Differences in the Microvascular Arrangement Lead to Improved Clinical Diagnostics of Esophageal Neoplasms: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Statements
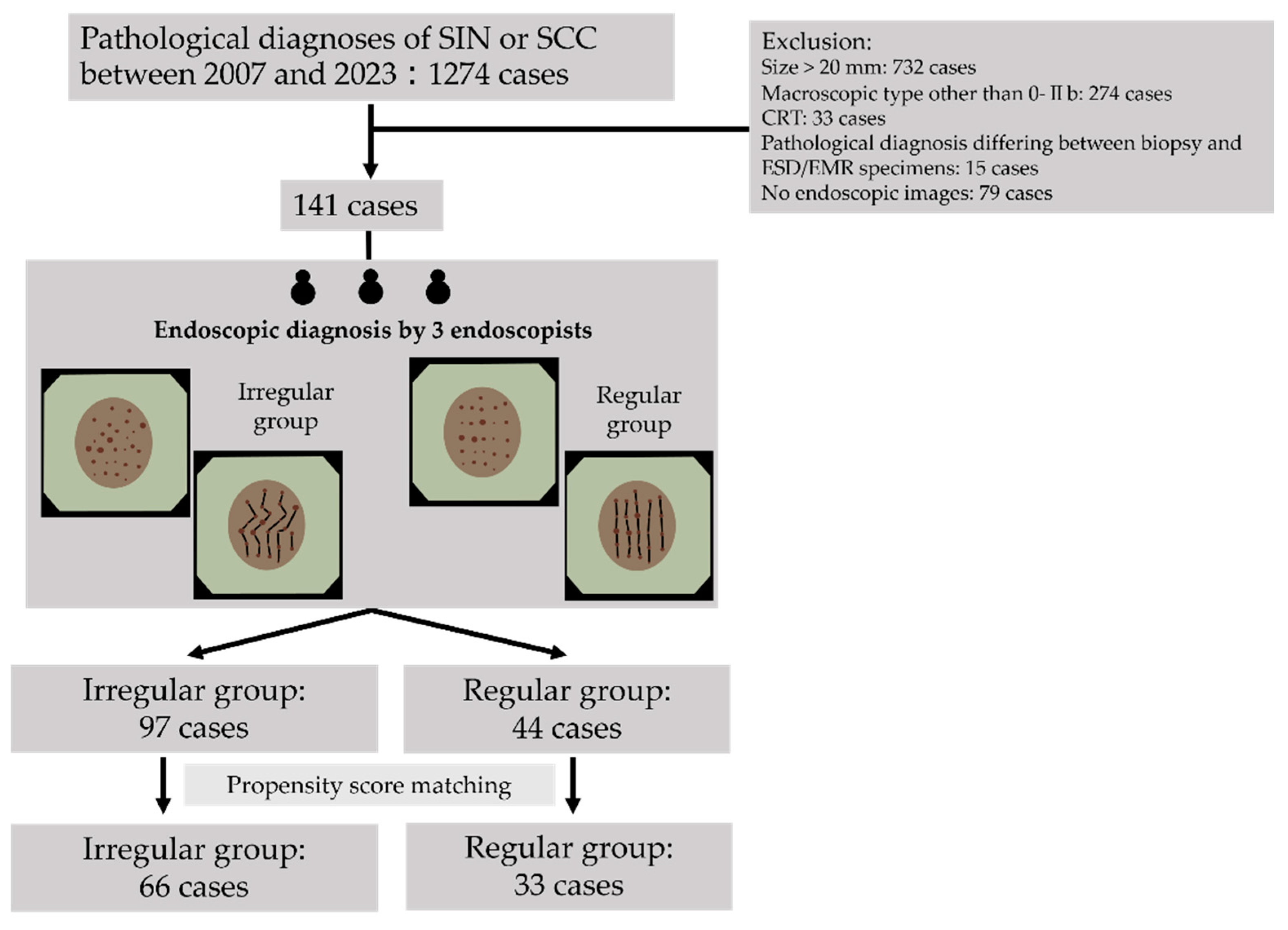
2.2. Patients
2.3. Endoscopic Procedure
2.4. Biopsy Protocol and EMR/ESD Procedure
2.5. Pathological Diagnosis
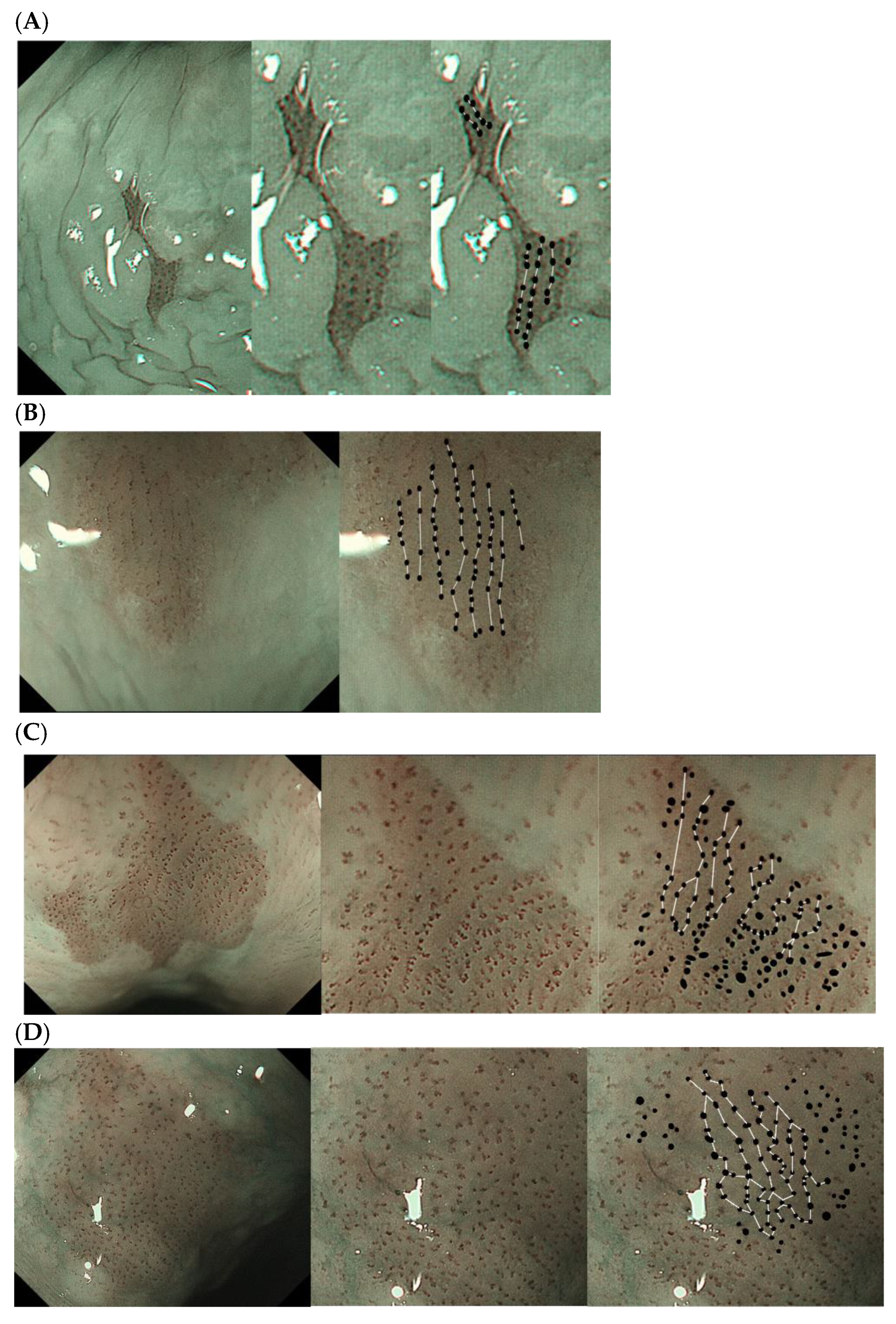
2.6. Definition and Evaluation of Vascular Arrangement
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Inter-Observer Agreement Regarding Vascular Arrangements
3.3. Pathological Characteristics of Patients Included After PS Matching
3.4. Comparison Between Regular and Irregular Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyama, T.; Inoue, H.; Arima, M.; Momma, K.; Omori, T.; Ishihara, R.; Hirasawa, D.; Takeuchi, M.; Tomori, A.; Goda, K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017, 14, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Inoue, T.; Uedo, N.; Yamamoto, S.; Kawada, N.; Tsujii, Y.; Kanzaki, H.; Hanafusa, M.; Hanaoka, N.; Takeuchi, Y.; et al. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J. Gastroenterol. Hepatol. 2010, 25, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002, 51, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer: Part II. In Esophagus, 12th ed.; Springer: Berlin/Heidelberg, Germany, 2024; Volume 21, pp. 216–269. [Google Scholar]
- Tachimori, Y.; Ozawa, S.; Numasaki, H.; Fujishiro, M.; Matsubara, H.; Oyama, T.; Shinoda, M.; Toh, Y.; Udagawa, H.; Uno, T. Comprehensive registry of esophageal cancer in Japan, 2008. Esophagus 2015, 12, 130–157. [Google Scholar] [CrossRef][Green Version]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: A prospective cohort study. Gut 2010, 59, 39–48. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, R.J.; Itabashi, M.; Kato, Y.; Lewin, K.J.; Riddell, R.H.; Shimoda, T.; Sipponen, P.; Stolte, M.; Watanabe, H.; Takahashi, H.; et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and Western pathologists. Lancet 1997, 349, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, R.J.; Itabashi, M.; Kato, Y.; Lewin, K.J.; Riddell, R.H.; Shimoda, T.; Sipponen, P.; Stolte, M.; Watanabe, H. Differences in the diagnostic criteria used by Japanese and Western pathologists to diagnose colorectal carcinoma. Cancer 1998, 82, 60–69. [Google Scholar] [CrossRef]
