Abstract
Several serum biomarkers for fibrosis assessment have been proposed in various liver diseases, but in autoimmune hepatitis (AIH) or overlap with primary biliary cholangitis (PBC; AIH-PBC) patients, the data are scarce. This retrospective cross-sectional study was conducted to validate six non-invasive biomarkers in the diagnosis of cirrhosis (F4 fibrosis) in such patients. We included adult patients diagnosed with AIH or AIH-PBC overlap syndrome who underwent a liver biopsy between 2011 and 2021. Laboratory data were collected to calculate the following scores: red cell distribution width to platelet ratio (RPR), aspartate aminotransferase/platelet ratio index (APRI), Fibrosis-4 index (FIB-4), aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-platelet ratio (LPR). A total of 139 patients were eligible (111 AIH and 28 AIH-PBC). The prevalence of cirrhosis was 35.3% (36% in AIH and 32.1% in AIH-PBC). The AUROCs of the RPR, FIB-4, APRI, AAR, LPR, and NLR in all patients were 0.742, 0.724, 0.650, 0.640, 0.609, and 0.585, respectively. RPR was significantly superior to APRI, NLR, and LPR. Moreover, RPR showed the highest AUROC (0.915) in the overlap AIH-PBC subgroup. In conclusion, RPR yielded the highest diagnostic accuracy to predict cirrhosis in AIH and AIH-PBC overlap syndrome patients, while FIB-4 was considerably optimal.
1. Introduction
Autoimmune hepatitis (AIH) is a chronic, non-resolving immune-mediated inflammatory liver disease that affects patients of all ages and ethnicities, with a female preponderance. The diagnosis of AIH can be ascertained by a combination of clinical characteristics, laboratory findings, and liver histology. The diagnostic scoring systems of the International Autoimmune Hepatitis Group (IAIHG), both the revised scoring system (1999) and the simplified scoring system (2008), were affirmed for use in the diagnosis of AIH, according to the clinical practice guidelines from the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) [1,2].
Nonetheless, there might be more than one autoimmune liver disease feature in a single person, a so-called ‘overlap syndrome’. The most commonly found overlap syndrome is AIH and primary biliary cholangitis (or the previous nomenclature was primary biliary cirrhosis) in combination (AIH-PBC), and the Paris criteria are generally recommended for use in the diagnosis of such a condition [1,2,3,4]. Once diagnosed, the incidence of liver cirrhosis detected at initial presentation is about 30–80% [5,6] in patients with AIH, and in those without cirrhosis at the diagnosis, 10% developed cirrhosis during follow-up or even after biochemical remission is detected, which means life-long monitoring is needed in these patients [7]. In addition, in AIH-PBC overlap syndrome, patients tend to present with more advanced fibrosis and portal hypertension-related complications than in patients with AIH alone or conventional PBC alone [2,3,4].
Accordingly, fibrosis and cirrhosis assessments at first diagnosis and during the follow-up period are of clinical importance. Once cirrhosis is diagnosed, it triggers a cascade of cirrhosis care, for example, screening for gastro-esophageal varices, either by esophagogastroduodenoscopy (EGD) or using non-invasive methods such as transient elastography in combination with platelet counts as per BAVENO VI recommendation, and hepatocellular carcinoma surveillance every 6 months [8,9,10]. Moreover, for the decision of therapeutic options in autoimmune liver diseases, budesonide is generally not recommended in patients who already have cirrhosis, as it increases the risk of portal vein thrombosis and systemic side effects regarding portosystemic shunting [1]. Liver biopsy is still a reference standard for the evaluation of the fibrosis stage. Despite high diagnostic accuracy, liver biopsy is an invasive procedure, and it comes with a risk of severe life-threatening complications [5]. A study regarding percutaneous liver biopsy in AIH patients with cirrhosis found a complication rate of 5.8% with some serious bleeding, even in patients with Child–Pugh class A patients [11]. Thus, a liver biopsy is not a suitable tool for a dynamic evaluation of the liver fibrosis stage during the follow-up period [12,13]. And recently, a study concerning the role of a liver biopsy for the diagnosis of AIH in patients with typical laboratory features concluded that a liver biopsy might be unnecessary in patients with compatible clinical criteria [14]. Hence, the non-invasive liver fibrosis assessment methods should be used to avoid the complications of a liver biopsy and aid in dynamic fibrosis evaluation [13].
