2023).
- Pfizer Inc. Pfizer and Sangamo Therapeutics Announce Phase 3 Trial of Investigational Gene Therapy for Hemophilia A Has Re-Opened Recruitment. 2022. Available online: https://www.pfizer.com/news/announcements/pfizer-and-sangamo-therapeutics-announce-phase-3-trial-investigational-gene (accessed on 17 April 2023).
- Badulescu, O.V.; Sirbu, P.D.; Filip, N.; Bordeianu, G.; Cojocaru, E.; Budacu, C.C.; Badescu, M.C.; Bararu-Bojan, I.; Veliceasa, B.; Ciocoiu, M. Hereditary Thrombophilia in the Era of COVID-19. Healthcare 2022, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Badulescu, O.V.; Sirbu, P.D.; Ungureanu, C.; Pȋnzariu, A.; Cojocaru, E.; Filip, N.; Bararu-Bojan, I.; Vladeanu, M.; Ciocoiu, M. Orthopedic surgery in hemophilic patients with musculoskeletal disorders: A systematic review. Exp. Ther. Med. 2021, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
| Severity | Clotting Factor Level | Bleeding Episodes |
|---|---|---|
| Mild | >5% | Rare, often after surgery or trauma |
| Moderate | 1–5% | Occasional, usually with injury or surgery |
| Severe | <1% | Spontaneous, frequent, and severe bleeding |
| Bleeding Disorder | Patients | Type of Product | VTE | Thrombo-Phlebitis | Thrombotic AEs/Patients (%) | Thrombotic AEs/ Total AEs (%) |
|---|---|---|---|---|---|---|
| Hemophilia A | 4420 | pdFVIII or rFVIII | 0 | 2 | 2/4420 (0.045) | 2/423 (0.47) |
| Hemophilia B | 748 | pdFIX or rFIX | 0 | 11 | 11/748 (1.47) | 2/104 (1.92) |
| von Willebrand disease | 361 | pdVWF/FVIII or rVWF/FVIII | 2 | 5 | 7/361 (1.94) | 14.0 |
| All | 5528 | pd and r products | 2 | 18 | 20/5528 (0.36) | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badulescu, O.V.; Badescu, M.C.; Bojan, I.B.; Vladeanu, M.; Filip, N.; Dobreanu, S.; Tudor, R.; Ciuntu, B.-M.; Tanevski, A.; Ciocoiu, M. Thrombotic Disease in Hemophilic Patients: Is This a Paradox in a State of Hypocoagulability? Diagnostics 2024, 14, 286. https://doi.org/10.3390/diagnostics14030286
Badulescu OV, Badescu MC, Bojan IB, Vladeanu M, Filip N, Dobreanu S, Tudor R, Ciuntu B-M, Tanevski A, Ciocoiu M. Thrombotic Disease in Hemophilic Patients: Is This a Paradox in a State of Hypocoagulability? Diagnostics. 2024; 14(3):286. https://doi.org/10.3390/diagnostics14030286
Chicago/Turabian StyleBadulescu, Oana Viola, Minerva Codruta Badescu, Iris Bararu Bojan, Maria Vladeanu, Nina Filip, Stefan Dobreanu, Razvan Tudor, Bogdan-Mihnea Ciuntu, Adelina Tanevski, and Manuela Ciocoiu. 2024. "Thrombotic Disease in Hemophilic Patients: Is This a Paradox in a State of Hypocoagulability?" Diagnostics 14, no. 3: 286. https://doi.org/10.3390/diagnostics14030286
APA StyleBadulescu, O. V., Badescu, M. C., Bojan, I. B., Vladeanu, M., Filip, N., Dobreanu, S., Tudor, R., Ciuntu, B.-M., Tanevski, A., & Ciocoiu, M. (2024). Thrombotic Disease in Hemophilic Patients: Is This a Paradox in a State of Hypocoagulability? Diagnostics, 14(3), 286. https://doi.org/10.3390/diagnostics14030286