- Schlemper, R.J.; Dawsey, S.M.; Itabashi, M.; Iwashita, A.; Kato, Y.; Koike, M.; Lewin, K.J.; Riddell, R.H.; Shimoda, T.; Sipponen, P.; et al. Differences in diagnostic criteria for esophageal squamous cell carcinoma between Japanese and Western pathologists. Cancer 2000, 88, 996–1006. [Google Scholar] [CrossRef]
- Itabashi, M. Pathology Comments for I-4 Superfcial Carcinoma of the Esophagus. I. Case Presentations: Clinical Data, Endos Copy, and Pathology. Early Cancer of the Gastrointestinal Tract; Springer: Tokyo, Japan, 2006; pp. 100–129. [Google Scholar]
- The WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, Digestive System Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- Yoshimura, N.; Goda, K.; Tajiri, H.; Yoshida, Y.; Kato, T.; Seino, Y.; Ikegami, M.; Urashima, M. Diagnostic utility of narrow-band imaging endoscopy for pharyngeal superficial carcinoma. World J. Gastroenterol. 2011, 17, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inoue, H.; Usui, S.; Satodate, H.; Fukami, N.; Kudo, S.E. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest. Endosc. 2004, 59, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, Y.; Saito, Y.; Kobori, A.; Ban, H.; Shioya, M.; Nishimura, T.; Inatomi, O.; Bamba, S.; Tsujikawa, T.; Ishida, M.; et al. Magnified endoscopy combined with narrow band imaging of minimal superficial esophageal neoplasia-indicators to differentiate intraepithelial neoplasias. J. Gastrointest. Cancer 2012, 43, 599–606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubota, Y.; Kaneko, K.; Konishi, K.; Ito, H.; Yamamoto, T.; Katagiri, A.; Muramoto, T.; Yano, Y.; Kobayashi, Y.; Oyama, T.; et al. The onset of angiogenesis in a multistep process of esophageal squamous cell carcinoma. Front. Biosci. 2009, 14, 3872–3878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumagai, Y.; Toi, M.; Kawada, K.; Kawano, T. Angiogenesis in superficial esophageal squamous cell carcinoma: Magnifying endoscopic observation and molecular analysis. Dig. Endosc. 2010, 22, 259–267. [Google Scholar] [CrossRef] [PubMed]

| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Regular (n = 44) | Irregular (n = 97) | p-Value | Regular (n = 33) | Irregular (n = 66) | p-Value | |
| Age, mean ± SD | 73.3 ± 9.87 | 71.3 ± 9.34 | 0.278 | 72.9 ± 9.00 | 72.6 ± 8.71 | 0.866 |
| Sex, n (%) | 0.812 | 0.789 | ||||
| Male | 36 (81.8) | 81 (83.5) | 26(79) | 54(82) | ||
| Female | 8 (18.2) | 16 (16.5) | 7 (21) | 12(18) | ||
| Lesion location, n (%) | 0.082 | 0.246 | ||||
| Cervical esophagus | 3 (6.8) | 7 (7.2) | 2 (6.1) | 2 (3.0) | ||
| Upper esophagus | 5 (11.4) | 29 (29.9) | 4 (12.1) | 19 (28.8) | ||
| Middle esophagus | 27 (61.4) | 42 (43.4) | 19 (57.6) | 30 (45.5) | ||
| Lower esophagus | 9 (20.5) | 19 (19.6) | 8 (24.2) | 15 (22.7) | ||
| Lesion diameter (mm) | 10.8 (3–20) | 10.8 (2–20) | 0.983 | 11.1 (3–20) | 11.4 (2–20) | 0.84 |
| Smoking, pack years | 28.4 (0–110) | 27.8 (0–162) | 0.911 | 26.4 (0–100) | 25.9 (0–162) | 0.923 |
| Alcohol, g/week | 293 (0–1000) | 327(0–1400) | 0.495 | 308 (0–630) | 294(0–1400) | 0.786 |
| Alcohol, years | 36 (0–70) | 42 (0–74) | 0.118 | 39 (0–70) | 41 (0–74) | 0.727 |
| PPI use, n (%) | 15 (34) | 27 (28) | 0.551 | 11 (33) | 22 (33) | 1.00 |
| Prior iodine staining, n (%) | 0 (0) | 0 (0) | 1.00 | 0 (0) | 0 (0) | 1.00 |
| Past history: esophageal | 23 (52) | 50 (52) | 1.00 | 20 (61) | 33 (50) | 0.394 |
| EMR/ESD, n (%) |
| Squamous Cell Carcinoma | Squamous Intraepithelial Neoplasia | p-Value | |
|---|---|---|---|
| Number of lesions | 64 | 35 | |
| Lesion location | 0.59 | ||
| Cervical esophagus | 2 | 2 | |
| Upper esophagus | 17 | 6 | |
| Middle esophagus | 32 | 17 | |
| Lower esophagus | 13 | 10 | |
| Lesion diameter (mm), median (range) | 12.8 (3–20) | 8.5 (2–20) | <0.001 |
| Macroscopic type: 0-IIb | 64 | 35 | |
| Depth of invasion, n | |||
| EP | 46 | - | |
| LPM | 16 | - | |
