It Looks Like a Zebra but Is Not: [18F]FDG PET/CT in a Giant Cutaneous Malignant Melanoma Mimicking Squamous Cell Carcinoma
Abstract
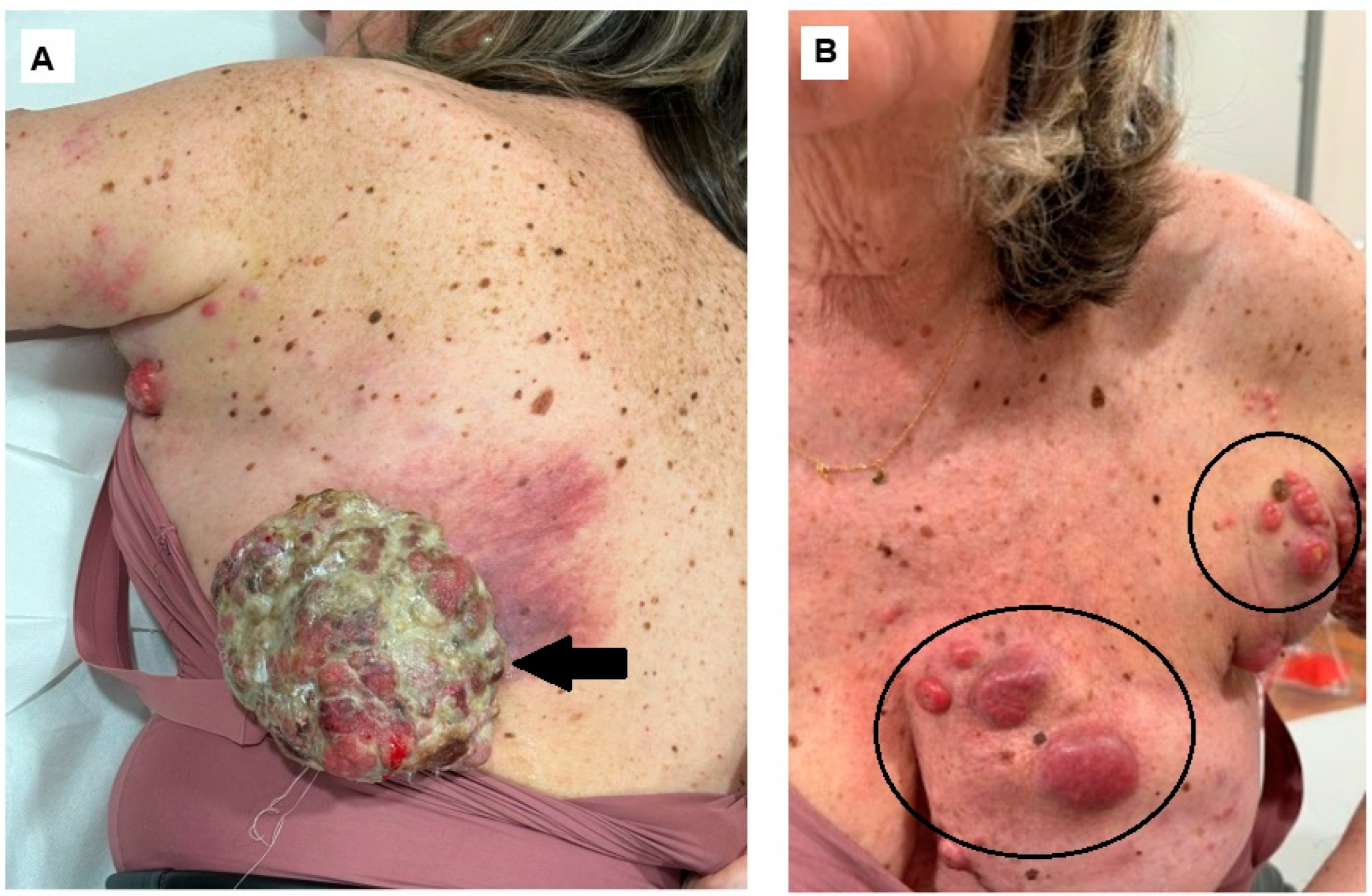

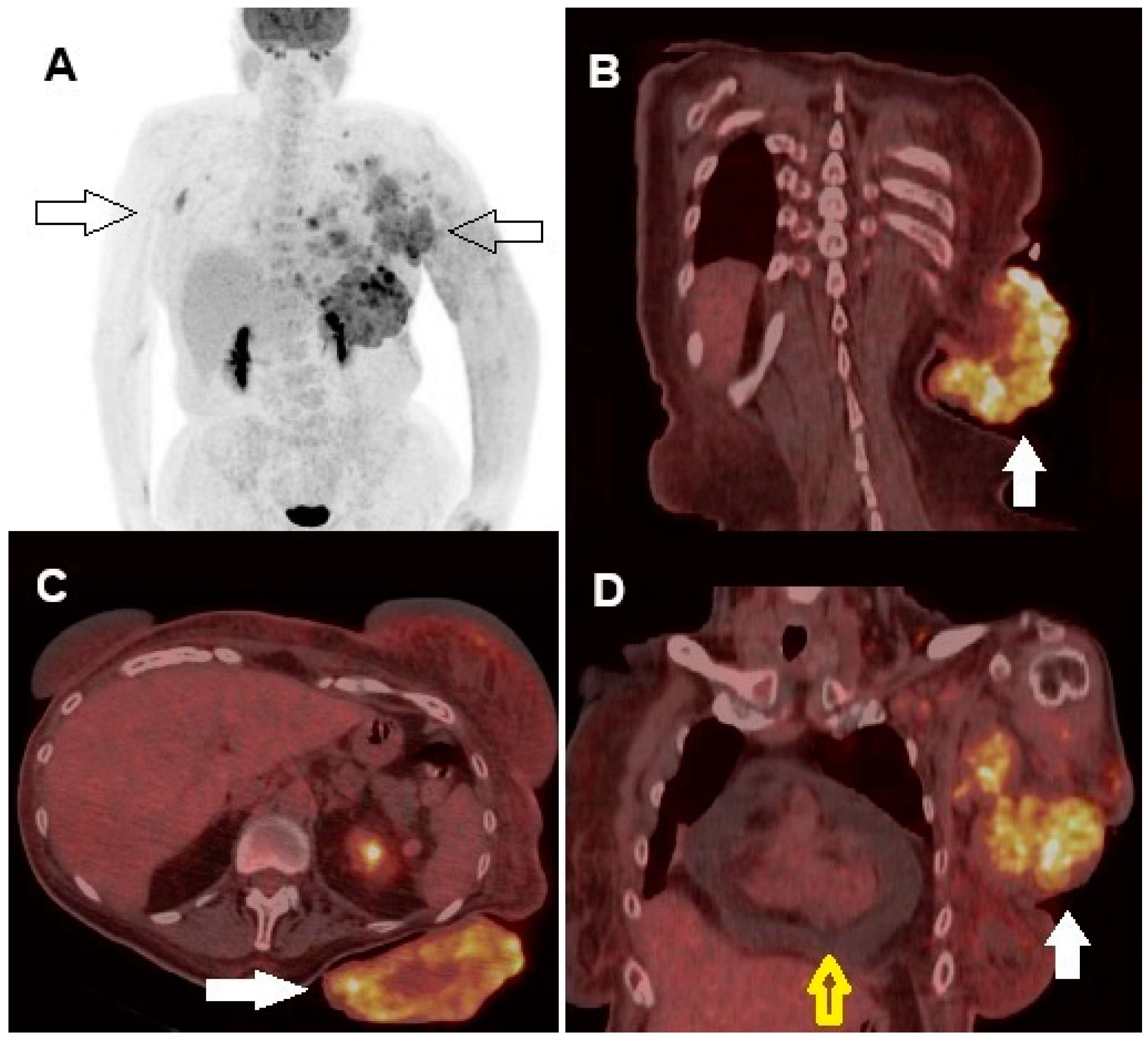
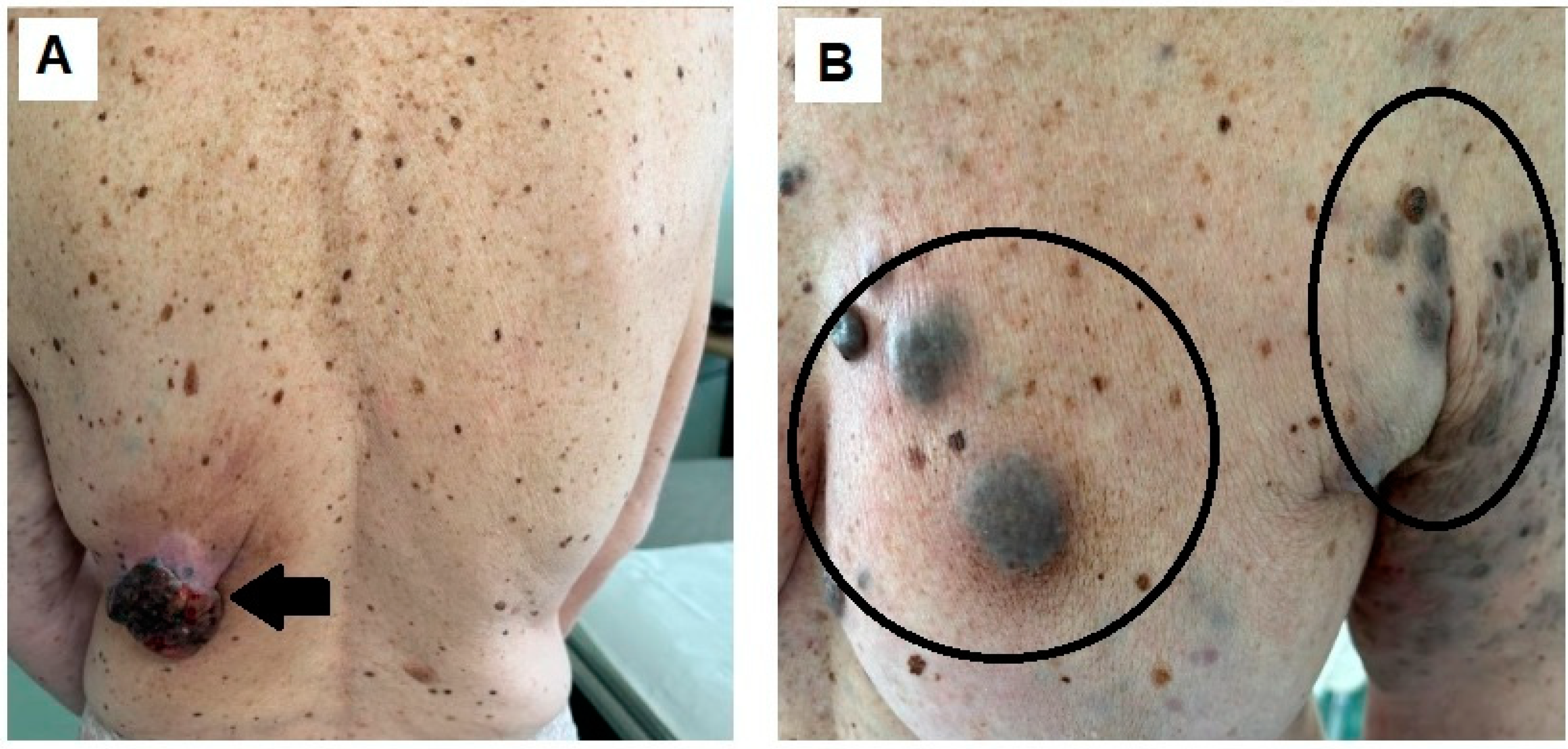

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nielsen, J.B.; Kristiansen, I.S.; Thapa, S. Increasing Melanoma Incidence with Unchanged Mortality: More Sunshine, Better Treatment, Increased Diagnostic Activity, Overdiagnosis or Lowered Diagnostic Threshold? Br. J. Dermatol. 2024, 191, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Julião, M.J.; Tellechea, O. Acantholytic-Like Pattern in a Recurrent Melanoma Mimicking Acantholytic Squamous Cell Carcinoma: A Further Example Highlighting the Potential for Misdiagnosis. Am. J. Dermatopathol. 2016, 38, 324–326. [Google Scholar] [CrossRef]
- Haruna, K.; Suga, Y.; Mizuno, Y.; Ikeda, S. Malignant Melanoma with a Seborrheic Keratosis-like Clinical Presentation. Indian J. Dermatol. 2009, 54, 387. [Google Scholar] [CrossRef] [PubMed]
- Moshe, M.; Levi, A.; Ad-El, D.; Ben-Amitai, D.; Mimouni, D.; Didkovsky, E.; Feinmesser, M.; Lapidoth, M. Malignant Melanoma Clinically Mimicking Pyogenic Granuloma: Comparison of Clinical Evaluation and Histopathology. Melanoma Res. 2018, 28, 363–367. [Google Scholar] [CrossRef]