Since there is a gap in the knowledge concerning the high-performance non-invasive serum biomarkers for cirrhosis assessment in AIH and AIH-PBC overlap syndrome patients, in this study, we aim to evaluate the diagnostic performance of six non-patented serum biomarkers in the diagnosis of cirrhosis (stage 4 fibrosis) in AIH and AIH-PBC overlap syndrome patients.
2. Materials and Methods
We conducted a single-center retrospective cross-sectional study at our institute, which is a tertiary care university hospital in Thailand. The study protocol was approved by the Institutional Human Research Ethics Committee (HREC), Faculty of Medicine, Prince of Songkla University, Thailand (REC. 64-429-14-1). The study was conducted under the ethical guidelines of the 1975 Declaration of Helsinki.
2.1. Study Population
We included all adult patients (aged at least 18 years old) diagnosed with AIH or AIH-PBC overlap syndrome who underwent a liver biopsy at our center between 2011 and 2021 with available laboratory data to calculate serum biomarkers of interest. The exclusion criteria were as follows: (1) concomitant with hepatitis B virus (HBV), hepatitis C virus (HCV), significant alcohol consumption, or sclerosing cholangitis; (2) presence of the liver tumor at the time of the liver biopsy; (3) active infection, hematologic disease, or other inflammatory disease that may interfere with the results of the biomarkers of interest; and (4) inadequate tissue sampling from the liver biopsy for a re-evaluation.
2.2. Data Collection
All histopathological specimens were reviewed by a single pathologist who was blinded to clinical and laboratory data (JT). Baseline demographic data of the patients, e.g., age, sex, weight, height, underlying diseases, and laboratory data at the day of liver biopsy were obtained from the Hospital Information System (HIS) for the calculation of the non-invasive serum biomarker scores. Cirrhosis was defined by the presence of F4 fibrosis by METAVIR system on histopathological re-evaluation.
2.3. Non-Invasive Biomarkers of Interest
Aiming for the generalizability of the biomarkers’ utility in the real-world context, we focused only on non-patented biomarkers using only baseline demographic data and routine laboratory variables. The following scores were calculated and evaluated for the diagnostic performances in our study: red cell distribution width to platelet ratio (RPR), [15] aspartate aminotransferase/platelet ratio index (APRI), Fibrosis-4 index (FIB-4) [16], aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR) [17], neutrophil-to-lymphocyte ratio (NLR) [18], and lymphocyte-to-platelet ratio (LPR) [17]. The formulas of the scores are as follows:
- RPR: RDW (%)/PLT count (109/L).
- APRI: (AST (U/L)/ULN of AST)/PLT count (109/L) × 100.
- FIB-4: (age (years) × AST (U/L))/((PLT count (109/L) × (ALT (U/L))1/2).
- AAR: AST (U/L)/ALT (U/L) [16].
- NLR: Absolute neutrophil count (cells/μL)/Absolute lymphocyte count (cells/μL).
- LPR: Absolute lymphocyte count (cells/μL)/PLT count (109/L).
3. Statistical Analysis
All statistical analyses were performed using R program version 4.1.0 (Vienna, Austria). Descriptive statistics were used for baseline demographic data. Quantitative measurements were shown as mean ± SD or median with interquartile range (IQR) according to the distribution of observed values. To compare the groups of patients with and without cirrhosis, Chi-square test for categorical variables and Wilcoxon rank-sum test or t-test for continuous variables were used for the analysis. The diagnostic performance of each biomarker was analyzed using areas under receiver operating curve (AUROCs) for the ability to discriminate between cirrhosis and non-cirrhosis. DeLong’s test was used for the comparison of AUROC. A p-value of <0.05 was considered statistically significant.
