Regression of the Flow Signal from the Neovascular Network in AMD Neovascular Membranes Treated with Faricimab
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcome Measures
2.2. Statistical Analysis
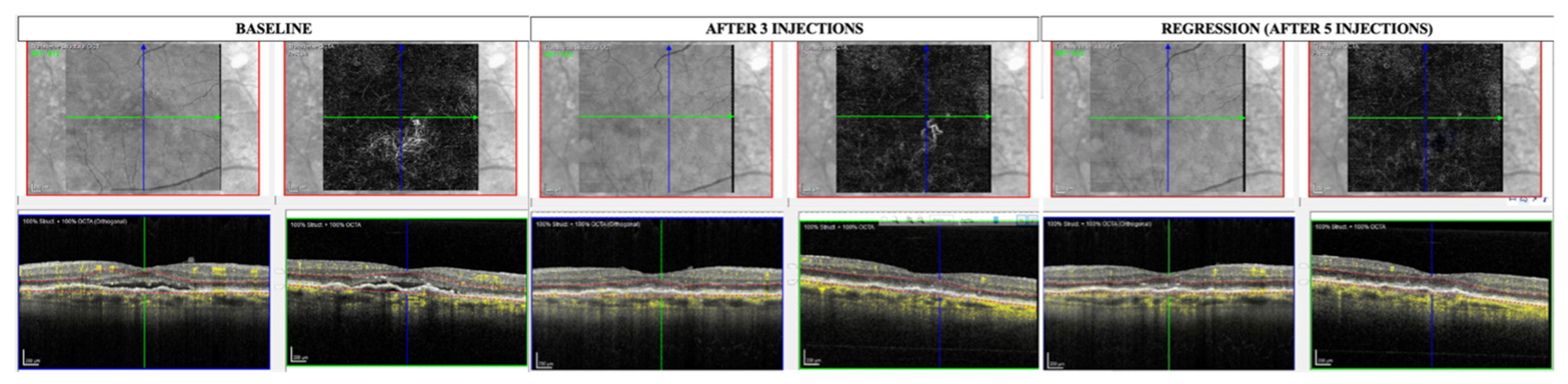
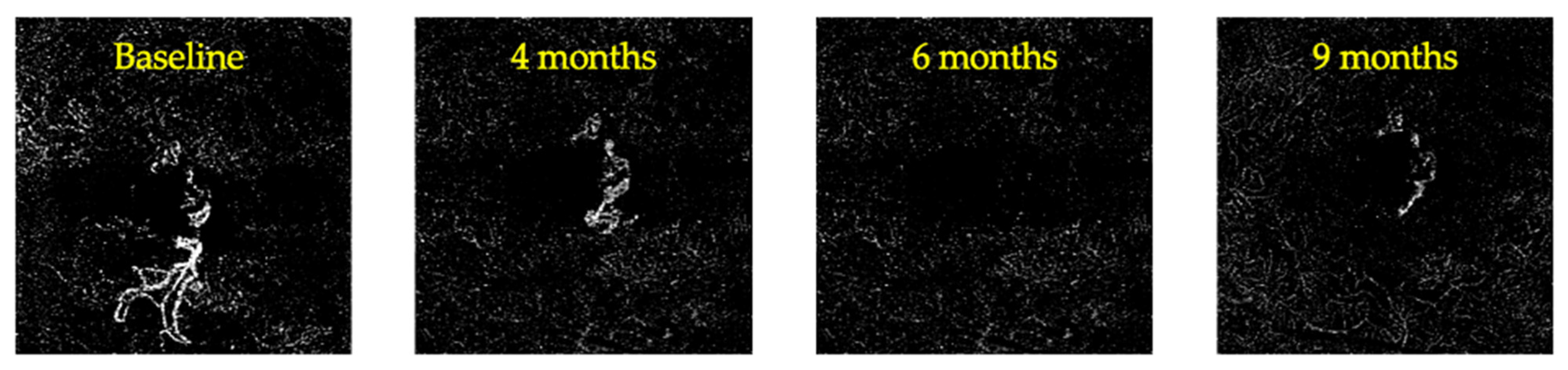
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Jung, J.J.; Chen, C.Y.; Mrejen, S.; Gallego-Pinazo, R.; Xu, L.; Marsiglia, M.; Boddu, S.; Freund, K.B. The Incidence of Neovascular Subtypes in Newly Diagnosed Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2014, 158, 769–779.e2. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Bressler, S.B.; Bressler, N.M. Impact of availability of anti-vascular endothelial growth factor therapy on visual impairment and blindness due to neovascular age-related macular degeneration. Arch. Ophthalmol. 2012, 130, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Eandi, C.M.; Alovisi, C.; De Sanctis, U.; Grignolo, F.M. Treatment for neovascular age related macular degeneration: The state of the art. Eur. J. Pharmacol. 2016, 787, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab. Cells 2020, 9, 1869. [Google Scholar] [CrossRef]
- Khanani, A.M.; Heier, J.; Quezada Ruiz, C.; Lin, H.; Silverman, D.; Brittain, C.; Ives, J.; Swaminathan, B.; Basu, K.; Wong, T.Y. Faricimab in Neovascular Age-Related Macular Degeneration: 1-Year Efficacy, Safety, and Durability in the Phase 3 TENAYA and LUCERNE Trials. Investig. Ophthalmol. Vis. Sci. 2021, 62, 428. [Google Scholar]
- Khanani, A.M.; Guymer, R.H.; Basu, K.; Boston, H.; Heier, J.S.; Korobelnik, J.-F.; Kotecha, A.; Lin, H.; Silverman, D.; Swaminathan, B.; et al. TENAYA and LUCERNE: Rationale and Design for the Phase 3 Clinical Trials of Faricimab for Neovascular Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100076. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Khanani, A.M.; Aziz, A.A.; Khan, H.; Gupta, A.; Mojumder, O.; Saulebayeva, A.; Abbey, A.M.; Almeida, D.R.P.; Avery, R.L.; Banda, H.K.; et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: The TRUCKEE study—6 month results. Eye 2023, 37, 3574–3581. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 2022, 92, 101113. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization with Antiangiogenic Therapy for Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef]
- la Sala, A.; Pontecorvo, L.; Agresta, A.; Rosano, G.; Stabile, E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol. Med. 2012, 18, 494–501. [Google Scholar] [CrossRef]
- Markomichelakis, N.N.; Theodossiadis, P.G.; Sfikakis, P.P. Regression of neovascular age-related macular degeneration following infliximab therapy. Am. J. Ophthalmol. 2005, 139, 537–540. [Google Scholar] [CrossRef]