- Broggi, G.; Russo, A.; Reibaldi, M.; Russo, D.; Varricchio, S.; Bonfiglio, V.; Spatola, C.; Barbagallo, C.; Foti, P.V.; Avitabile, T.; et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Appl. Sci. 2020, 22, 8081. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Hsieh, M.; Hsu, S.-K.; Liu, T.-Y.; Wu, C.-Y.; Chiu, C.-C. Melanoma Biology and Treatment: A Review of Novel Regulated Cell Death-Based Approaches. Cancer Cell Int. 2024, 24, 63. [Google Scholar] [CrossRef]
- Myers, D.J.; Hyde, E.A. Aggressive Nodular Malignant Melanoma. Cureus 2021, 13, e16819. [Google Scholar] [CrossRef]
- Cappilli, S.; Paradisi, A.; Di Stefani, A.; Palmisano, G.; Pellegrino, L.; D’Onghia, M.; Ricci, C.; Tognetti, L.; Verzì, A.E.; Rubegni, P.; et al. Line-Field Confocal Optical Coherence Tomography: A New Skin Imaging Technique Reproducing a “Virtual Biopsy” with Evolving Clinical Applications in Dermatology. Diagnostics 2024, 14, 1821. [Google Scholar] [CrossRef]
- Chong, T.A.; Strazzula, L.; Hoang, M.P.; Arakaki, R.; Kroshinsky, D. Giant Primary Melanoma With No Apparent Metastases: A Report of 2 Cases. JAMA Dermatol. 2014, 150, 574. [Google Scholar] [CrossRef] [PubMed]