4. Results
4.1. Baseline Characteristics
From 1377 liver biopsy specimens of non-malignant liver tissue in the HIS during the study period, a total of 139 patients were eligible and included for analysis. Of those, 111 (79.9%) were AIH patients, and 28 (20.1%) were overlap AIH-PBC patients. The prevalence of cirrhosis was 35.3% overall (36% in AIH and 32.1% in AIH-PBC). In the cirrhosis group, Child–Turcotte–Pugh (CTP) scores were class A 57.1%, class B 36.7%, and class C 6.1%, with a median model for end-stage liver disease (MELD) score of 9 (IQR: 7.5,16). The mean age of the entire cohort was 55 years; however, the mean age of patients in the cirrhosis group was significantly higher than the non-cirrhosis group (57 vs. 52 years, p = 0.005), and 80.6% were female. No significant difference between the cirrhosis group and non-cirrhosis group in baseline characteristics such as body weight, body mass index (BMI), and medical co-morbidities except for a higher proportion of diabetes was found in the cirrhosis group (34.7%) compared to the other group (6.7%, p < 0.001), and around one third of patients in both groups were treatment-experienced before undergoing a liver biopsy, as shown in Table 1.

Table 1.
Baseline clinical characteristics between the non-cirrhosis group and the cirrhosis group in patients with autoimmune hepatitis (AIH) and overlap with primary biliary cholangitis (PBC; AIH-PBC overlap syndrome).
4.2. Liver Biopsy Complications
Liver biopsy complications were reported in four (2.9%) patients; three were immediate post-procedural bleeding; however, blood transfusion was required in only one patient. One patient experienced severe pain with desaturation following the procedure, which improved after receiving pain control and oxygen support. No fatal cases related to liver biopsy procedures feature in this study.
4.3. Laboratory Results
For the complete blood count (CBC) results, patients with cirrhosis had significantly lower hematocrit (Hct) levels (mean 34.1% vs. 36.7%, p = 0.012) and platelet counts (median 166 × 103/μL vs. 235 × 103/μL, p < 0.001) than those with non-cirrhosis. The red cell distribution width (RDW) was also significantly different between both groups (median 15.8 vs. 14.2 fL, p = 0.034), while mean corpuscular volume (MCV), white blood cell count, and lymphocyte proportion were comparable between groups. For liver chemistries, the only variable showing a statistically significant difference between those with and without cirrhosis was serum albumin level (median 3.6 vs. 4 g/dL, p = 0.001, respectively), whereas bilirubin, AST, ALT, alkaline phosphatase (ALP), and globulin level were not significantly different as shown in Table 1. The median values of non-invasive serum biomarkers of interest were significantly different between the cirrhosis and non-cirrhosis groups, as shown in Table 2.

Table 2.
Comparisons of values of six non-invasive biomarkers between patients with and without cirrhosis (N = 139).
4.4. Diagnostic Performance of Serum Biomarkers
In this study, we investigated six non-invasive serum biomarkers to predict cirrhosis in patients with AIH or overlap AIH-PBC patients, including APRI, FIB-4, AAR, NLR, LPR, and RPR, as mentioned earlier. In the entire cohort, the AUROCs of the biomarkers shown in descending order are as follows: RPR (0.742), FIB-4 (0.724), APRI (0.650), AAR (0.640), LPR (0.609), and NLR (0.585), respectively (Figure 1). RPR was significantly superior to APRI (p = 0.029), NLR (p = 0.023), and LPR (p = 0.016) for the diagnosis of cirrhosis status, while it was non-significantly higher than FIB-4 and AAR. For the subgroup of AIH-PBC overlap syndrome patients, the AUROC of the RPR was still the highest among all the evaluated biomarkers (0.915). The AUROCs of all biomarkers in the AIH and AIH-PBC subgroups are shown in Table 3.
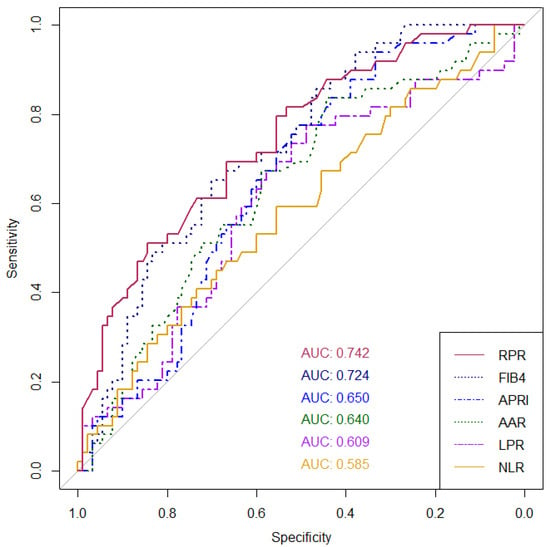
Figure 1.
