Multiple Red Flags of Cardiac Amyloidosis in a Single Patient: Clinical Manifestations of an Underdiagnosed Disease
Abstract
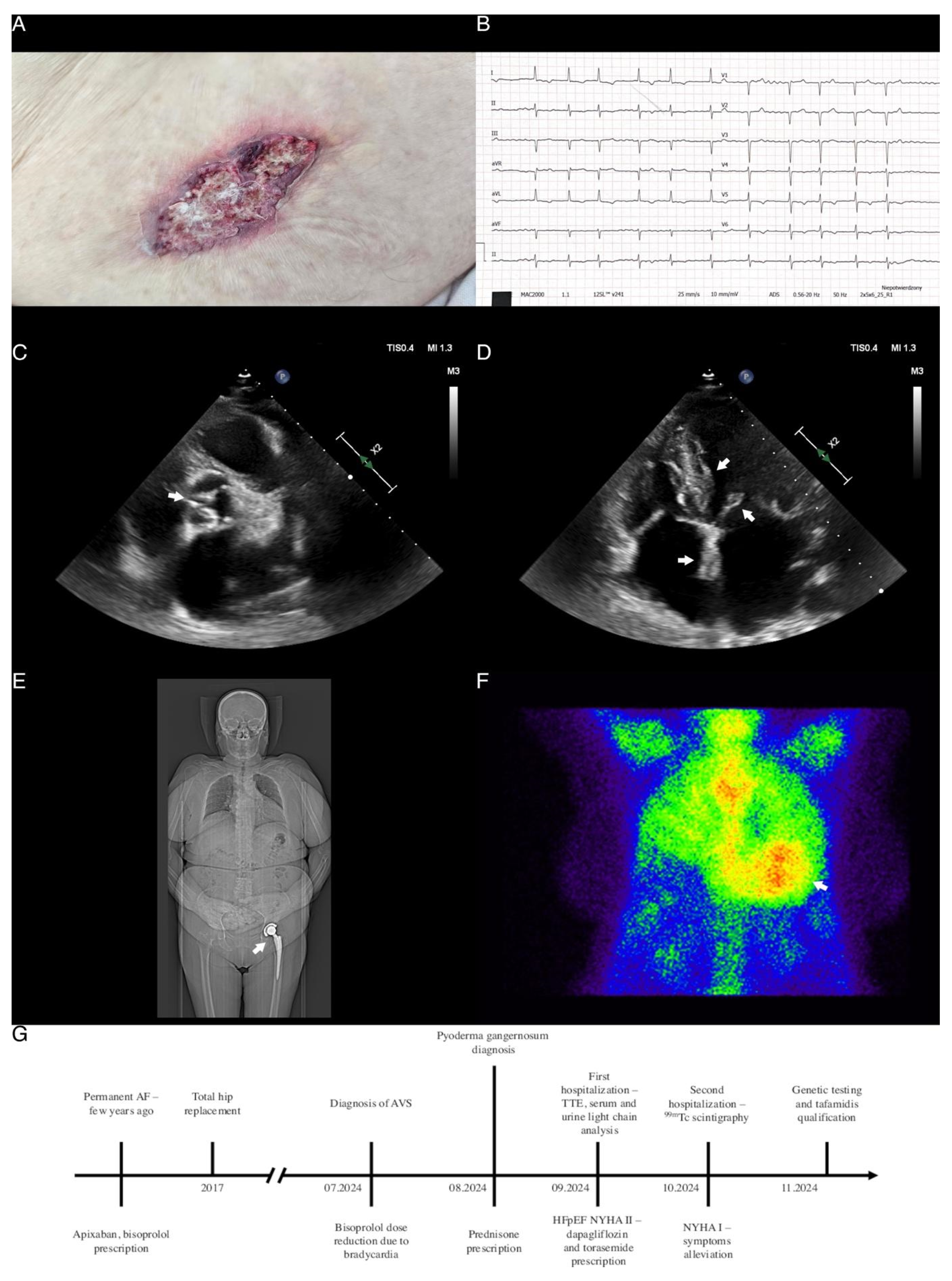
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, J.; Podolec, P.; Holcman, K.; Gawor-Prokopczyk, M.; Jankowska, E.; Kostkiewicz, M.; Dąbrowska-Kugacka, A.; Lipowska, M.; Mazurkiewicz, Ł.; Rajtar-Salwa, R.; et al. Stanowisko ekspertów Polskiego Towarzystwa Kardiologicznego dotyczące diagnostyki i leczenia amyloidozy transtyretynowej serca. Pol. Heart J. (Kardiol. Pol.) 2023, 81, 129–150. [Google Scholar]
- Brito, D.; Albrecht, F.C.; de Arenaza, D.P.; Bart, N.; Better, N.; Carvajal-Juarez, I.; Conceição, I.; Damy, T.; Dorbala, S.; Fidalgo, J.-C.; et al. World Heart Federation Consensus on Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM). Glob. Heart 2023, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Healy, L.; Giblin, G.; Gray, A.; Starr, N.; Murphy, L.; O’Sullivan, D.; Kavanagh, E.; Howley, C.; Tracey, C.; Morrin, E.; et al. Prevalence of transthyretin cardiac amyloidosis in undifferentiated heart failure with preserved ejection fraction. ESC Heart Fail. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Damy, T.; Piriou, N.; Barriales-Villa, R.; Cappelli, F.; Bahus, C.; Munteanu, C.; Keohane, D.; Mallaina, P.; Elliott, P.; et al. Prevalence and characteristics of transthyretin amyloid cardiomyopathy in hypertrophic cardiomyopathy. ESC Heart Fail 2024, 11, 4314–4324. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Berk, J.L.; Gillmore, J.D.; Witteles, R.M.; Grogan, M.; Drachman, B.; Damy, T.; Garcia-Pavia, P.; Taubel, J.; Solomon, S.D.; et al. Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. New Engl. J. Med. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, E.J.; Kozłowska, W.U.; Lipska, P.O.; Matys, U.; Pogorzelski, S.; Kożuch, M.; Dobrzycki, S. Multiple Red Flags of Cardiac Amyloidosis in a Single Patient: Clinical Manifestations of an Underdiagnosed Disease. Diagnostics 2024, 14, 2812. https://doi.org/10.3390/diagnostics14242812
Dąbrowski EJ, Kozłowska WU, Lipska PO, Matys U, Pogorzelski S, Kożuch M, Dobrzycki S. Multiple Red Flags of Cardiac Amyloidosis in a Single Patient: Clinical Manifestations of an Underdiagnosed Disease. Diagnostics. 2024; 14(24):2812. https://doi.org/10.3390/diagnostics14242812
Chicago/Turabian StyleDąbrowski, Emil Julian, Wiktoria Urszula Kozłowska, Patrycja Oliwia Lipska, Urszula Matys, Szymon Pogorzelski, Marcin Kożuch, and Sławomir Dobrzycki. 2024. "Multiple Red Flags of Cardiac Amyloidosis in a Single Patient: Clinical Manifestations of an Underdiagnosed Disease" Diagnostics 14, no. 24: 2812. https://doi.org/10.3390/diagnostics14242812
APA StyleDąbrowski, E. J., Kozłowska, W. U., Lipska, P. O., Matys, U., Pogorzelski, S., Kożuch, M., & Dobrzycki, S. (2024). Multiple Red Flags of Cardiac Amyloidosis in a Single Patient: Clinical Manifestations of an Underdiagnosed Disease. Diagnostics, 14(24), 2812. https://doi.org/10.3390/diagnostics14242812




