Non-Invasive and Quantitative Evaluation for Disuse Muscle Atrophy Caused by Immobilization After Limb Fracture Based on Surface Electromyography Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.1.1. Animals and Ethics
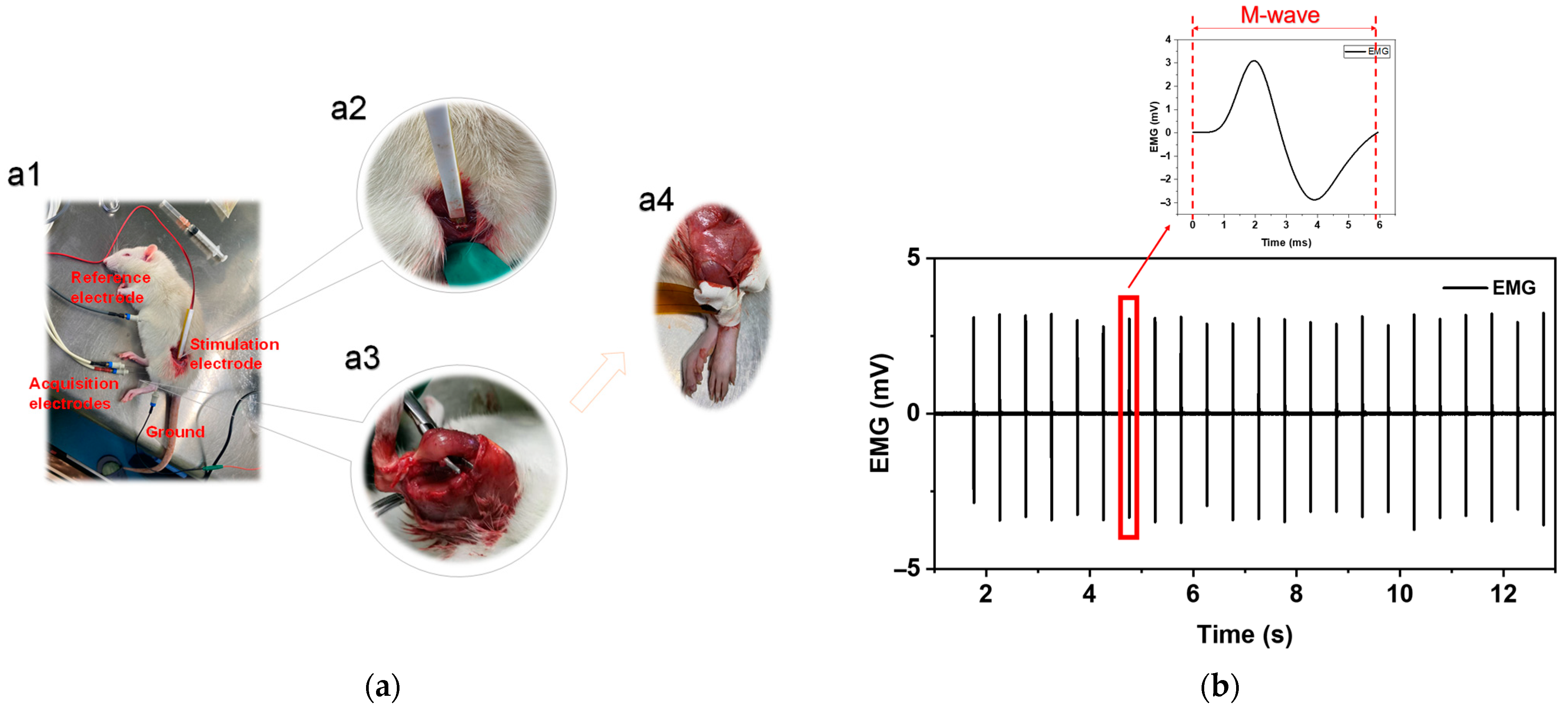
2.1.2. Disuse Atrophy Modeling
2.1.3. Experimental Design
2.1.4. EMG Acquisition and Processing
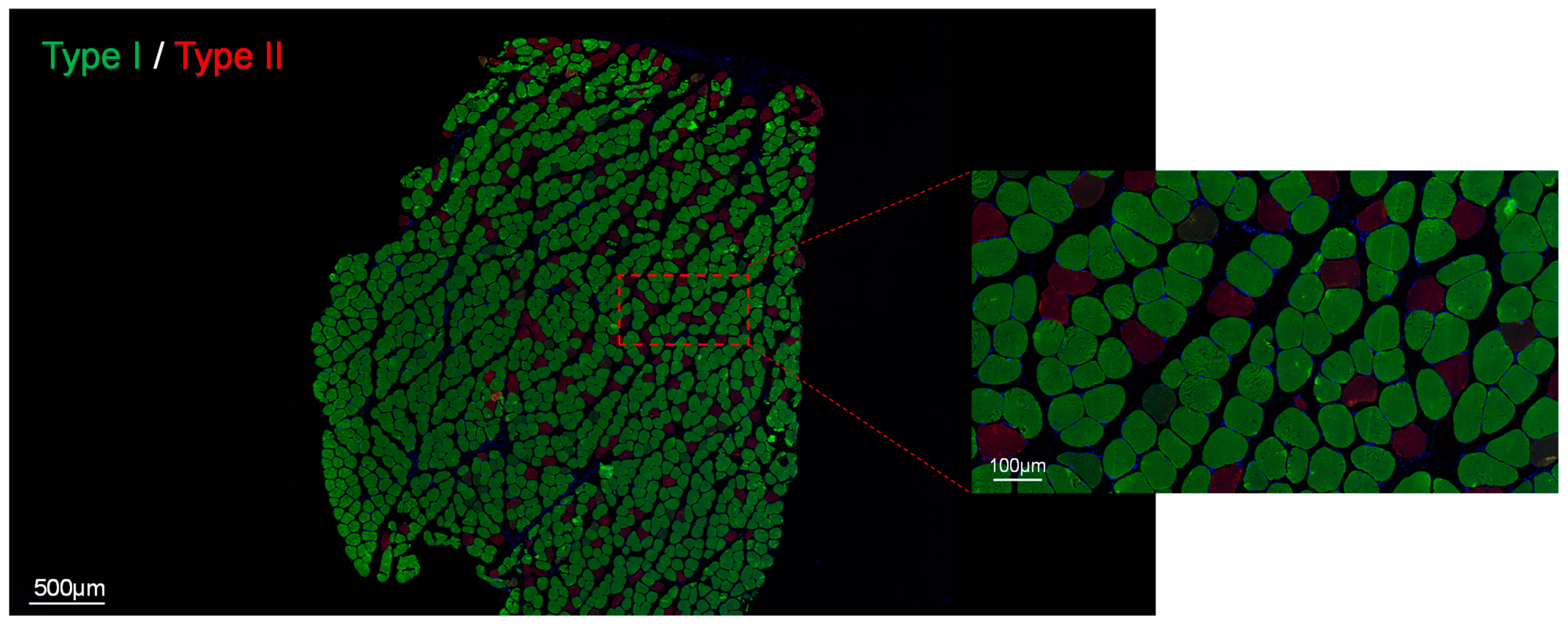
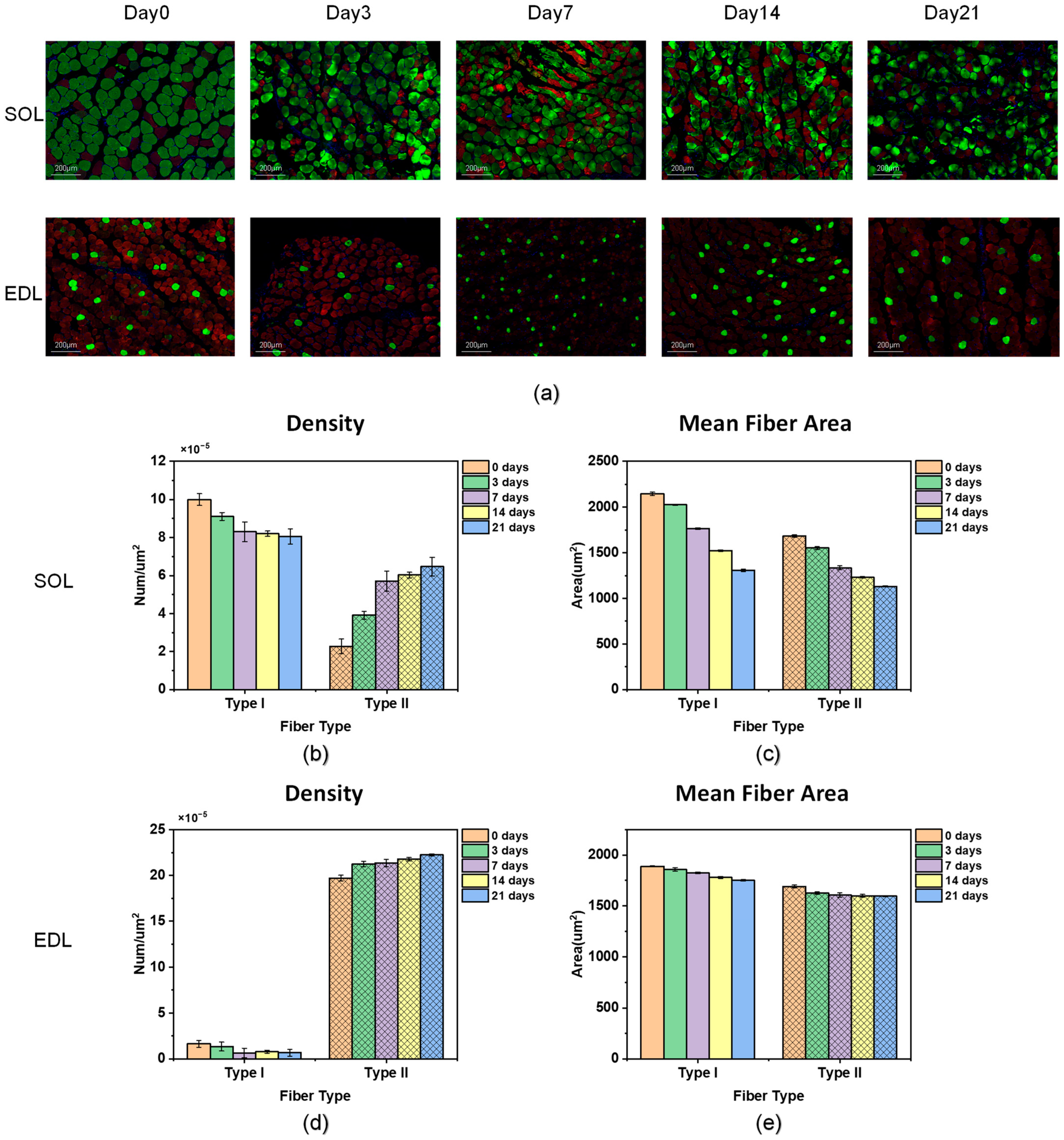
2.1.5. Fiber-Type Composition
2.2. Human Testing
2.2.1. Subject Selection
2.2.2. EMG Acquisition and Processing
3. Results
3.1. Animal Experiments
3.1.1. Fiber-Type Composition
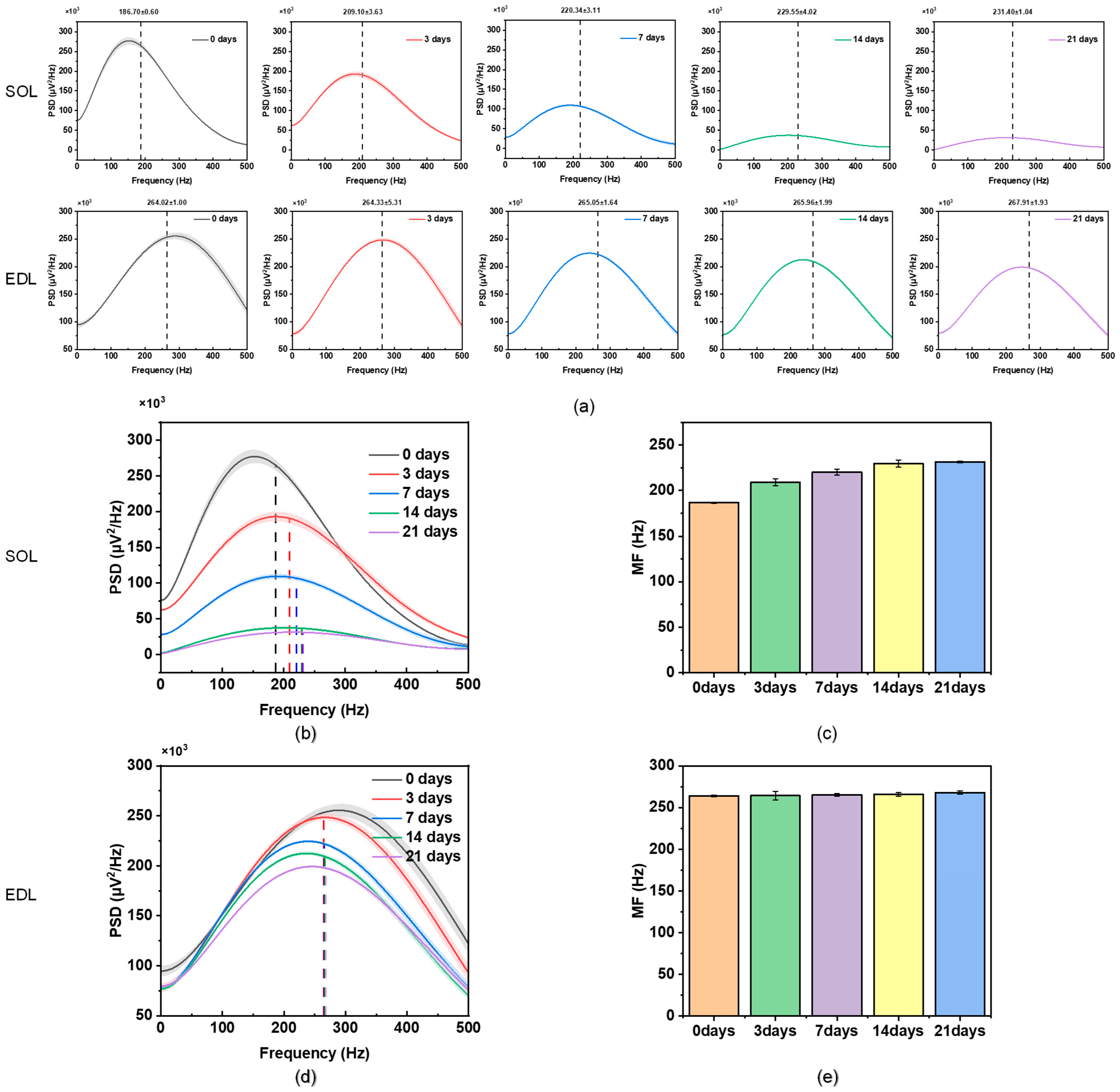
3.1.2. Analysis of sEMG
3.2. Human Testing
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance—A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C. Disuse-Induced Muscle Wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.M. Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth. Int. J. Mol. Sci. 2020, 21, 7940. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.I. Fracture Disease and Related Contractures. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 845–858. [Google Scholar] [CrossRef]
- Ferreira, R.; Neuparth, M.J.; Ascensão, A.; Magalhães, J.; Vitorino, R.; Duarte, J.A.; Amado, F. Skeletal Muscle Atrophy Increases Cell Proliferation in Mice Gastrocnemius during the First Week of Hindlimb Suspension. Eur. J. Appl. Physiol. 2006, 97, 340–346. [Google Scholar] [CrossRef]
- Kawashima, S.; Akima, H.; Kuno, S.; Gunji, A.; Fukunaga, T. Human Adductor Muscles Atrophy after Short Duration of Unweighting. Eur. J. Appl. Physiol. 2004, 92, 602–605. [Google Scholar] [CrossRef]
- Stevens, L.; Firinga, C.; Gohlsch, B.; Bastide, B.; Mounier, Y.; Pette, D. Effects of Unweighting and Clenbuterol on Myosin Light and Heavy Chains in Fast and Slow Muscles of Rat. Am. J. Physiol. Cell Physiol. 2000, 279, C1558–C1563. [Google Scholar] [CrossRef]
