Oscillometry Assesses Small Airway Disease and Reveals Peripheral Lung Pathology in Early Pulmonary Fibrosis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
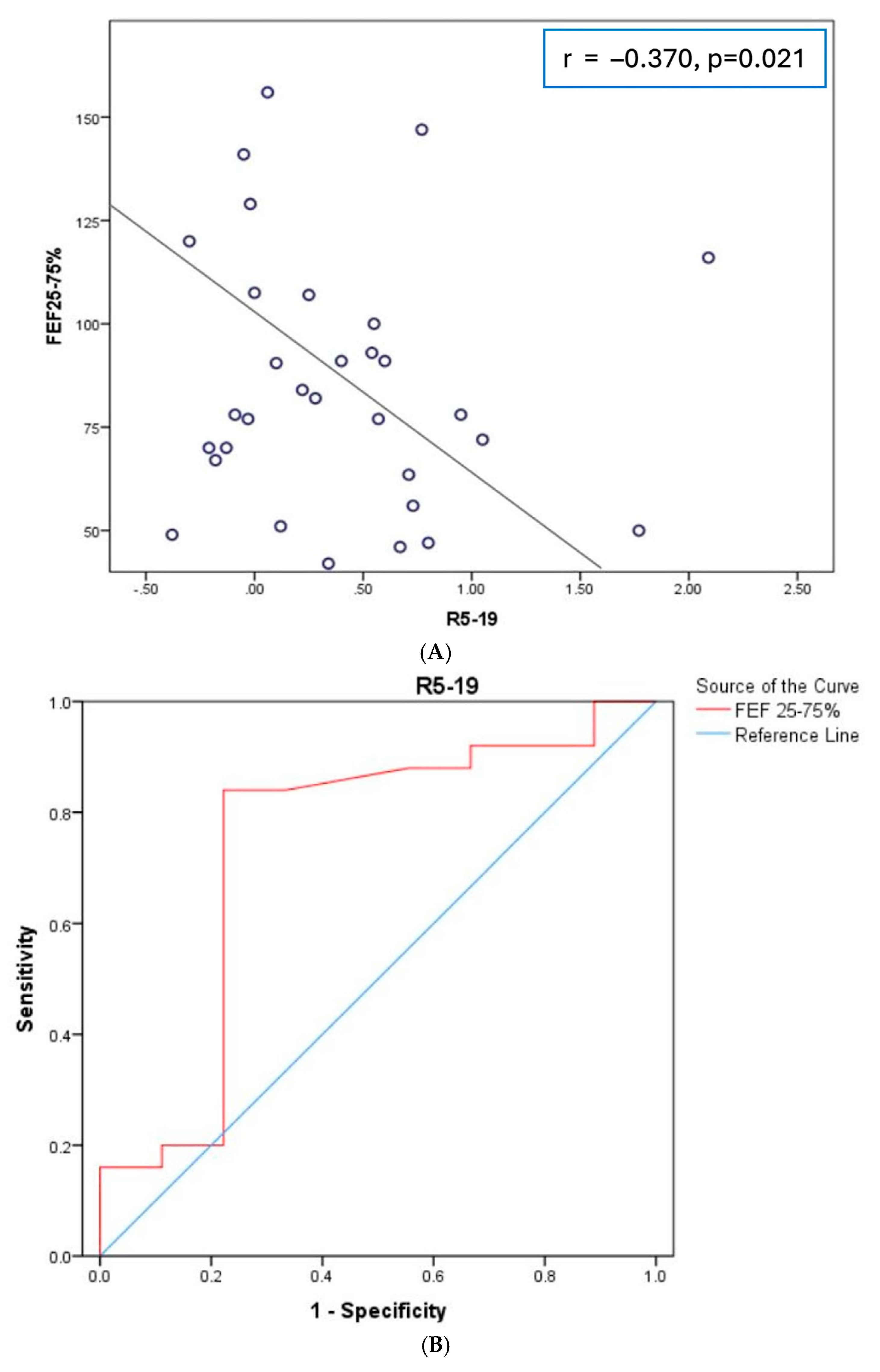
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, R.J.; Djukanovic, R.; Tashkin, D.P.; Millar, A.B.; Du Bois, R.M.; Corris, P.A. The role of small airways in lung disease. Respir. Med. 2002, 96, 67–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicholson, A.G.; Fulford, L.G.; Colby, T.V.; du Bois, R.M.; Hansell, D.M.; Wells, A.U. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2002, 166, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, K.; Hackett, T.L.; Peterson, S.; Prins, D.; Hague, C.J.; Murphy, D.; LeDoux, S.; Chu, F.; Xu, F.; Cooper, J.D.; et al. Small Airway Reduction and Fibrosis Is an Early Pathologic Feature of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 1048–1059. [Google Scholar] [CrossRef]
- Yin, C.; Xie, H.; He, X.; Zhang, Y.; Zhang, A.; Li, H. Small airway dysfunction in idiopathic pulmonary fibrosis. Front. Pharmacol. 2022, 13, 1025814. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, B.; Ban, C.; Ren, Y.; Ye, Q.; Zhu, M.; Liu, Y.; Zhang, S.; Geng, J.; Jiang, D.; et al. Small airway dysfunction in Chinese patients with idiopathic pulmonary fibrosis. BMC Pulm. Med. 2022, 22, 297. [Google Scholar] [CrossRef]
- Kaminsky, D.A.; Simpson, S.J.; Berger, K.I.; Calverley, P.; de Melo, P.L.; Dandurand, R.; Dellacà, R.L.; Farah, C.S.; Farré, R.; Hall, G.L.; et al. Clinical significance and applications of oscillometry. Eur. Respir. Rev. 2022, 31, 210208. [Google Scholar] [CrossRef]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; De Melo, P.L.; Dellacà, R.L.; Farre, R.; Hall, G.; Ioan, I.; Irvin, C.G.; et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef] [PubMed]
- Goh, N.S.L.; Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Copley, S.J.; Maher, T.M.; Corte, T.J.; Sander, C.R.; Ratoff, J.; Devaraj, A.; et al. Interstitial lung disease in systemic sclerosis. Am. J. Respir. Crit. Care Med. 2008, 177, 1248–1254. [Google Scholar] [CrossRef]
- Wells, A.U.; Desai, S.R.; Rubens, M.B.; Goh, N.S.; Cramer, D.; Nicholson, A.G.; Colby, T.V.; Du Bois, R.M.; Hansell, D.M. Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003, 167, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic pulmonary fibrosis (an update) and Progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Pisi, R.; Aiello, M.; Vigna, M.; Tzani, P.; Torres, A.; Bertorelli, G.; Chetta, A. Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration 2017, 93, 32–41. [Google Scholar] [CrossRef]
