The Prognostic Significance of CD47, CD68, and CD163 Expression Levels and Their Relationship with MLR and MAR in Locally Advanced and Oligometastatic Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Selection, and Collection of the Data
2.2. Treatment Details and Response Assessment
2.3. Immunohistochemical Study and CD47-CD68-CD163 Scoring System
2.4. Statistical Analysis
3. Results
3.1. Cut-Off Values of the Laboratory Parameters
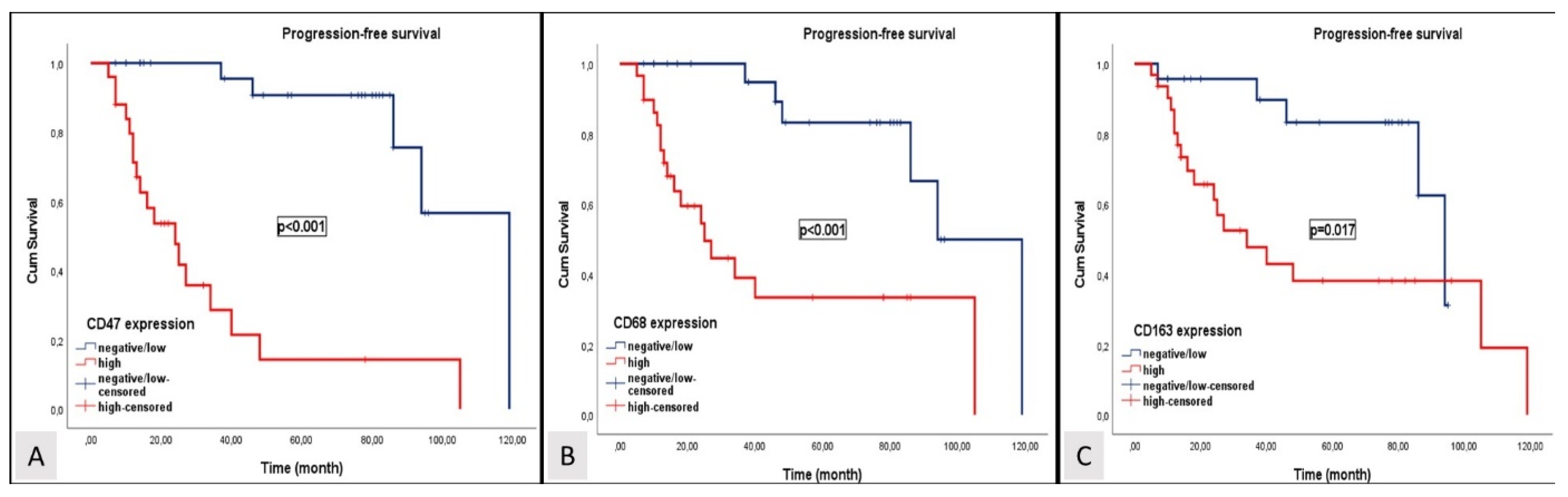
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Ye, W.; Zeng, Y.-X.; Adami, H.-O. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rumgay, H.; Li, M.; Cao, S.; Chen, W. Nasopharyngeal Cancer Incidence and Mortality in 185 Countries in 2020 and the Projected Burden in 2040: Population-Based Global Epidemiological Profiling. JMIR Public Health Surveill. 2023, 9, e49968. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Siak, P.Y.; Lwin, Y.Y.; Cheah, S.-C. Epidemiology of Nasopharyngeal Carcinoma: Current Insights and Future Outlook. Cancer Metastasis Rev. 2024, 43, 919–939. [Google Scholar] [CrossRef]
- London, A.O.; Gallagher, L.W.; Sharma, R.K.; Spielman, D.; Golub, J.S.; Overdevest, J.B.; Yan, C.H.; DeConde, A.; Gudis, D.A. Impact of Race, Ethnicity, and Socioeconomic Status on Nasopharyngeal Carcinoma Disease-Specific and Conditional Survival. J. Neurol. Surg. B Skull Base 2022, 83, 451–460. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal Carcinoma Ecology Theory: Cancer as Multidimensional Spatiotemporal “Unity of Ecology and Evolution” Pathological Ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal Carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Wong, K.C.W.; Hui, E.P.; Lo, K.-W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.-F.; King, A.D.; et al. Nasopharyngeal Carcinoma: An Evolving Paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef]
- Jiromaru, R.; Nakagawa, T.; Yasumatsu, R. Advanced Nasopharyngeal Carcinoma: Current and Emerging Treatment Options. Cancer Manag. Res. 2022, 14, 2681–2689. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Zhao, C.; Wang, J.; Wang, K.; Wang, L.; Miao, J.; Cao, C.; Jin, T.; Zhang, Y.; et al. Adding Concurrent Chemotherapy to Intensity-Modulated Radiotherapy Does Not Improve Treatment Outcomes for Stage II Nasopharyngeal Carcinoma: A Phase 2 Multicenter Clinical Trial. Front. Oncol. 2020, 10, 1314. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, G.-Q.; Zhang, N.; Zhu, X.-D.; Yang, K.-Y.; Jin, F.; Shi, M.; Chen, Y.-P.; Hu, W.-H.; et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Ismaila, N.; Chua, M.L.K.; Colevas, A.D.; Haddad, R.; Huang, S.H.; Wee, J.T.S.; Whitley, A.C.; Yi, J.-L.; Yom, S.S.; et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J. Clin. Oncol. 2021, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Y.; Li, W.-Z.; Wang, D.-S.; Liang, H.; Lv, X.; Ye, Y.-F.; Zhao, C.; Ke, L.-R.; Lv, S.-H.; Lu, N.; et al. Effect of Capecitabine Maintenance Therapy Plus Best Supportive Care vs Best Supportive Care Alone on Progression-Free Survival Among Patients With Newly Diagnosed Metastatic Nasopharyngeal Carcinoma Who Had Received Induction Chemotherapy. JAMA Oncol. 2022, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-Q.; Chen, Q.-Y.; Chen, D.; Hu, C.; Yang, K.; Wen, J.; Li, J.; Shi, Y.; Jin, F.; Xu, R.; et al. Toripalimab Plus Chemotherapy for Recurrent or Metastatic Nasopharyngeal Carcinoma. JAMA 2023, 330, 1961. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Siak, P.Y.; Leong, C.-O.; Cheah, S.-C. Nasopharyngeal Carcinoma and Its Microenvironment: Past, Current, and Future Perspectives. Front. Oncol. 2022, 12, 840467. [Google Scholar] [CrossRef]