- Harridge, S.D. Plasticity of Human Skeletal Muscle: Gene Expression to in Vivo Function. Exp. Physiol. 2007, 92, 783–797. [Google Scholar] [CrossRef]
- Boonyarom, O.; Inui, K. Atrophy and Hypertrophy of Skeletal Muscles: Structural and Functional Aspects. Acta Physiol. 2006, 188, 77–89. [Google Scholar] [CrossRef]
- Ding, S.; Dai, Q.; Huang, H.; Xu, Y.; Zhong, C. An Overview of Muscle Atrophy. In Muscle Atrophy; Springer: Singapore, 2018; pp. 3–19. [Google Scholar]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 2018, 9, 324534. [Google Scholar] [CrossRef]
- Schulze, K.; Gallagher, P.; Trappe, S. Resistance Training Preserves Skeletal Muscle Function during Unloading in Humans. Med. Sci. Sports Exerc. 2002, 34, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.E.; Walter, G.A.; Okereke, E.; Scarborough, M.T.; Esterhai, J.L.; George, S.Z.; Kelley, M.J.; Tillman, S.M.; Gibbs, J.D.; Elliott, M.A. Muscle Adaptations with Immobilization and Rehabilitation after Ankle Fracture. Med. Sci. Sports Exerc. 2004, 36, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Mijnarends, D.M.; Meijers, J.M.; Halfens, R.J.; ter Borg, S.; Luiking, Y.C.; Verlaan, S.; Schoberer, D.; Jentoft, A.J.C.; van Loon, L.J.; Schols, J.M. Validity and Reliability of Tools to Measure Muscle Mass, Strength, and Physical Performance in Community-Dwelling Older People: A Systematic Review. J. Am. Med. Dir. Assoc. 2013, 14, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Michael, K. Relationship of Skeletal Muscle Atrophy to Functional Status: A Systematic Research Review. Biol. Res. Nurs. 2000, 2, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, S.C.; Goodheart, G.J., Jr. On the Reliability and Validity of Manual Muscle Testing: A Literature Review. Chiropr. Osteopat. 2007, 15, 4. [Google Scholar] [CrossRef]