- Kruijff, S.; Vink, R.; Klaase, J. Salvage Surgery for a Giant Melanoma on the Back. Rare Tumors 2011, 3, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, M.L.; Melero, I.; Ascierto, P.A. Melanoma: From Incurable Beast to a Curable Bet. The Success of Immunotherapy. Front. Oncol. 2015, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Agarwala, S.S.; Messersmith, H.; Alluri, K.C.; Ascierto, P.A.; Atkins, M.B.; Bollin, K.; Chacon, M.; Davis, N.; Faries, M.B.; et al. Systemic Therapy for Melanoma: ASCO Guideline Update. JCO 2023, 41, 4794–4820. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Eggermont, A.M.M. Neoadjuvant Therapy in Melanoma: The next Step? Lancet Oncol. 2018, 19, 151–153. [Google Scholar] [CrossRef]
- Amaria, R.N.; Prieto, P.A.; Tetzlaff, M.T.; Reuben, A.; Andrews, M.C.; Ross, M.I.; Glitza, I.C.; Cormier, J.; Hwu, W.-J.; Tawbi, H.A.; et al. Neoadjuvant plus Adjuvant Dabrafenib and Trametinib versus Standard of Care in Patients with High-Risk, Surgically Resectable Melanoma: A Single-Centre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2018, 19, 181–193. [Google Scholar] [CrossRef]
- Petitprez, F.; Meylan, M.; de Reyniès, A.; Sautès-Fridman, C.; Fridman, W.H. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front. Immunol. 2020, 11, 784. [Google Scholar] [CrossRef]