- Forder, A.; Stewart, G.L.; Telkar, N.; Lam, W.L.; Garnis, C. New Insights into the Tumour Immune Microenvironment of Nasopharyngeal Carcinoma. Curr. Res. Immunol. 2022, 3, 222–227. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Bianconi, V.; Pirro, M.; Jaafari, M.R.; Hatamipour, M.; Sahebkar, A. CD47: Role in the Immune System and Application to Cancer Therapy. Cell. Oncol. 2020, 43, 19–30. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ye, Z.-H.; Huang, M.-Y.; Lu, J.-J. Regulation of CD47 Expression in Cancer Cells. Transl. Oncol. 2020, 13, 100862. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, H.; Xu, J.; Shen, C. CD47: The next Checkpoint Target for Cancer Immunotherapy. Crit. Rev. Oncol. Hematol. 2020, 152, 103014. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Yu, J.; Tian, W.; Song, Y. Targeting CD47 for Cancer Immunotherapy. J. Hematol. Oncol. 2021, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Li, C.; Liang, X.; Li, C.; Liu, Y.; Yi, Z.; Zhang, L.; Fu, S.; Zeng, Y. Role of CD47 in Tumor Immunity: A Potential Target for Combination Therapy. Sci. Rep. 2022, 12, 9803. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, Y.; Jin, K.; Fang, H.; Chen, Y.; Cao, Y.; Liu, X.; Lv, K.; He, X.; Lin, C.; et al. CD47 Expression in Gastric Cancer Clinical Correlates and Association with Macrophage Infiltration. Cancer Immunol. Immunother. 2021, 70, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, Y.; Wan, T.; Deng, T.; Huang, H.; Liu, J. Significance of CD47 and Its Association With Tumor Immune Microenvironment Heterogeneity in Ovarian Cancer. Front. Immunol. 2021, 12, 768115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Yang, L.; Li, H.; Li, R.; Yu, J.; Yang, L.; Wei, F.; Yan, C.; Sun, Q.; et al. Anti-CD47 Antibody As a Targeted Therapeutic Agent for Human Lung Cancer and Cancer Stem Cells. Front. Immunol. 2017, 8, 404. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Y.; Guan, J.-S.; Ngo, H.G.; Totoro, A.; Singh, A.P.; Chen, K.; Xu, Y.; Yang, E.S.; Zhou, L.; et al. Anti-CD47 Monoclonal Antibody–Drug Conjugate: A Targeted Therapy to Treat Triple-Negative Breast Cancers. Vaccines 2021, 9, 882. [Google Scholar] [CrossRef]
- Candas-Green, D.; Xie, B.; Huang, J.; Fan, M.; Wang, A.; Menaa, C.; Zhang, Y.; Zhang, L.; Jing, D.; Azghadi, S.; et al. Dual Blockade of CD47 and HER2 Eliminates Radioresistant Breast Cancer Cells. Nat. Commun. 2020, 11, 4591. [Google Scholar] [CrossRef]
- Chiang, Z.-C.; Fang, S.; Shen, Y.; Cui, D.; Weng, H.; Wang, D.; Zhao, Y.; Lin, J.; Chen, Q. Development of Novel CD47-Specific ADCs Possessing High Potency Against Non-Small Cell Lung Cancer in Vitro and in Vivo. Front. Oncol. 2022, 12, 857927. [Google Scholar] [CrossRef]