- Naqvi, U.; Sherman, A.L. Muscle Strength Grading. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lee, D.H.; Claussen, G.C.; Oh, S. Clinical Nerve Conduction and Needle Electromyography Studies. JAAOS J. Am. Acad. Orthop. Surg. 2004, 12, 276–287. [Google Scholar] [CrossRef]
- Cushman, D.M.; Strenn, Q.; Elmer, A.; Yang, A.J.; Onofrei, L. Complications Associated with Electromyography: A Systematic Review. Am. J. Phys. Med. Rehabil. 2020, 99, 149–155. [Google Scholar] [CrossRef]
- Venturelli, N.; Tordjman, M.; Ammar, A.; Chetrit, A.; Renault, V.; Carlier, R.-Y. Contribution of Muscle MRI for Diagnosis of Myopathy. Rev. Neurol. 2023, 179, 61–80. [Google Scholar] [CrossRef]
- Heckmatt, J.Z.; Leeman, S.; Dubowitz, V. Ultrasound imaging in the diagnosis of muscle disease. J. Pediatr. 1982, 101, 656–660. [Google Scholar] [CrossRef]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 333–560. [Google Scholar]
- Zierath, J.R.; Hawley, J.A. Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Stuart, C.A.; Brunder, D.G.; Hillman, G.R. Magnetic Resonance Imaging Quantitation of Changes in Muscle Volume during 7 Days of Strict Bed Rest. Aviat. Space Environ. Med. 1995, 66, 976–981. [Google Scholar] [PubMed]
- Stelzer, J.E.; Widrick, J.J. Effect of Hindlimb Suspension on the Functional Properties of Slow and Fast Soleus Fibers from Three Strains of Mice. J. Appl. Physiol. 2003, 95, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.; Zhou, M.; Ohira, Y.; Klitgaard, H.; Jiang, B.; Bell, G.; Harris, B.; Saltin, B.; Gollnick, P.; Roy, R. Human Fiber Size and Enzymatic Properties after 5 and 11 Days of Spaceflight. J. Appl. Physiol. 1995, 78, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W. Effect of Limb Immobilization on Skeletal Muscle. J. Appl. Physiol. 1982, 52, 1113–1118. [Google Scholar] [CrossRef]
