Biomarker Insights: Evaluation of Presepsin, Apelin, and Irisin Levels in Cutaneous Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Serum Presepsin
2.2. Measurement of Serum Apelin
2.3. Measurement of Serum Irisin
2.4. Statistical Analysis
3. Results
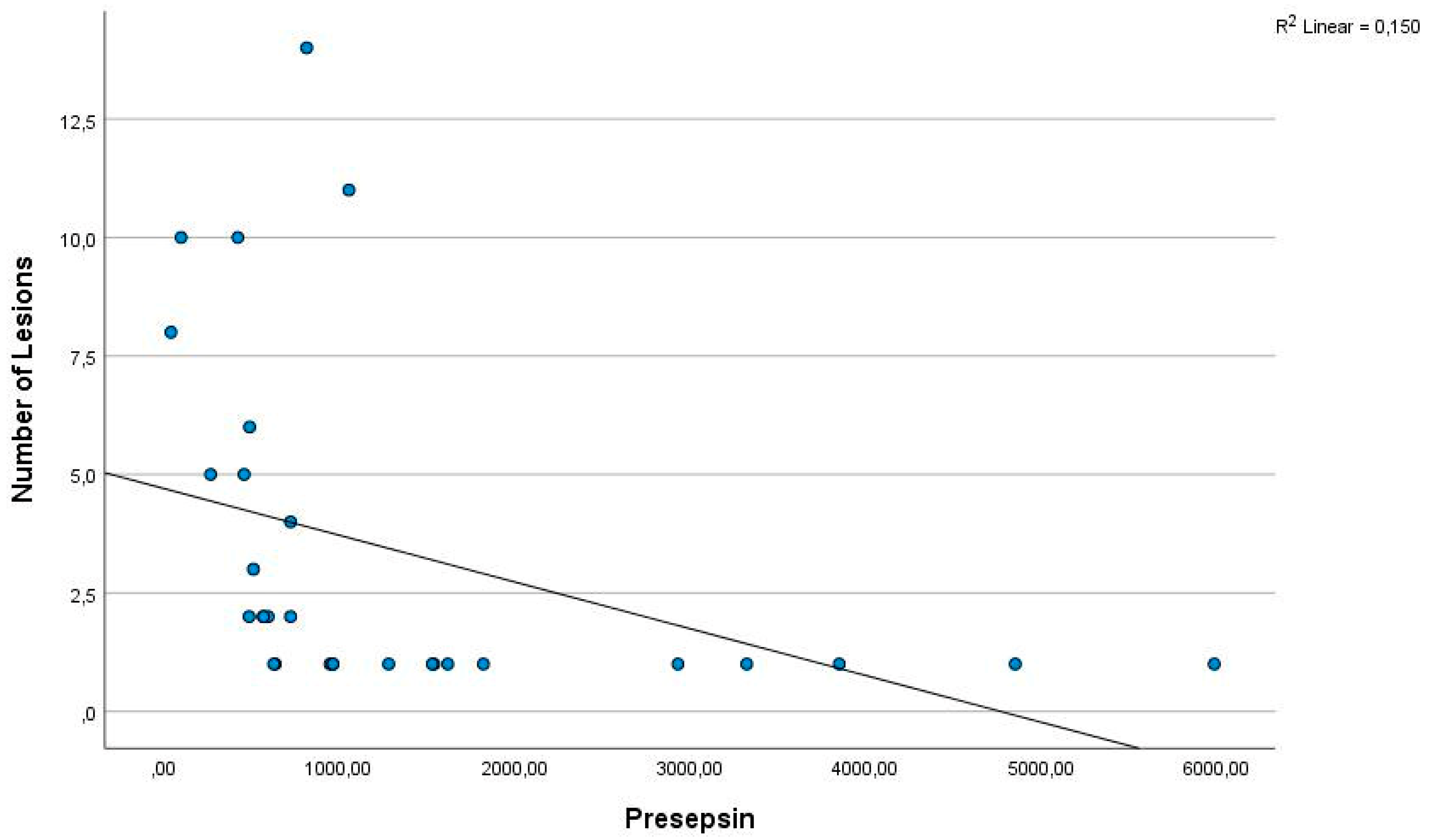
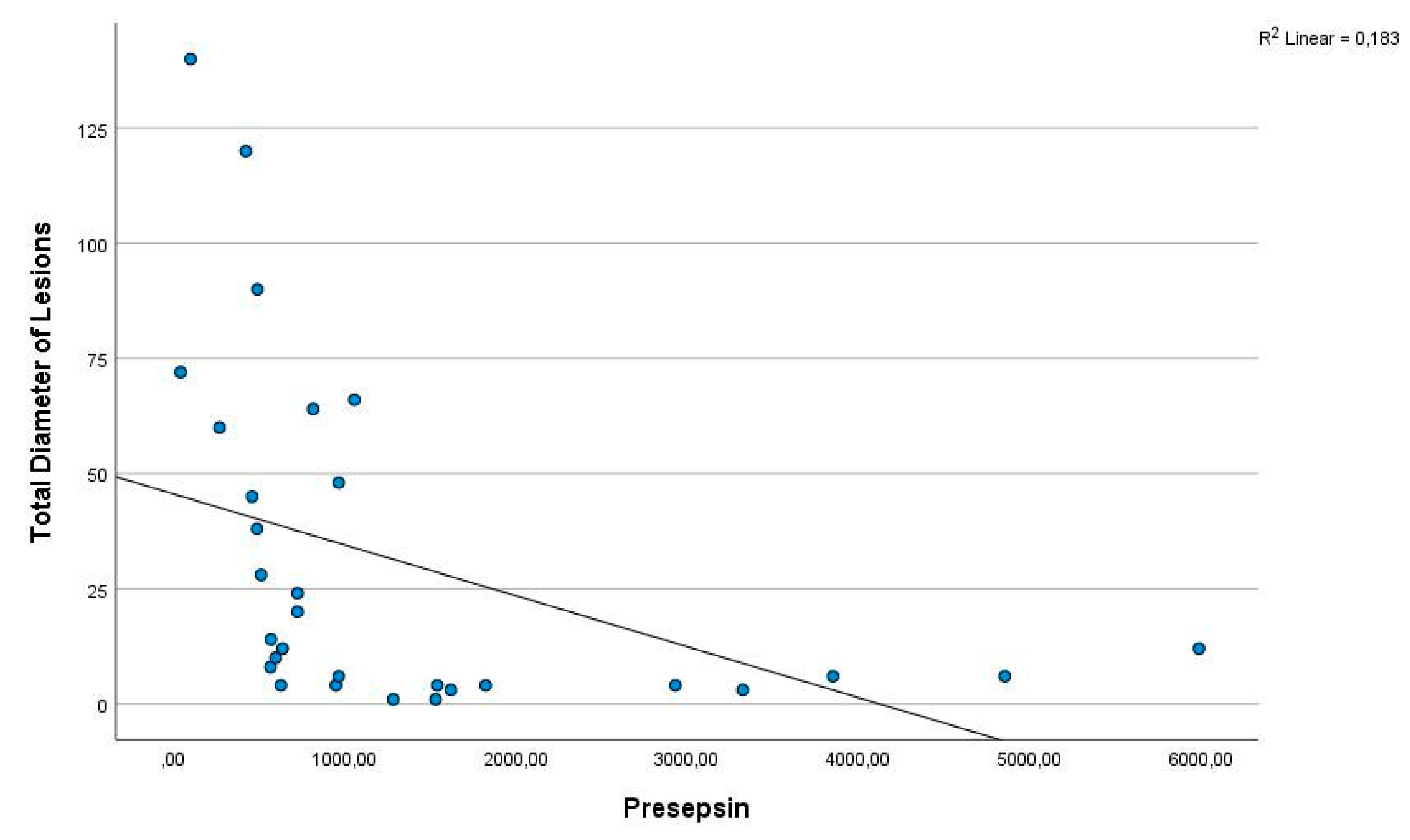
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sula, B.; Tekin, R. Use of hematological parameters in evaluation of treatment efficacy in cutaneous leishmaniasis. J. Microbil. Infect. Dis. 2016, 5, 167–172. [Google Scholar] [CrossRef][Green Version]
- Piyasiri, S.B.; Dewasurendra, R.; Samaranayake, N.; Karunaweera, N. Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review. Diagnostics 2023, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Sula, B.; Tekin, R.; Yolbaş, I.; Aktar, F.; Ucak, H. Ratio of Neutrophil/Lymphocyte and Platelet/Lymphocyte in Paediatric Patient with Cutaneous Leishmaniasis. West. Indian. Med. J. 2015; Epub ahead of print. [Google Scholar] [CrossRef][Green Version]
- Kondo, Y.; Umemura, Y.; Hayashida, K.; Hara, Y.; Aihara, M.; Yamakawa, K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: A systematic review and meta-analysis. J. Intensive Care 2019, 7, 22. [Google Scholar] [CrossRef]
- Formenti, P.; Gotti, M.; Palmieri, F.; Pastori, S.; Roccaforte, V.; Menozzi, A.; Galimberti, A.; Umbrello, M.; Sabbatini, G.; Pezzi, A. Presepsin in Critical Illness: Current Knowledge and Future Perspectives. Diagnostics 2024, 14, 1311. [Google Scholar] [CrossRef]
- Canpolat Erkan, R.E.; Tekin, R. Investigation of new inflammatory biomarkers in patients with brucella. PLoS ONE 2024, 19, e0297550. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef]