- Schierling, W.; Troidl, K.; Troidl, C.; Schmitz-Rixen, T.; Schaper, W.; Eitenmüller, I.K. The role of angiogenic growth factors in arteriogenesis. J. Vasc. Res. 2009, 46, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.L.; Berger, J.W.; Maguire, M.G.; Ho, A.C. Age-Related Macular Degeneration. N. Engl. J. Med. 2000, 342, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Costa, J.F.; Marques, M.; Cachulo, M.L.; Figueira, J.; Silva, R. Sequential Morphological Changes in the CNV Net after Intravitreal Anti-VEGF Evaluated with OCT Angiography. Ophthalmic Res. 2016, 55, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Dávila, J.P.; Rahimi, M.; Rebhun, C.B.; Alibhai, A.Y.; Waheed, N.K.; Sarraf, D. Long-term Progression of Type 1 Neovascularization in Age-related Macular Degeneration Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 187, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, E.; Catania, F.; Labbate, G.; Sacconi, R.; Ferrara, S.; Parravano, M.; Costanzo, E.; Querques, G. Microvascular changes in treatment naïve non-exudative macular neovascularization complicated by exudation. Retina 2022, 44, 1679–1687. [Google Scholar] [CrossRef]
- Crincoli, E.; Catania, F.; Sacconi, R.; Ribarich, N.; Ferrara, S.; Parravano, M.; Costanzo, E.; Querques, G. Deep learning for automatic prediction of early activation of treatment naïve non-exudative MNVs in AMD. Retina 2024, 44, 1360–1370. [Google Scholar] [CrossRef]
- Roisman, L.; Zhang, Q.; Wang, R.K.; Gregori, G.; Zhang, A.; Chen, C.-L.; Durbin, M.K.; An, L.; Stetson, P.F.; Robbins, G.; et al. Optical Coherence Tomography Angiography of Asymptomatic Neovascularization in Intermediate Age-Related Macular Degeneration. Ophthalmology 2016, 123, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Q.; Motulsky, E.H.; Thulliez, M.; Shi, Y.; Lyu, C.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Wang, R.K.; et al. Two-Year Risk of Exudation in Eyes with Nonexudative Age-Related Macular Degeneration and Subclinical Neovascularization Detected with Swept Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2019, 208, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, M.; Savastano, M.C.; Lumbroso, B. Capillary Network Anomalies in Branch Retinal Vein Occlusion on Optical Coherence Tomography Angiography. Retina 2015, 35, 2332–2338. [Google Scholar] [CrossRef]
- Wietecha, M.S.; Cerny, W.L.; DiPietro, L.A. Mechanisms of Vessel Regression: Toward an Understanding of the Resolution of Angiogenesis. In New Perspectives in Regeneration; Heber-Katz, E., Stocum, D.L., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–32. ISBN 978-3-642-35810-4. [Google Scholar]