- Bisschop, C.; de Heer, E.C.; Brouwers, A.H.; Hospers, G.A.P.; Jalving, M. Rational use of 18F-FDG PET/CT in patients with advanced cutaneous melanoma: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 153, 103044. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A. Monitoring of Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibitors Using PET–CT. Cancer Immunol. Immunother. 2019, 68, 813–822. [Google Scholar] [CrossRef]
- Filippi, L.; Bianconi, F.; Schillaci, O.; Spanu, A.; Palumbo, B. The Role and Potential of 18F-FDG PET/CT in Malignant Melanoma: Prognostication, Monitoring Response to Targeted and Immunotherapy, and Radiomics. Diagnostics 2022, 12, 929. [Google Scholar] [CrossRef]
- Martens, R.M.; Koopman, T.; Lavini, C.; Brug, T.V.D.; Zwezerijnen, G.J.C.; Marcus, J.T.; Vergeer, M.R.; Leemans, C.R.; Bree, R.D.; Graaf, P.D.; et al. Early Response Prediction of Multiparametric Functional MRI and 18F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation. Cancers 2022, 14, 216. [Google Scholar] [CrossRef]
- Annovazzi, A.; Ferraresi, V.; Rea, S.; Russillo, M.; Renna, D.; Carpano, S.; Sciuto, R. Prognostic Value of Total Metabolic Tumour Volume and Therapy-Response Assessment by [18F]FDG PET/CT in Patients with Metastatic Melanoma Treated with BRAF/MEK Inhibitors. Eur. Radiol. 