- Hadifar, S.; Masoudzadeh, N.; Heydari, H.; Mashayekhi Goyonlo, V.; Kerachian, M.; Daneshpazhooh, M.; Sadeghnia, A.; Tootoonchi, N.; Erfanian Salim, R.; Rafati, S.; et al. Intralesional gene expression profile of JAK-STAT signaling pathway and associated cytokines in Leishmania tropica-infected patients. Front. Immunol. 2024, 15, 1436029. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Alkhaldi, A.A.M.; Saleh, A.A. Host immune response against leishmaniasis and parasite persistence strategies: A review and assessment of recent research. Biomed. Pharmacother. 2021, 139, 111671. [Google Scholar] [CrossRef]
- Bahrami, F.; Harandi, A.M.; Rafati, S. Biomarkers of Cutaneous Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 222. [Google Scholar] [CrossRef]
- Khandibharad, S.; Nimsarkar, P.; Singh, S. Mechanobiology of immune cells: Messengers, receivers and followers in leishmaniasis aiding synthetic devices. Curr. Res. Immunol. 2022, 3, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Saidi, N.; Blaizot, R.; Prévot, G.; Aoun, K.; Demar, M.; Cazenave, P.A.; Bouratbine, A.; Pied, S. Clinical and immunological spectra of human cutaneous leishmaniasis in North Africa and French Guiana. Front. Immunol. 2023, 14, 1134020. [Google Scholar] [CrossRef] [PubMed]
- Divenuto, F.; Pavia, G.; Marascio, N.; Barreca, G.S.; Quirino, A.; Matera, G. Role of Treg, Breg and other cytokine sets in host protection and immunopathology during human leishmaniasis: Are they potential valuable markers in clinical settings and vaccine evaluation? Acta Trop. 2023, 240, 106849. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Gur, S.; Erel, O.; Gurel, M.S. Associations among plasma selenium, zinc, copper, and iron concentrations and immunoregulatory cytokine levels in patients with cutaneous leishmaniasis. Biol. Trace Elem. Res. 2002, 90, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Divenuto, F.; Marascio, N.; Quirino, A.; Giancotti, A.; Filice, S.; Gigliotti, S.; Campolo, M.P.; Campolo, M.; Barreca, G.S.; Lamberti, A.G.; et al. Cellular mediators in human leishmaniasis: Critical determinants in parasite killing or disease progression. Acta Trop. 2023, 248, 107037. [Google Scholar] [CrossRef]
- Chenevier-Gobeaux, C.; Borderie, D.; Weiss, N.; Mallet-Coste, T.; Claessens, Y.E. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin. Chim. Acta 2015, 450, 97–103. [Google Scholar] [CrossRef]
- Song, Q.; Wang, X.; Cao, Z.; Xin, C.; Zhang, J.; Li, S. The Apelin/APJ System: A Potential Therapeutic Target for Sepsis. J. Inflamm. Res. 2024, 17, 313–330. [Google Scholar] [CrossRef]
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: A multicenter prospective study. Crit. Care 2013, 17, R168. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgrò, S.; Fanizza, C.; et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial. Crit. Care 2014, 18, R6. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.D.; Song, J.; Shao, J. Diagnostic Value of Presepsin for Sepsis: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e2158. [Google Scholar] [CrossRef]
- Shiota, J.; Tagawa, H.; Ohura, N.; Kasahara, H. Presepsin is a potent biomarker for diagnosing skin wound infection in hemodialysis patients compared to white blood cell count, high-sensitivity C-reactive protein, procalcitonin, and soluble CD14. Ren. Replace. Ther. 2017, 3, 31. [Google Scholar] [CrossRef][Green Version]
- Ha, E.Y.; Park, I.R.; Chung, S.M.; Roh, Y.N.; Park, C.H.; Kim, T.G.; Kim, W.; Moon, J.S. The Potential Role of Presepsin in Predicting Severe Infection in Patients with Diabetic Foot Ulcers. J. Clin. Med. 2024, 13, 2311. [Google Scholar] [CrossRef] [PubMed]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin reduces inflammatory signaling pathways in inflammation-mediated metabolic syndrome. Mol. Cell. Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef] [PubMed]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, F.; Sanesi, L.; Oranger, A.; Barlusconi, C.; Dicarlo, M.; Pignataro, P.; Zerlotin, R.; Romita, P.; Favoino, E.; Cazzato, G.; et al. Circulating Irisin Levels in Patients with Chronic Plaque Psoriasis. Biomolecules 2022, 12, 1096. [Google Scholar] [CrossRef]
- Tang, L.; Yu, B.; Liao, Y.; Long, S.; Yan, H.; He, Q.; Li, C. Serum Irisin: A Potential Diagnostic Marker for Insulin Resistance in Acne Vulgaris. Indian. J. Dermatol. 2022, 67, 477. [Google Scholar] [CrossRef]
- Mantaka AKalyvianaki, K.; Kastritsi, O.; Kampa, M.; Koutroubakis, I.E. Increased Serum Apelin Levels in Patients with Inflammatory Bowel Disease. Gastroenterol. Insights 2024, 15, 255–265. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sekiguchi, A.; Fujiwara, C.; Uchiyama, A.; Uehara, A.; Ogino, S.; Torii, R.; Ishikawa, O.; Motegi, S.I. Inhibitory Regulation of Skin Fibrosis in Systemic Sclerosis by Apelin/APJ Signaling. Arthritis Rheumatol. 2018, 70, 1661–1672. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, S. Apelin/APJ system in inflammation. Int. Immunopharmacol. 2022, 109, 108822. [Google Scholar]
- Sandal, S.; Tekin, S. Adipoz Dokudan Salgılanan Bir Hormon: Apelin. Ann. Health Sci. Res. 2013, 2, 55–62. [Google Scholar]
- Yamazaki, S.; Sekiguchi, A.; Uchiyama, A.; Fujiwara, C.; Inoue, Y.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; Akai, R.; et al. Apelin/APJ signaling suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model. Sci. Rep. 2020, 10, 1349. [Google Scholar] [CrossRef]
| Total n = 30 (%) | |
|---|---|
| Age (year) | |
| 46.1 ± 17.2 | |