- Verleden, S.E.; Tanabe, N.; McDonough, J.E.; Vasilescu, D.M.; Xu, F.; Wuyts, W.A.; Piloni, D.; De Sadeleer, L.; Willems, S.; Mai, C.; et al. Small airways pathology in idiopathic pulmonary fibrosis: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 573–584. [Google Scholar] [CrossRef]
- Figueira de Mello, G.C.; Ribeiro Carvalho, C.R.; Adib Kairalla, R.; Saldiva, P.H.N.; Fernezlian, S.; Silva, L.F.F.; Dolhnikoff, M.; Mauad, T. Small airway remodeling in idiopathic interstitial pneumonias: A pathological study. Respiration 2010, 79, 322–332. [Google Scholar] [CrossRef]
- Katsoulis, K.; Kostikas, K.; Kontakiotis, K. Techniques for assessing small airways function: Possible applications in asthma and COPD. Respir. Med. 2016, 119, e2–e9. [Google Scholar] [CrossRef]
- Galant, S.P.; Komarow, H.D.; Shin, H.W. The case for impulse oscillometry in the management of asthma in children and adults Ann Allergy Asthma Immunol. Ann. Allergy Asthma Immunol. Ann. Allergy Asthma Immunol. 2017, 118, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, B.W.; Goldring, R.M.; Herberg, M.E.; Hofer, I.S.; Reyfman, P.A.; Liautaud, S.; Rom, W.N.; Reibman, J.; Berger, K.I. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007, 132, 1275–1282. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Hsiao, Y.-H.; Su, K.-C.; Lee, Y.-C.; Ko, H.-K.; Perng, D.-W. Small airway dysfunction by impulse oscillometry in symptomatic patients with preserved pulmonary function. J. Allergy Clin. Immunol. Pract. 2020, 8, 229–235.e3. [Google Scholar] [CrossRef]
- Chan, R.; Lipworth, B. Interactions between spirometry and oscillometry in patients with moderate to severe asthma. Eur. Respir. J. 2022, 60, 2200543. [Google Scholar] [CrossRef]
- Horita, N.; Yamamoto, S.; Mizuki, Y.; Kawagoe, T.; Mihara, T.; Yamashiro, T. Minimal clinically important difference (MCID) of effect sizes other than mean difference. J. Clin. Quest. 2024. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Miki, K.; Tsujino, K.; Kuge, T.; Okabe, F.; Kawasaki, T.; Matsuki, T.; Kagawa, H.; Miki, M.; Kida, H. Oscillometry and computed tomography findings in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2020, 6, 00391–02020. [Google Scholar] [CrossRef] [PubMed]
- Mikamo, M.; Fujisawa, T.; Oyama, Y.; Kono, M.; Enomoto, N.; Nakamura, Y.; Inui, N.; Sumikawa, H.; Johkoh, T.; Suda, T. Clinical significance of forced oscillation technique for evaluation of small airway disease in interstitial lung diseases. Lung 2016, 194, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Barkas, G.I.; Daniil, Z.; Kotsiou, O.S. The role of small airway disease in pulmonary fibrotic diseases. J. Pers. Med. 2023, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M. Small airways in idiopathic pulmonary fibrosis: Quiet but not forgotten. Am. J. Respir. Crit. Care Med. 2021, 204, 1010–1011. [Google Scholar] [CrossRef] [PubMed]