- Kitson, S.; McAllister, A. A case of hypertensive uveitis with intravitreal faricimab. Retin. Cases Brief Rep. 2023. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Younis, S.; Bedan Hamoud, A.; Fabozzi, L. Uveitis Following Intravitreal Injections of Faricimab: A Case Report. Ocul. Immunol. Inflamm. 2023, 32, 1873–1877. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Phasukkijwatana, N.; Sarraf, D.; Freund, K.B. Regression of Type 2 Neovascularization into a Type 1 Pattern After Intravitreal Anti-Vascular Endothelial Growth Factor Therapy for Neovascular Age-Related Macular Degeneration. Retina 2017, 37, 222. [Google Scholar] [CrossRef]
- Channa, R.; Sophie, R.; Bagheri, S.; Shah, S.M.; Wang, J.; Adeyemo, O.; Sodhi, A.; Wenick, A.; Ying, H.S.; Campochiaro, P.A. Regression of Choroidal Neovascularization Results in Macular Atrophy in Anti-Vascular Endothelial Growth Factor-Treated Eyes. Am. J. Ophthalmol. 2015, 159, 9–19.e2. [Google Scholar] [CrossRef]
- Rai, B.B.; Essex, R.W.; Sabeti, F.; Maddess, T.; Rohan, E.M.F.; van Kleef, J.P.; Carle, C.F. An Objective Perimetry Study of Central Versus Peripheral Sensitivities and Delays in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef]
- Sabeti, F.; Lane, J.; Rohan, E.M.F.; Rai, B.B.; Essex, R.W.; McKone, E.; Maddess, T. Correlation of Central Versus Peripheral Macular Structure-Function with Acuity in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Haas, A.M.; Ahmed, D.; Stattin, M.; Graf, A.; Krepler, K.; Ansari-Shahrezaei, S. Comparison of macular neovascularization lesion size by the use of Spectral-Domain Optical Coherence Tomography Angiography and Swept-Source Optical Coherence Tomography Angiography versus Indocyanine Green Angiography. Acta Ophthalmol. 2021, 99, e260–e266. [Google Scholar] [CrossRef]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Lommatzsch, C.; Spital, G.; Gutfleisch, M.; Pauleikhoff, D.; Lommatzsch, A. Quantitative Comparison of the Vascular Structure of Macular Neovascularizations Between Swept-Source and Spectral-Domain Optical Coherence Tomography Angiography. Clin. Ophthalmol. 2020, 14, 3179–3186. [Google Scholar] [CrossRef] [PubMed]

| Sex | Treatment-Naïve | Type of MNV | Type of Neovascular Network | MNV Height at Baseline (μm) | MNV GLD at Baseline (μm) | Time to Remission of Exudation (Months) | Time to Regression of the MNV (Months) | |
|---|---|---|---|---|---|---|---|---|
| 1 | M | Yes | I | Glomerular Immature | 105.1 | 1223 | 2 | 4 |
| 2 | F | No | I | Tree-like Mature | 167.7 | 1127 | 3 | 4 |
| 3 | M | Yes | I | Fragmented Mature | 98.0 | 1608 | 4 | 6 |
| 4 | M | Yes | I | Fragmented Hypermature | 124.8 | 1098 | 1 | 4 |
| 5 | F | Yes | I | Glomerular Immature | 73.2 | 1455 | 3 | 6 |
| 6 | M | No | I | Tree-like Mature | 68.2 | 1397 | 4 | 6 |
| 7 | F | No | I | Fragmented Hypermature | 178.4 | 1290 | 5 | 8 |
| 8 | M | No | II | Tree-like Mature | 136.3 | 1505 | 6 | 8 |
| 9 | F | Yes | I | Glomerular Immature | 109.1 | 1560 | 5 | 8 |
| 10 | F | No | II | Tree-like Immature | 129.3 | 1081 | 4 | 6 |