- Booth, F.W.; Gollnick, P.D. Effects of Disuse on the Structure and Function of Skeletal Muscle. Med. Sci. Sports Exerc. 1983, 15, 415–420. [Google Scholar] [CrossRef]
- Baldwin, K.M. Effect of Spaceflight on the Functional, Biochemical, and Metabolic Properties of Skeletal Muscle. Med. Sci. Sports Exerc. 1996, 28, 983–987. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Haddad, F. Effects of Different Activity and Inactivity Paradigms on Myosin Heavy Chain Gene Expression in Striated Muscle. J. Appl. Physiol. 2001, 90, 345–357. [Google Scholar] [CrossRef]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Functional and Structural Adaptations of Skeletal Muscle to Microgravity. J. Exp. Biol. 2001, 204, 3201–3208. [Google Scholar] [CrossRef]
- Ohira, Y.; Yoshinaga, T.; Ohara, M.; Kawano, F.; Wang, X.D.; Higo, Y.; Terada, M.; Matsuoka, Y.; Roy, R.R.; Edgerton, V.R. The Role of Neural and Mechanical Influences in Maintaining Normal Fast and Slow Muscle Properties. Cells Tissues Organs 2006, 182, 129–142. [Google Scholar] [CrossRef]
- Kupa, E.; Roy, S.; Kandarian, S.; De Luca, C. Effects of Muscle Fiber Type and Size on EMG Median Frequency and Conduction Velocity. J. Appl. Physiol. 1995, 79, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Retortillo, S.; Rizzo, R.; Wang, J.W.; Sitges, C.; Ivanov, P.C. Universal Spectral Profile and Dynamic Evolution of Muscle Activation: A Hallmark of Muscle Type and Physiological State. J. Appl. Physiol. 2020, 129, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Natanek, S.A.; Gosker, H.R.; Slot, I.G.; Marsh, G.S.; Hopkinson, N.S.; Man, W.D.; Tal-Singer, R.; Moxham, J.; Kemp, P.R.; Schols, A.M. Heterogeneity of Quadriceps Muscle Phenotype in Chronic Obstructive Pulmonary Disease (COPD); Implications for Stratified Medicine? Muscle Nerve 2013, 48, 488–497. [Google Scholar] [CrossRef]