| R5 Act. | X5 Act. | Fres Act. | AX act. | R5-19 Act. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | r | p | r | p | r | p | r | p | r | p |
| FEV1, % pred. | −0.290 | 0.096 | 0.374 | 0.064 | −0.396 | 0.020 | −0.364 | 0.034 | −0.240 | 0.172 |
| FEV1, L | −0.601 | <0.001 | 0.666 | 0.224 | −0.552 | 0.001 | −0.500 | 0.003 | −0.483 | 0.004 |
| FVC, % pred. | −0.287 | 0.099 | 0.397 | 0.047 | −0.454 | 0.007 | −0.515 | 0.002 | −0.254 | 0.148 |
| FVC, L | −0.588 | <0.001 | 0.671 | <0.001 | −0.562 | 0.001 | −0.515 | 0.002 | −0.481 | 0.004 |
| FEV1/FVC, % | 0.218 | 0.216 | −0.289 | 0.549 | 0.243 | 0.166 | 0.245 | 0.163 | 0.174 | 0.324 |
| FEF25-75, % pred. | −0.366 | 0.033 | 0.337 | 0.736 | −0.228 | 0.195 | −0.166 | 0.347 | −0.370 | 0.021 |
| DLCO, % pred. | −0.134 | 0.458 | 0.208 | 0.405 | −0.251 | 0.159 | −0.184 | 0.304 | −0.234 | 0.190 |
| DLCO/VA, % pred. | 0.084 | 0.680 | 0.069 | 0.767 | 0.049 | 0.785 | 0.081 | 0.654 | −0.085 | 0.639 |
| RV/TLC, % pred. | 0.074 | 0.680 | −0.106 | 0.750 | 0.036 | 0.842 | 0.081 | 0.653 | −0.017 | 0.924 |
| RV/TLC, % | 0.076 | 0.690 | −0.145 | 0.973 | 0.238 | 0.205 | 0.159 | 0.401 | 0.131 | 0.468 |
| TLC, % pred. | −0.324 | 0.066 | 0.448 | 0.100 | −0.479 | 0.005 | −0.405 | 0.019 | −0.311 | 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogali, A.; Gkrepi, G.; Kyriakopoulos, C.; Tatsis, K.; Katsoulis, K.; Tselepi, C.; Kostikas, K. Oscillometry Assesses Small Airway Disease and Reveals Peripheral Lung Pathology in Early Pulmonary Fibrosis: A Cross-Sectional Study. Diagnostics 2024, 14, 2873. https://doi.org/10.3390/diagnostics14242873
Gogali A, Gkrepi G, Kyriakopoulos C, Tatsis K, Katsoulis K, Tselepi C, Kostikas K. Oscillometry Assesses Small Airway Disease and Reveals Peripheral Lung Pathology in Early Pulmonary Fibrosis: A Cross-Sectional Study. Diagnostics. 2024; 14(24):2873. https://doi.org/10.3390/diagnostics14242873
Chicago/Turabian StyleGogali, Athena, Georgia Gkrepi, Christos Kyriakopoulos, Konstantinos Tatsis, Konstantinos Katsoulis, Chara Tselepi, and Konstantinos Kostikas. 2024. "Oscillometry Assesses Small Airway Disease and Reveals Peripheral Lung Pathology in Early Pulmonary Fibrosis: A Cross-Sectional Study" Diagnostics 14, no. 24: 2873. https://doi.org/10.3390/diagnostics14242873
APA StyleGogali, A., Gkrepi, G., Kyriakopoulos, C., Tatsis, K., Katsoulis, K., Tselepi, C., & Kostikas, K. (2024). Oscillometry Assesses Small Airway Disease and Reveals Peripheral Lung Pathology in Early Pulmonary Fibrosis: A Cross-Sectional Study. Diagnostics, 14(24), 2873. https://doi.org/10.3390/diagnostics14242873