- Nishiga, Y.; Drainas, A.P.; Baron, M.; Bhattacharya, D.; Barkal, A.A.; Ahrari, Y.; Mancusi, R.; Ross, J.B.; Takahashi, N.; Thomas, A.; et al. Radiotherapy in Combination with CD47 Blockade Elicits a Macrophage-Mediated Abscopal Effect. Nat. Cancer 2022, 3, 1351–1366. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, X.; Ma, W. Opportunities and Challenges of CD47-Targeted Therapy in Cancer Immunotherapy. Oncol. Res. 2024, 32, 49–60. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The Complex Role of Tumor-Infiltrating Macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Furgiuele, S.; Descamps, G.; Lechien, J.R.; Dequanter, D.; Journe, F.; Saussez, S. Immunoscore Combining CD8, FoxP3, and CD68-Positive Cells Density and Distribution Predicts the Prognosis of Head and Neck Cancer Patients. Cells 2022, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Liu, F.; Yang, K. Role of CD68 in Tumor Immunity and Prognosis Prediction in Pan-Cancer. Sci. Rep. 2022, 12, 7844. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizadeh, F.; Tajik, F.; Talebi, M.; Taha, S.R.; Shariat Zadeh, M.; Farhangnia, P.; Hosseini, H.S.; Nazari, A.; Mollazadeh Ghomi, S.; Kamrani Mousavi, S.M.; et al. Unraveling the Potential of CD8, CD68, and VISTA as Diagnostic and Prognostic Markers in Patients with Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2024, 15, 1283364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-R.; Zhang, N.; Chen, S.-T.; He, J.; Liu, Y.-H.; Han, Y.-Q.; Shi, X.-Q.; Yang, J.-J.; Mu, D.-Y.; Fu, G.-H.; et al. PD-1-Positive Tumor-Associated Macrophages Define Poor Clinical Outcomes in Patients With Muscle Invasive Bladder Cancer Through Potential CD68/PD-1 Complex Interactions. Front. Oncol. 2021, 11, 679928. [Google Scholar] [CrossRef]
- Kato, S.; Okamura, R.; Kumaki, Y.; Ikeda, S.; Nikanjam, M.; Eskander, R.; Goodman, A.; Lee, S.; Glenn, S.T.; Dressman, D.; et al. Expression of TIM3/VISTA Checkpoints and the CD68 Macrophage-Associated Marker Correlates with Anti-PD1/PDL1 Resistance: Implications of Immunogram Heterogeneity. Oncoimmunology 2020, 9, 1708065. [Google Scholar] [CrossRef]
- Etzerodt, A.; Tsalkitzi, K.; Maniecki, M.; Damsky, W.; Delfini, M.; Baudoin, E.; Moulin, M.; Bosenberg, M.; Graversen, J.H.; Auphan-Anezin, N.; et al. Specific Targeting of CD163+ TAMs Mobilizes Inflammatory Monocytes and Promotes T Cell–Mediated Tumor Regression. J. Exp. Med. 2019, 216, 2394–2411. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Tian, J.; Man, H.; Li, P.; Shan, B. High Expression of B7-H3 and CD163 in Cancer Tissues Indicates Malignant Clinicopathological Status and Poor Prognosis of Patients with Urothelial Cell Carcinoma of the Bladder. Oncol. Lett. 2018, 15, 6519–6526. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, D.; Gong, B.; Wang, P.; Liu, F. CD163 as a Novel Target Gene of STAT3 Is a Potential Therapeutic Target for Gastric Cancer. Oncotarget 2017, 8, 87244–87262. [Google Scholar] [CrossRef]
- Ma, C.; Horlad, H.; Ohnishi, K.; Nakagawa, T.; Yamada, S.; Kitada, S.; Motoshima, T.; Kamba, T.; Nakayama, T.; Fujimoto, N.; et al. CD163-Positive Cancer Cells Are Potentially Associated with High Malignant Potential in Clear Cell Renal Cell Carcinoma. Med. Mol. Morphol. 2018, 51, 13–20. [Google Scholar] [CrossRef]