AUROCs for the ability of non-invasive serum biomarkers to discriminate between cirrhosis and non-cirrhosis in patients with autoimmune hepatitis (AIH) or overlap with primary biliary cholangitis (PBC; AIH-PBC overlap syndrome). Abbreviations: RPR, red cell distribution width to platelet ratio; APRI, aspartate aminotransferase/platelet ratio index: FIB-4, Fibrosis-4 index; AAR, aspartate aminotransferase to alanine aminotransferase ratio; NLR, neutrophil-to-lymphocyte ratio; and LPR, lymphocyte-to-platelet ratio.

Table 3.
Comparison of the AUROCs of non-invasive serum biomarkers to discriminate between cirrhosis and non-cirrhosis in patients with autoimmune hepatitis (AIH) or overlap with primary biliary cholangitis (PBC; AIH-PBC overlap syndrome).
As only RPR and FIB-4 demonstrated good diagnostic performance (AUROCs > 0.70), we then evaluated the sensitivity and specificity of RPR and FIB-4 at the cutoffs mentioned in prior studies for the diagnosis of cirrhosis (Table 4). According to Wang et al.’s study, the optimal cutoff values of RPR were 0.083, 0.084, and 0.127 in identifying significant fibrosis, advanced fibrosis, and cirrhosis in patients with autoimmune hepatitis, respectively; therefore, we use RPR at 0.127 for the cutoff in the diagnosis of cirrhosis in this study [15]. In the current cohort, at the cutoff levels of RPR (≥0.127) and FIB-4 (≥3.24) [12], the RPR showed a significantly higher specificity for ruling in cirrhosis than that in FIB-4 (93.33% vs. 66.67%, p < 0.001, respectively).

Table 4.
Sensitivities and specificities of RPR and FIB-4 in the diagnosis of cirrhosis at a given cutoffs for an entire cohort.
5. Discussion
Our study shows that in patients with biopsy-proven AIH and overlap AIH-PBC, the prevalence of F4 fibrosis or cirrhosis was around one-third, and among the currently available non-invasive biomarkers, RPR showed a good diagnostic ability to distinguish between the cirrhosis and non-cirrhosis stages at the AUROC of 0.742, which is better than other biomarkers.
The epidemiology of AIH varies worldwide; the estimated prevalence is 15–25 cases/105 persons in Europe [19], 4 cases/105 persons in Singapore [20], and 2.5% of chronic hepatitis patients in Thailand [6]. The clinical manifestations of AIH are presented in a broad spectrum from asymptomatic (25–34%) to acute hepatitis (25–75%) to acute liver failure (3–6%). [1,2] In untreated patients, progression to liver fibrosis or cirrhosis with complications often occurs.
In this study, most of the patients with AIH and AIH-PBC overlap syndrome were female (80.6%), and the prevalence of biopsy-proven cirrhosis was 35.3%, which was similar to previous studies. [6,21] AIH was diagnosed in almost 80%, while overlap AIH-PBC patients were around one-fifth of the entire cohort. The baseline laboratory results also showed significantly lower platelet counts and albumin levels in AIH or overlap AIH-PBC patients with cirrhosis, as well as in general cirrhotic patients from other causes.
Abdominal ultrasonography, although it is commonly used to diagnose the cirrhotic status of patients in routine clinical practice, the accuracy of an ultrasound in the diagnosis of cirrhosis has been reported to be only 64–79% [22]. Moreover, if signs of portal hypertension, e.g., splenomegaly, ascites, or collateral vessels, were not considered, using only liver parenchymal echogenicity or surface characteristics on ultrasound showed limited sensitivity and specificity to ascertain the diagnosis of cirrhosis [23].