- Whittom, F.; Jobin, J.; Simard, P.-M.; Leblanc, P.; Simard, C.; Bernard, S.; Belleau, R.; Maltais, F. Histochemical and Morphological Characteristics of the Vastus Lateralis Muscle in Patients with Chronic Obstructive Pulmonary Disease. Med. Sci. Sports Exerc. 1998, 30, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Gosker, H.R.; Zeegers, M.P.; Wouters, E.F.; Schols, A.M. Muscle Fibre Type Shifting in the Vastus Lateralis of Patients with COPD Is Associated with Disease Severity: A Systematic Review and Meta-Analysis. Thorax 2007, 62, 944–949. [Google Scholar] [CrossRef]
- Kapchinsky, S.; Vuda, M.; Miguez, K.; Elkrief, D.; de Souza, A.R.; Baglole, C.J.; Aare, S.; MacMillan, N.J.; Baril, J.; Rozakis, P. Smoke-induced Neuromuscular Junction Degeneration Precedes the Fibre Type Shift and Atrophy in Chronic Obstructive Pulmonary Disease. J. Physiol. 2018, 596, 2865–2881. [Google Scholar] [CrossRef]
- Gosselin, N.; Matecki, S.; Poulain, M.; Ramonatxo, M.; Ceugniet, F.; Préfaut, C.; Varray, A. Electrophysiologic Changes during Exercise Testing in Patients with Chronic Obstructive Pulmonary Disease. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2003, 27, 170–179. [Google Scholar] [CrossRef]
- Allaire, J.; Maltais, F.; Doyon, J.; Noel, M.; LeBlanc, P.; Carrier, G.; Simard, C.; Jobin, J. Peripheral Muscle Endurance and the Oxidative Profile of the Quadriceps in Patients with COPD. Thorax 2004, 59, 673–678. [Google Scholar] [CrossRef]
- Marquis, N.; Debigare, R.; Bouyer, L.; Saey, D.; Laviolette, L.; Brouillard, C.; Maltais, F. Physiology of Walking in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Med. Sci. Sports Exerc. 2009, 41, 1540–1548. [Google Scholar] [CrossRef]
- Ovechkin, A.V.; Sayenko, D.G.; Ovechkina, E.N.; Aslan, S.C.; Pitts, T.; Folz, R.J. Respiratory Motor Training and Neuromuscular Plasticity in Patients with Chronic Obstructive Pulmonary Disease: A Pilot Study. Respir. Physiol. Neurobiol. 2016, 229, 59–64. [Google Scholar] [CrossRef][Green Version]