| Gender | |
| Female | 14 (43) |
| Male | 16 (57) |
| Time of Lesion (month) | |
| <6 months | 22 (73) |
| 6–12 months | 6 (20) |
| >12 months | 2 (7) |
| Location of Lesion | |
| Head and neck | 17 (57) |
| Multiple location | 7 (23) |
| Lower limbs | 4 (14) |
| Upper limbs | 2 (7) |
| Number of Lesions | |
| Single | 15 (50) |
| Two | 5 (17) |
| Multiple >3 | 10 (33) |
| Size of Lesion | |
| <15 mm | 17 (57) |
| 15–30 mm | 3 (10) |
| 30–45 mm | 2 (7) |
| >45 mm | 8 (26) |
| Type of Lesion | |
| Dry | 18 (60) |
| Wet | 12 (40) |
| Cutaneous Leishmania Patients (n = 30) | Controls (n = 30) | p | |
|---|---|---|---|
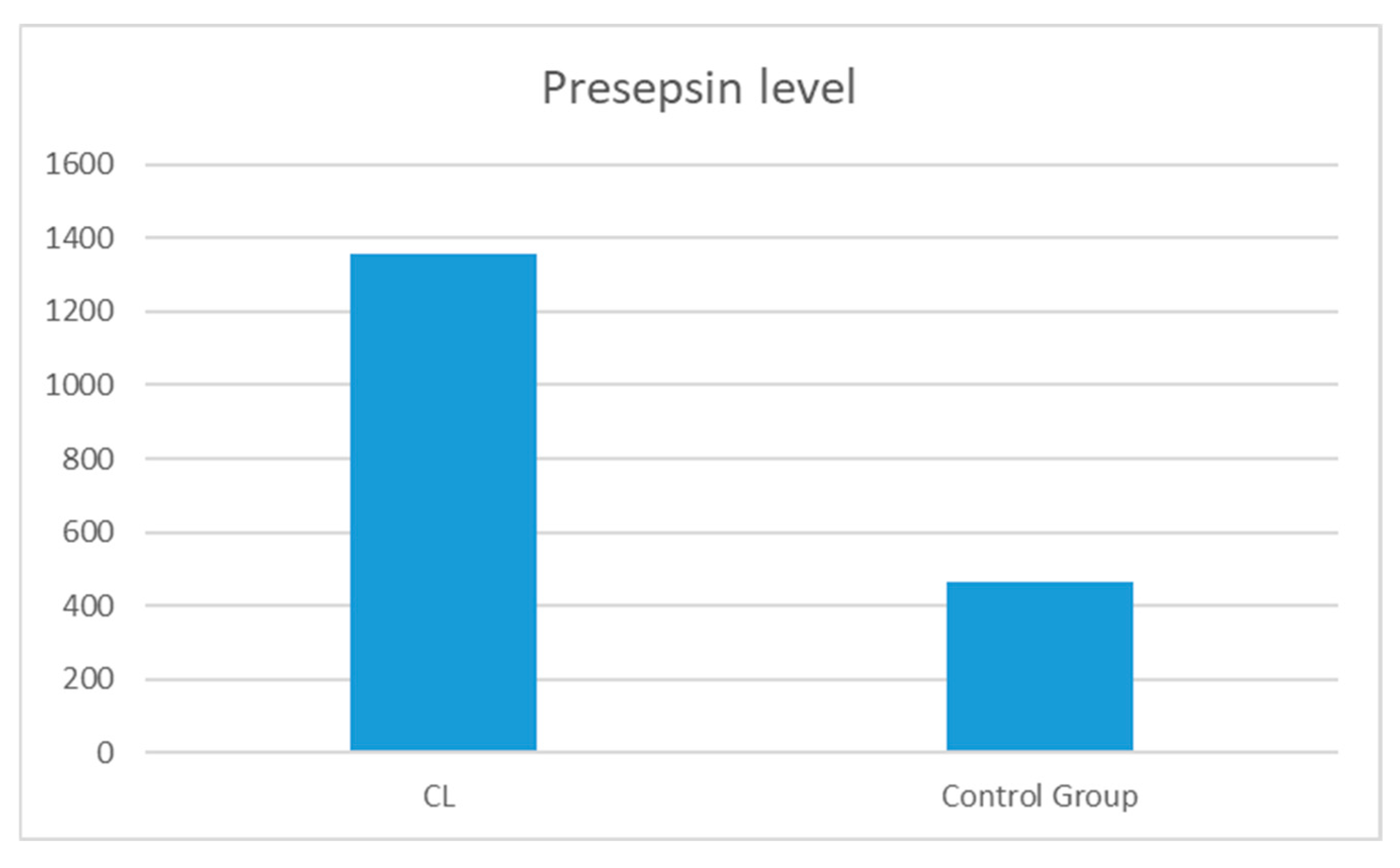
| Presepsin (pg/mL) | 1358.12 ± 1431.7 | 466.6 ± 531.1 | 0.000 |
| Irisin (ng/mL) | 7.1 ± 9.1 | 5.5 ± 2.6 | 0.096 |
| Apelin (ng/mL) | 18.2 ± 2.65 | 17.8 ± 9.8 | 0.836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canpolat-Erkan, R.E.; Tekin, R.; Sula, B. Biomarker Insights: Evaluation of Presepsin, Apelin, and Irisin Levels in Cutaneous Leishmaniasis. Diagnostics 2024, 14, 2869. https://doi.org/10.3390/diagnostics14242869
Canpolat-Erkan RE, Tekin R, Sula B. Biomarker Insights: Evaluation of Presepsin, Apelin, and Irisin Levels in Cutaneous Leishmaniasis. Diagnostics. 2024; 14(24):2869. https://doi.org/10.3390/diagnostics14242869
Chicago/Turabian StyleCanpolat-Erkan, Revsa Evin, Recep Tekin, and Bilal Sula. 2024. "Biomarker Insights: Evaluation of Presepsin, Apelin, and Irisin Levels in Cutaneous Leishmaniasis" Diagnostics 14, no. 24: 2869. https://doi.org/10.3390/diagnostics14242869
APA StyleCanpolat-Erkan, R. E., Tekin, R., & Sula, B. (2024). Biomarker Insights: Evaluation of Presepsin, Apelin, and Irisin Levels in Cutaneous Leishmaniasis. Diagnostics, 14(24), 2869. https://doi.org/10.3390/diagnostics14242869