2022, 32, 3398–3407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proietti, I.; Azzella, G.; Dirzu, D.; Di Cristofano, C.; Bagni, O.; Potenza, C.; Filippi, L. It Looks Like a Zebra but Is Not: [18F]FDG PET/CT in a Giant Cutaneous Malignant Melanoma Mimicking Squamous Cell Carcinoma. Diagnostics 2024, 14, 2860. https://doi.org/10.3390/diagnostics14242860
Proietti I, Azzella G, Dirzu D, Di Cristofano C, Bagni O, Potenza C, Filippi L. It Looks Like a Zebra but Is Not: [18F]FDG PET/CT in a Giant Cutaneous Malignant Melanoma Mimicking Squamous Cell Carcinoma. Diagnostics. 2024; 14(24):2860. https://doi.org/10.3390/diagnostics14242860
Chicago/Turabian StyleProietti, Ilaria, Giulia Azzella, Diana Dirzu, Claudio Di Cristofano, Oreste Bagni, Concetta Potenza, and Luca Filippi. 2024. "It Looks Like a Zebra but Is Not: [18F]FDG PET/CT in a Giant Cutaneous Malignant Melanoma Mimicking Squamous Cell Carcinoma" Diagnostics 14, no. 24: 2860. https://doi.org/10.3390/diagnostics14242860
APA StyleProietti, I., Azzella, G., Dirzu, D., Di Cristofano, C., Bagni, O., Potenza, C., & Filippi, L. (2024). It Looks Like a Zebra but Is Not: [18F]FDG PET/CT in a Giant Cutaneous Malignant Melanoma Mimicking Squamous Cell Carcinoma. Diagnostics, 14(24), 2860. https://doi.org/10.3390/diagnostics14242860