Currently, non-invasive tests (NITs) for fibrosis stage assessment in liver diseases are the subject of extensive research. Device-assisted elastography, such as vibration-controlled transient elastography, is the most widely used and validated method with high performance for the diagnosis of cirrhosis, but it requires a dedicated device, which might not be easily accessible in general practice and requires an experienced operator [5,13]. Another point of concern is that the diagnostic performance of vibration-controlled transient elastography may be reduced in cases of narrow intercostal spaces, ascites, or the presence of inflammation, venous congestion, or obstructive cholestasis, which results in an overestimation of fibrosis [5,24]. The same concern was also observed in other methods, e.g., shear wave elastography or magnetic resonance elastography. Data from a large multicenter cohort study by Llovet et al. also indicate that liver stiffness measurement is not accurate to predict AIH cirrhosis in the long term, according to the decrease in necro-inflammation with liver remodeling or fibrosis resorption after immunosuppressive therapy, which may deteriorate the diagnostic yield [7].
Hence, in this study, we selected only non-patented serum biomarkers to be the NITs of interest in our study, owing to their wide availability, good reproducibility, high applicability, and lack of extra costs from routine clinical practice. We specifically focused on the diagnosis of F4 fibrosis rather than significant (≥F2) or advanced (≥F3) fibrosis in this study, as the main shift in management of AIH and overlap AIH-PBC patients occurs when patients have cirrhosis, not at the lower fibrosis stage; for example, such diseases should be treated regardless of the fibrosis stage, but having cirrhosis precludes the use of budesonide treatment, and it sets off further cirrhotic care bundle for the patients. In other liver diseases, the threshold for commencing treatment may start at F2 or higher.
The APRI and FIB-4 scores are well-known NITs among hepatologists, and recently, LPR [17], AAR, NLR, and RPR [15,25,26,27,28,29,30,31,32] have been introduced to the field of liver fibrosis assessment. The diagnostic performances of these non-invasive serum markers in fibrosis assessment were mostly validated in HBV, HCV, or non-alcoholic fatty liver disease patients (NAFLD; or the most recently updated nomenclature of metabolic dysfunction-associated steatotic liver disease: MASLD). Nonetheless, when it comes to autoimmune liver diseases, the aforementioned biomarkers were examined to a considerably lesser extent. And previous limited data have indicated suboptimal to moderate discriminatory accuracy, hence precluding their recommendation for use in patients with AIH and PBC according to the current guidance [1,5]. In the more recent studies, Yuan X et al. [17] conducted an analysis of the LPR in patients with AIH in 2020 and reported that the LPR had a high diagnostic performance for liver cirrhosis, with an AUROC of 0.936. In contrast, Wang H. et al. [15] reported in the same year that the RPR demonstrated a statistically significant improvement over APRI and FIB-4 in the identification of cirrhosis among patients with AIH. The most recent study, published in 2022, reported on a meta-analysis examining the diagnostic accuracy of APRI and FIB-4 in predicting cirrhosis among 759 patients with AIH. The results indicated that both APRI and FIB-4 demonstrated modest diagnostic accuracy, with mean AUROC values of 0.644 and 0.732, respectively [12]. The existing body of data has not yielded a definitive outcome on the efficacy of NITs in individuals with AIH.
We evaluated all the aforementioned serum NITs in Thai AIH and overlap AIH-PBC patients. Our data showed that the AUROCs of those NITs range from 0.58 to 0.74, and RPR yielded the highest AUROCs for the diagnosis of cirrhosis, significantly better than APRI, NLR, and LPR, although it was not significantly different from FIB-4 and AAR. For the LPR, the AUROC was the lowest (0.585) in contrast to the study by Yuan X et al. [17], but the high diagnostic performance of the RPR was consistent with the Wang H. et al. study [15]. The difference in the results of LPR performance between our study and the study by Yuan X et al. [17] might be due to the difference in the study population. In the prior study, Yuan X et al. [17] compared AIH patients and healthy controls, whereas we studied the more homogenous group of only AIH and overlap AIH-PBC patients, in which we considered that this represented the exact population of interest in the real clinical situation. In the study by Wang H. et al. [15], although the population of the patients was almost the same as that in this study and showed concordant results, the novelty of our study was a remarkably high AUROC of RPR in overlap AIH-PBC patients.