- Boccia, G.; Coratella, G.; Dardanello, D.; Rinaldo, N.; Lanza, M.; Schena, F.; Rainoldi, A. Severe COPD Alters Muscle Fiber Conduction Velocity during Knee Extensors Fatiguing Contraction. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Dardanello, D.; Rinaldo, N.; Coratella, G.; Schena, F.; Rainoldi, A. Electromyographic Manifestations of Fatigue Correlate with Pulmonary Function, 6-Minute Walk Test, and Time to Exhaustion in COPD. Respir. Care 2015, 60, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, I.M.M.; Basso-Vanelli, R.P.; Beltrame, T.; Frade, M.C.M.; de Abreu, R.M.; Cid, M.M.; Catai, A.M.; Oliveira, A.B.; Jamami, M. Acute Effects of the 6-Minute Pegboard and Ring Test in COPD. Respir. Care 2020, 65, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Casabona, A.; Valle, M.S.; Laudani, L.; Crimi, C.; Russo, C.; Malaguarnera, L.; Crimi, N.; Cioni, M. Is the Power Spectrum of Electromyography Signal a Feasible Tool to Estimate Muscle Fiber Composition in Patients with COPD? J. Clin. Med. 2021, 10, 3815. [Google Scholar] [CrossRef] [PubMed]
- Roudier, E.; Gineste, C.; Wazna, A.; Dehghan, K.; Desplanches, D.; Birot, O. Angio-adaptation in Unloaded Skeletal Muscle: New Insights into an Early and Muscle Type-specific Dynamic Process. J. Physiol. 2010, 588, 4579–4591. [Google Scholar] [CrossRef]
- Pei, X.C.; Jin, H.; Dong, S.R.; Lou, D.; Ma, L.; Wang, X.G.; Cheng, W.W.; Wong, H. Flexible wireless skin impedance sensing system for wound healing assessment. Vacuum 2019, 168, 108808. [Google Scholar] [CrossRef]
- YY/T 1095-2015; China Food and Drug Administration. Pharmaceutical Industry Standard of the People’s Republic of China, Electromyographic Biofeedback Device. China Standards Press: Beijing, China, 2015.