| 11 | F | Yes | I | Glomerular Immature | 77.5 | 1107 | 3 | 6 |
| 12 | M | No | I | Glomerular Mature | 112 | 1055 | 3 | 6 |
| CR Group (12/110) | PR Group (67/110) | Stable Group (31/110) | p | |
|---|---|---|---|---|
| Age | 71.3 ± 6.9 | 70.1 ± 7.2 | 70.9 ± 7.0 | 0.996 |
| Sex | 6/12 (50.0%) | 32/67 (47.7%) | 14/31 (45.2%) | 0.952 |
| Follow-up (months) | 7.9 ± 1.2 | 8.2 ± 1.4 | 8.0 ± 1.3 | 0.865 |
| Naïve eyes | 6/12 (50.0%) | 11/67 (16.4%) | 9/31 (29.1%) | 0.029 * |
| MNV type | I = 10/12 (83.3%) II = 2/12 (16.7%) | I = 58/67 (86.6%) II = 9/67 (13.4%) | I = 25/31 (80.6%) II = 6/31 (19.3%) | 0.675 |
| MNV pattern | Glomerular = 5/12 (41.6%) Tree-like = 4/12 (33.3%) Fragmented = 3/12 (25.0%) | Glomerular = 23/67 (34.3%) Tree-like = 24/67 (35.8%) Fragmented = 20/67 (29.8%) | Glomerular = 8/31 (25.8%) Tree-like = 10/31 (32.3%) Fragmented = 13/31 (41.9%) | 0.744 |
| MNV morphology | Immature = 5/12 (41.6%) Mature = 5/12 (41.6%) Hypermature = 2/12 (16.7%) | Immature = 25/67 (37.3%) Mature = 22/67 (32.8%) Hypermature = 20/67 (29.9%) | Immature = 7/31 (22.6%) Mature = 12/31 (38.7%) Hypermature = 12/31 (38.7%) | 0.490 |
| MNV height at baseline (μm) | 1114.9 ± 33.3 | 1190.8 ± 42.0 | 1074.9 ± 35.6 | 0.784 |
| GLD at baseline (μm) | 1292.2 ± 195.6 | 1324.6 ± 135.6 | 1412.5 ± 110.9 | 0.003 * |
| VD at baseline (%) | 40.34 ± 6.1 | 40.92 ± 5.8 | 39.93 ± 5.7 | 0.592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savastano, M.C.; Crincoli, E.; Toto, L.; Grassi, M.O.; Chiosi, F.; Savastano, A.; Rizzo, C.; Mastropasqua, R.; Boscia, F.; Rizzo, S. Regression of the Flow Signal from the Neovascular Network in AMD Neovascular Membranes Treated with Faricimab. Diagnostics 2024, 14, 2653. https://doi.org/10.3390/diagnostics14232653
Savastano MC, Crincoli E, Toto L, Grassi MO, Chiosi F, Savastano A, Rizzo C, Mastropasqua R, Boscia F, Rizzo S. Regression of the Flow Signal from the Neovascular Network in AMD Neovascular Membranes Treated with Faricimab. Diagnostics. 2024; 14(23):2653. https://doi.org/10.3390/diagnostics14232653
Chicago/Turabian StyleSavastano, Maria Cristina, Emanuele Crincoli, Lisa Toto, Maria Oliva Grassi, Flavia Chiosi, Alfonso Savastano, Clara Rizzo, Rodolfo Mastropasqua, Francesco Boscia, and Stanislao Rizzo. 2024. "Regression of the Flow Signal from the Neovascular Network in AMD Neovascular Membranes Treated with Faricimab" Diagnostics 14, no. 23: 2653. https://doi.org/10.3390/diagnostics14232653
APA StyleSavastano, M. C., Crincoli, E., Toto, L., Grassi, M. O., Chiosi, F., Savastano, A., Rizzo, C., Mastropasqua, R., Boscia, F., & Rizzo, S. (2024). Regression of the Flow Signal from the Neovascular Network in AMD Neovascular Membranes Treated with Faricimab. Diagnostics, 14(23), 2653. https://doi.org/10.3390/diagnostics14232653