It is not entirely understood why RPR is superior to other NITs in diagnosing cirrhosis in patients with AIH. RPR consists of two routine laboratory variables, RDW and platelets, reported in daily clinical practice. RDW abnormality was hypothetically linked to cirrhosis as portal hypertension leads to hypersplenism and, consequently, a shorter survival of red blood cells (RBC) [29]. Additionally, the chronic inflammatory status produces proinflammatory cytokines that inhibit RBC maturation, and impaired iron metabolism and erythropoiesis in chronic inflammation may also contribute to the increased RDW [33]. Low platelet counts, on the other hand, are already well-known to be associated with liver fibrosis and cirrhosis owing to the impairment of thrombopoietin production and hypersplenism in such conditions. Not only in patients with AIH, RPR has also been validated in other liver diseases such as HBV, HCV, and PBC patients, as well as chronic hepatitis patients with mixed etiology [25,26,34,35] and yielded an AUROC of 0.71–0.9 in predicting cirrhosis. For the NLR, in 2018, Li X et al. demonstrated an AUROC of 0.637 for predicting advanced liver fibrosis in AIH patients; later, the study by Zeng T et al. also showed the same trend with an AUROC of 0.680 for the diagnosis of cirrhosis [27,28]. When it comes to NLR and LPR, both of them are indicators of the immune response to systemic inflammation [27,36] rather than reflecting the cirrhosis consequences; thus, this could be a possible explanation for the superiority of the RPR over these two biomarkers.
Due to the exclusion of AIH-PBC overlap syndrome patients from most studies’ eligibility criteria, no validated serum markers for fibrosis assessment are currently available for this subgroup so far. This is the first study to provide novel information regarding non-invasive serum biomarkers for predicting cirrhosis in patients with AIH-PBC overlap syndrome. In this subgroup population, RPR still produced the highest AUROC (0.91), and in the same manner, followed by FIB-4.
The virtues of our study are that all patients had biopsy-proven AIH or overlap AIH-PBC, and the liver biopsy specimens were reviewed by a single pathologist who was blinded to the patients’ data. The laboratory data were also available in all patients on the same day of liver biopsy as per institutional protocol before the procedure, and they were well documented in the HIS. Additionally, the sample size in this study was quite large among the studies regarding AIH patients, and we did not include healthy controls for the comparison. We studied only in the homogenous group of patients with autoimmune liver diseases, reflecting the real clinical practice encountered for the differentiation between cirrhotic and non-cirrhotic AIH in both treatment-naïve and treatment-experienced patients. And lastly, this is the first study to evaluate the NITs in patients with AIH-PBC overlap syndrome.
Nonetheless, we acknowledge some limitations of the present study. Firstly, although the sample size was quite large among studies in AIH patients, the total sample size of 139 patients may not be high enough to demonstrate the difference in diagnostic performances among RPR and other NITs, especially FIB-4 and AAR. Moreover, our study was conducted in a single-center university hospital; thus, this may limit the generalizability of our findings. Secondly, the design of the study is retrospective; thus, potentially unrecognized confounders interfering with the laboratory results, e.g., concomitant other drugs or herbal use or patients’ co-morbidity status, which could affect the NIT results, may exist. However, we had already excluded patients with hepatocellular carcinoma or other liver tumors and those with active infection, inflammation or hematologic diseases in which there could be a clinically significant alteration of the laboratory results used in the calculation of non-invasive scores. Lastly, we didn’t have available transient elastography data in all patients to compare or combine with serum biomarkers to improve validation. Anyway, one of our purposes is to seek out simple tools for cirrhosis evaluation that are practical to use even in resource-limited areas.
In conclusion, we have learned from our cohort that RPR yielded the highest diagnostic accuracy to predict the cirrhosis status in AIH and especially in the subgroup of AIH-PBC overlap syndrome patients compared with other available NITs, while FIB-4 was considerably optimal. High RPR (≥0.127) showed a high specificity of >90% in the ascertainment of cirrhosis in these patients. Nonetheless, further prospective multicenter studies are needed to validate the usefulness of RPR and to provide strong evidence-based data before endorsing it in routine clinical practice guidelines.