| MM-SM1 | 2 | - | |
| Tissue sampling, n | <0.001 | ||
| EMR + ESD | 64 | 20 | |
| Biopsy | 0 | 15 |
| Endoscopic Diagnosis | p-Value | |||
|---|---|---|---|---|
| Regular | Irregular | |||
| Pathological diagnosis | SIN | 21 | 14 | |
| SCC | 12 | 52 | <0.001 | |
| Category | Diagnosis | Clinical Management |
|---|---|---|
| 1 | Negative for neoplasia | Optional follow-up |
| 2 | Indefinite for neoplasia | Follow-up |
| 3 | Mucosal low-grade neoplasia | Endoscopic resection or follow-up |
| Low-grade adenoma | ||
| Low-grade dysplasia | ||
| 4 | Mucosal high-grade neoplasia | Endoscopic or surgical local resection |
| 4.1 High-grade adenoma/dysplasia | ||
| 4.2 Non-invasive carcinoma | ||
| 4.3 Suspicious for invasive carcinoma | ||
| 4.4 Intramucosal carcinoma | ||
| 5 | Submucosal invasion by carcinoma | Surgical resection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, R.; Noma, E.; Moriguchi, Y.; Horiguchi, S.; Iizuka, T. Differences in the Microvascular Arrangement Lead to Improved Clinical Diagnostics of Esophageal Neoplasms: A Single-Center Retrospective Study. Diagnostics 2024, 14, 2852. https://doi.org/10.3390/diagnostics14242852
Minami R, Noma E, Moriguchi Y, Horiguchi S, Iizuka T. Differences in the Microvascular Arrangement Lead to Improved Clinical Diagnostics of Esophageal Neoplasms: A Single-Center Retrospective Study. Diagnostics. 2024; 14(24):2852. https://doi.org/10.3390/diagnostics14242852
Chicago/Turabian StyleMinami, Ryogo, Eriko Noma, Yoshiaki Moriguchi, Shinichiro Horiguchi, and Toshiro Iizuka. 2024. "Differences in the Microvascular Arrangement Lead to Improved Clinical Diagnostics of Esophageal Neoplasms: A Single-Center Retrospective Study" Diagnostics 14, no. 24: 2852. https://doi.org/10.3390/diagnostics14242852
APA StyleMinami, R., Noma, E., Moriguchi, Y., Horiguchi, S., & Iizuka, T. (2024). Differences in the Microvascular Arrangement Lead to Improved Clinical Diagnostics of Esophageal Neoplasms: A Single-Center Retrospective Study. Diagnostics, 14(24), 2852. https://doi.org/10.3390/diagnostics14242852