- Nepomuceno, A.C.; Politani, E.L.; Silva, E.G.d.; Salomone, R.; Longo, M.V.L.; Salles, A.G.; Faria, J.C.M.d.; Gemperli, R. Tibial and Fibular Nerves Evaluation Using Intraoperative Electromyography in Rats. Acta Cirúrgica Bras. 2016, 31, 542–548. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG. A practical introduction to kinesiological electromyography. Noraxon 2005, 1, 30–35. [Google Scholar]
- Wang, C.; Yue, F.; Kuang, S. Muscle Histology Characterization Using H&E Staining and Muscle Fiber Type Classification Using Immunofluorescence Staining. Bio-Protocol 2017, 7, e2279. [Google Scholar]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Merletti, R.; Vieira, T.; Farina, D. Techniques for Information Extraction from the Surface EMG Signalhigh-Density Surface EMG. In Surface Electromyography: Physiology, Engineering and Applications; Wiley: Hoboken, NJ, USA, 2016; pp. 126–157. [Google Scholar]
- Rojas-Martínez, M.; Serna, L.Y.; Jordanic, M.; Marateb, H.R.; Merletti, R.; Mañanas, M.Á. High-density surface electromyography signals during isometric contractions of elbow muscles of healthy humans. Sci. Data 2020, 7, 397. [Google Scholar] [CrossRef] [PubMed]


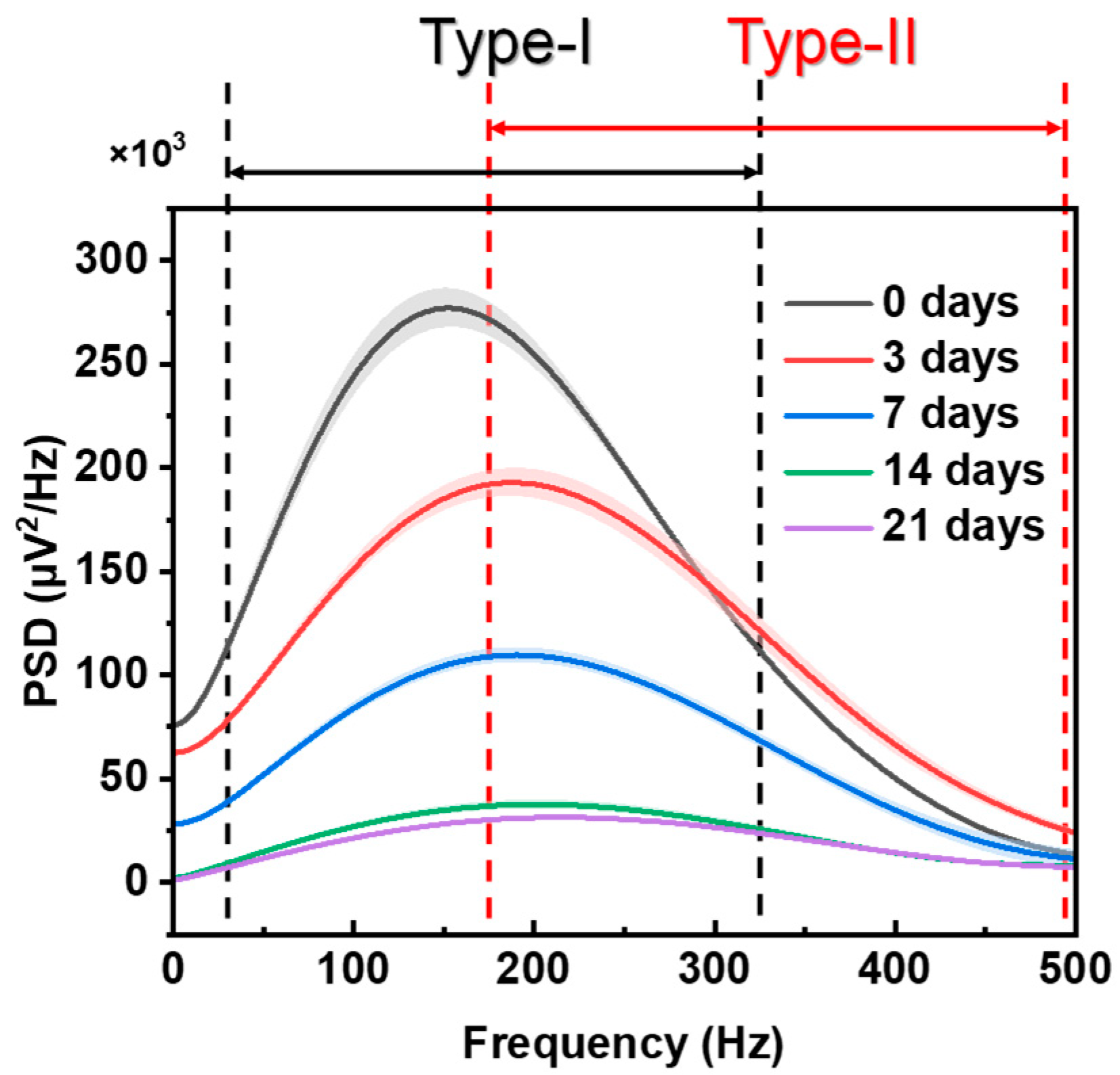
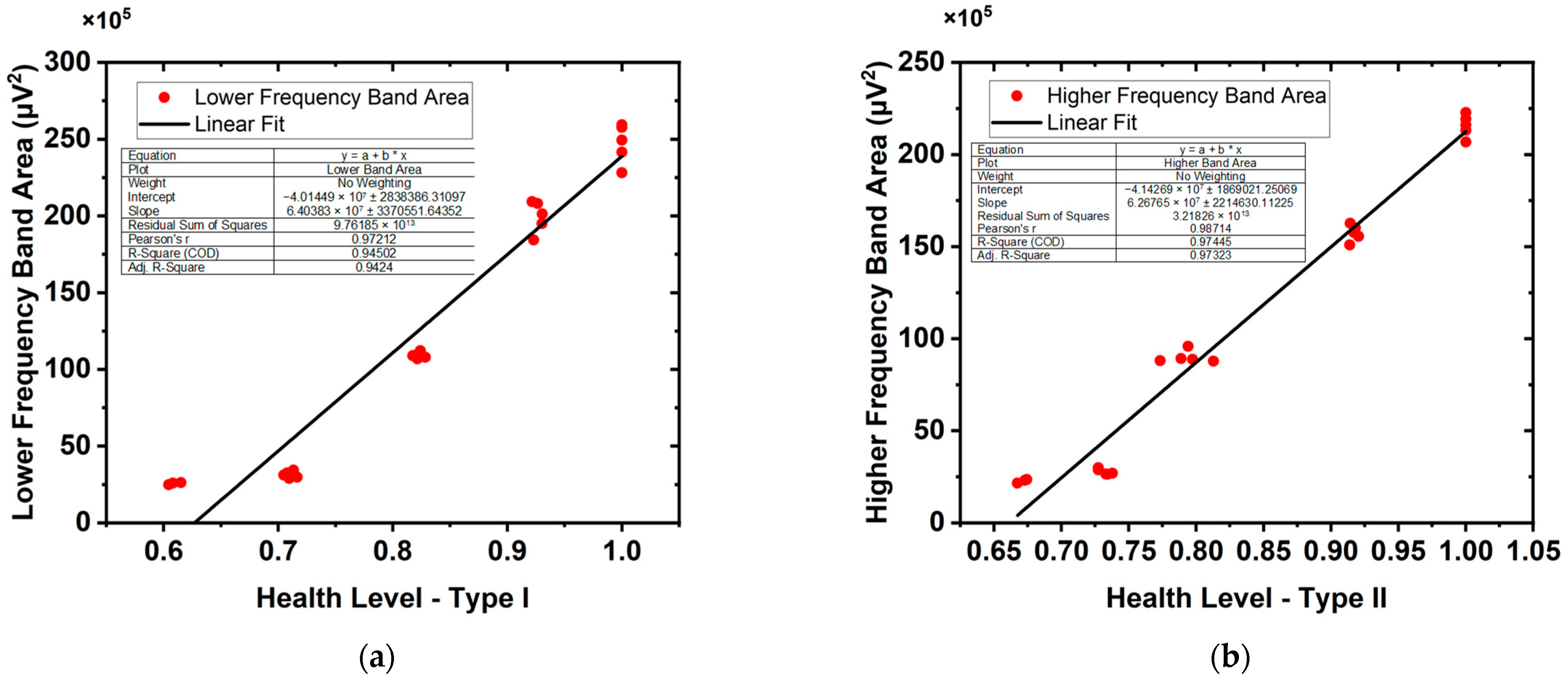

| Term/Muscle | SOL | EDL | |
|---|---|---|---|
| Main fiber component | Type I | Type II | |
| Fiber changes of atrophy process | Common | Mean fiber area of both type I and type II decreases Type II density increases and type I density decreases A shift from type I to type II | |