Author Contributions
Guarantor of the article: P.S.; P.S. and S.N. made a substantial contribution to the study’s concept and design. P.S. is responsible for data analysis, interpretation of the data, and critical revision of the manuscript. S.N. is responsible for data acquisition, interpretation and drafting the manuscript. J.T. is responsible for reviewing liver histopathology and data collection. S.A. also collected the data and drafted the manuscript. P.S., A.K. and N.C. are senior authors who responsible for study supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Faculty of Medicine, Prince of Songkla University (Funding number 64-068-1).
Institutional Review Board Statement
Approval of the research protocol: The Institutional Human Research Ethics Committee (HREC), Faculty of Medicine, Prince of Songkla University, Thailand (REC. 64-429-14-1), approval date: 27 September 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to personal health information privacy policy and ethical restrictions but deidentification data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AAR, aspartate aminotransferase to alanine aminotransferase ratio; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APRI, aspartate aminotransferase/platelet ratio index; AST, Aspartate aminotransferase; AUROCs, areas under receiver operating curve; BMI, body mass index; CBC; complete blood count; CTP, Child–Turcotte–Pugh; FIB-4, Fibrosis-4 index; HBV, hepatitis B virus; Hct, Hematocrit; HCV, hepatitis C virus, HREC, Human Research Ethics Committee; IQR; interquartile range; LPR, lymphocyte-to-platelet ratio, MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; PBC, primary biliary cholangitis; RPR, red cell distribution width to platelet ratio.
References
- Mack, C.L.; Adams, D.; Assis, D.N.; Kerkar, N.; Manns, M.P.; Mayo, M.J.; Vierling, J.M.; Alsawas, M.; Murad, M.H.; Czaja, A.J. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines from the American Association for the Study of Liver Diseases. Hepatology 2020, 72, 671–722. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019, 69, 394–419. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel; Berzigotti, A.; Tsochatzis, E., on behalf of the EASL Governing Board; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Maja Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Tanwandee, T.; Wanichapol, S.; Vejbaesya, S.; Chainuvati, S.; Chotiyaputta, W. Association between HLA class II alleles and autoimmune hepatitis type 1 in Thai patients. J. Med. Assoc. Thai. 2006, 89 (Suppl. S5), S73–S78. [Google Scholar] [PubMed]
- Llovet, L.P.; Gratacos-Gines, J.; Tellez, L.; Gomez-Outomuro, A.; Navascues, C.A.; Riveiro-Barciela, M.; Vinuesa, R.; Gomez-Camarero, J.; Garcia-Retortillo, M.; Diaz-Fontenla, F.; et al. Noninvasive Prediction of Outcomes in Autoimmune Hepatitis-Related Cirrhosis. Hepatol. Commun. 2022, 6, 1392–1402. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef]
- de Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Sripongpun, P.; Pongpaibul, A.; Charatcharoenwitthaya, P. Value and risk of percutaneous liver biopsy in patients with cirrhosis and clinical suspicion of autoimmune hepatitis. BMJ Open Gastroenterol. 2021, 8, e000701. [Google Scholar] [CrossRef]
- Dong, B.; Chen, Y.; Lyu, G.; Yang, X. Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis-4 Index for Detecting Liver Fibrosis in Patients with Autoimmune Hepatitis: A Meta-Analysis. Front. Immunol. 2022, 13, 892454. [Google Scholar] [CrossRef]
- Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Talwalkar, J.; Treeprasertsuk, S.; Neuhauser, M.; Lindor, K. Patients with typical laboratory features of autoimmune hepatitis rarely need a liver biopsy for diagnosis. Clin. Gastroenterol. Hepatol. 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Xia, J.; Yan, X.; Feng, Y.; Li, L.; Chen, J.; Liu, D.; Ding, W.; Yang, Y.; et al. Red cell distribution width to platelet ratio predicts liver fibrosis in patients with autoimmune hepatitis. Medicine 2020, 99, e21408. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Duan, S.Z.; Cao, J.; Gao, N.; Xu, J.; Zhang, L. Noninvasive inflammatory markers for assessing liver fibrosis stage in autoimmune hepatitis patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy. 2001, 102, 5–14. [Google Scholar]