| Difference | Changing trend of fiber density in the SOL is greater | ||
| PSD changes of atrophy process | Obviously shifting to the high frequency | Basically no shift | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Hong, Y.; Zhang, S.; Jin, H.; Wang, S.; Feng, G. Non-Invasive and Quantitative Evaluation for Disuse Muscle Atrophy Caused by Immobilization After Limb Fracture Based on Surface Electromyography Analysis. Diagnostics 2024, 14, 2695. https://doi.org/10.3390/diagnostics14232695
Shi L, Hong Y, Zhang S, Jin H, Wang S, Feng G. Non-Invasive and Quantitative Evaluation for Disuse Muscle Atrophy Caused by Immobilization After Limb Fracture Based on Surface Electromyography Analysis. Diagnostics. 2024; 14(23):2695. https://doi.org/10.3390/diagnostics14232695
Chicago/Turabian StyleShi, Lvgang, Yuyin Hong, Shun Zhang, Hao Jin, Shengming Wang, and Gang Feng. 2024. "Non-Invasive and Quantitative Evaluation for Disuse Muscle Atrophy Caused by Immobilization After Limb Fracture Based on Surface Electromyography Analysis" Diagnostics 14, no. 23: 2695. https://doi.org/10.3390/diagnostics14232695
APA StyleShi, L., Hong, Y., Zhang, S., Jin, H., Wang, S., & Feng, G. (2024). Non-Invasive and Quantitative Evaluation for Disuse Muscle Atrophy Caused by Immobilization After Limb Fracture Based on Surface Electromyography Analysis. Diagnostics, 14(23), 2695. https://doi.org/10.3390/diagnostics14232695