- Casal Moura, M.; Liberal, R.; Cardoso, H.; Horta, E.V.A.M.; Macedo, G. Management of autoimmune hepatitis: Focus on pharmacologic treatments beyond corticosteroids. World J. Hepatol. 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Lee, Y.M.; Teo, E.K.; Ng, T.M.; Khor, C.; Fock, K.M. Autoimmune hepatitis in Singapore: A rare syndrome affecting middle-aged women. J. Gastroenterol. Hepatol. 2001, 16, 1384–1389. [Google Scholar] [CrossRef]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef]
- Huber, A.; Ebner, L.; Heverhagen, J.T.; Christe, A. State-of-the-art imaging of liver fibrosis and cirrhosis: A comprehensive review of current applications and future perspectives. Eur. J. Radiol. Open 2015, 2, 90–100. [Google Scholar] [CrossRef]
- Allan, R.; Thoirs, K.; Phillips, M. Accuracy of ultrasound to identify chronic liver disease. World J. Gastroenterol. 2010, 16, 3510–3520. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, L.; Hu, L.; Zhou, H.; Yu, L.; Liang, W. Transient Elastography (FibroScan) Performs Better Than Non-Invasive Markers in Assessing Liver Fibrosis and Cirrhosis in Autoimmune Hepatitis Patients. Med. Sci. Monit. 2017, 23, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, H.; Qu, L.; Wang, X.; Wu, R.; Gao, X.; Jin, Q.; Niu, J. Red blood cell distribution width and globulin, noninvasive indicators of fibrosis and inflammation in chronic hepatitis patients. Eur. J. Gastroenterol. Hepatol. 2016, 28, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, X.; Yang, Y.; Chang, H.; Jia, B.; Zhao, X.A.; Chen, G.; Xia, J.; Liu, Y.; Chen, Y.; et al. A novel predictive model using routinely clinical parameters to predict liver fibrosis in patients with chronic hepatitis B. Oncotarget 2017, 8, 59257–59267. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Yu, J.; Tan, L.; Wu, Y.; Tian, Y.; Wu, Q.; Duan, X.; Yu, L. Noninvasive indices for monitoring disease course in Chinese patients with autoimmune hepatitis. Clin. Chim. Acta 2018, 486, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Gao, P. Red Blood Cell Distribution Width-to-Platelet Ratio and Other Laboratory Indices Associated with Severity of Histological Hepatic Fibrosis in Patients with Autoimmune Hepatitis: A Retrospective Study at a Single Center. Med. Sci. Monit. 2020, 26, e927946. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, J.; Zhong, Z.; Guo, Z.; Jiang, Y.; Bai, Y.; Xu, J. Noninvasive indicators predict advanced liver fibrosis in autoimmune hepatitis patients. J. Clin. Lab. Anal. 2019, 33, e22922. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, Z.; Zhou, J.; Zeng, N.; He, Z.; Zhan, S.; Jia, J.; You, H. Systematic review: Diagnostic accuracy of non-invasive tests for staging liver fibrosis in autoimmune hepatitis. Hepatol. Int. 2019, 13, 91–101. [Google Scholar] [CrossRef]
- Liu, X.D.; Wu, J.L.; Liang, J.; Zhang, T.; Sheng, Q.S. Globulin-platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J. Gastroenterol. 2012, 18, 2784–2792. [Google Scholar] [CrossRef]
- Li, Q.; Lu, C.; Li, W.; Huang, Y.; Chen, L. Globulin-platelet model predicts significant fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated alanine transaminase levels. Clin. Exp. Med. 2018, 18, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Ye, B.; Lin, S.; Fu, Y.; Chen, Y.; Chen, Y. Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment. ASAIO J. 2010, 56, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Xu, H.; Qu, L.; Zhang, D.; Gao, P. Platelet count to spleen thickness ratio is related to histologic severity of primary biliary cholangitis. Medicine 2018, 97, e9843. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; He, Q.; Qin, X.; Li, S.; Li, T.; Xie, L.; Deng, Y.; He, Y.; Chen, Y.; Wei, Z. The Relationship between Inflammatory Marker Levels and Hepatitis C Virus Severity. Gastroenterol. Res. Pract. 2016, 2016, 2978479. [Google Scholar] [CrossRef]
- Meng, X.; Wei, G.; Chang, Q.; Peng, R.; Shi, G.; Zheng, P.; He, F.; Wang, W.; Ming, L. The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int. J. Infect. Dis. 2016, 45, 72–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).