- Matsubara, E.; Komohara, Y.; Shinchi, Y.; Mito, R.; Fujiwara, Y.; Ikeda, K.; Shima, T.; Shimoda, M.; Kanai, Y.; Sakagami, T.; et al. CD163-positive Cancer Cells Are a Predictor of a Worse Clinical Course in Lung Adenocarcinoma. Pathol. Int. 2021, 71, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Maisel, B.A.; Yi, M.; Peck, A.R.; Sun, Y.; Hooke, J.A.; Kovatich, A.J.; Shriver, C.D.; Hu, H.; Nevalainen, M.T.; Tanaka, T.; et al. Spatial Metrics of Interaction between CD163-Positive Macrophages and Cancer Cells and Progression-Free Survival in Chemo-Treated Breast Cancer. Cancers 2022, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhao, Y.; Liu, X.; Sun Zhang, A.; Zhang, H.; Hu, G.; Sun, X.-F. CD163 as a Potential Biomarker in Colorectal Cancer for Tumor Microenvironment and Cancer Prognosis: A Swedish Study from Tissue Microarrays to Big Data Analyses. Cancers 2022, 14, 6166. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhou, L.; Li, X.; Bao, W.; Chen, T.; Xi, X.; He, Y.; Wan, X. Preoperative Monocyte-to-Lymphocyte Ratio in Peripheral Blood Predicts Stages, Metastasis, and Histological Grades in Patients with Ovarian Cancer. Transl. Oncol. 2017, 10, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-T.; Chen, X.-X.; Yang, X.-M.; He, S.-C.; Qian, F.-H. Application of Monocyte-to-Albumin Ratio and Neutrophil Percentage-to-Hemoglobin Ratio on Distinguishing Non-Small Cell Lung Cancer Patients from Healthy Subjects. Int. J. Gen. Med. 2023, 16, 2175–2185. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Gao, Z.; Zhao, Y.; Wen, Z.; Han, H.; Li, Y.; Hu, H.; Chen, H. Combination of CD47 and CD68 Expression Predicts Survival in Eastern-Asian Patients with Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 739–747. [Google Scholar] [CrossRef]
- Sugimura-Nagata, A.; Koshino, A.; Inoue, S.; Matsuo-Nagano, A.; Komura, M.; Riku, M.; Ito, H.; Inoko, A.; Murakami, H.; Ebi, M.; et al. Expression and Prognostic Significance of CD47–SIRPA Macrophage Checkpoint Molecules in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2690. [Google Scholar] [CrossRef]
- Yuan, J.; He, H.; Chen, C.; Wu, J.; Rao, J.; Yan, H. Combined High Expression of CD47 and CD68 Is a Novel Prognostic Factor for Breast Cancer Patients. Cancer Cell Int. 2019, 19, 238. [Google Scholar] [CrossRef]
- Ni, C.; Yang, L.; Xu, Q.; Yuan, H.; Wang, W.; Xia, W.; Gong, D.; Zhang, W.; Yu, K. CD68- and CD163-Positive Tumor Infiltrating Macrophages in Non-Metastatic Breast Cancer: A Retrospective Study and Meta-Analysis. J. Cancer 2019, 10, 4463–4472. [Google Scholar] [CrossRef]
- Jamiyan, T.; Kuroda, H.; Yamaguchi, R.; Abe, A.; Hayashi, M. CD68- and CD163-Positive Tumor-Associated Macrophages in Triple Negative Cancer of the Breast. Virchows Arch. 2020, 477, 767–775. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Pei, X.-F.; Zhu, Z.-Q.; Lin, Z.; Mao, Y.-Y.; Xu, X.-L.; Luo, Y.-L.; Zhang, L.; Peng, P.-J. CD47 Overexpression Is Associated with Epstein–Barr Virus Infection and Poor Prognosis in Patients with Nasopharyngeal Carcinoma. Onco Targets Ther. 2020, 13, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ke, L.; Xia, W.-X.; Xiang, Y.; Lv, X.; Bu, J. Elevated Levels of TNF-α and Decreased Levels of CD68-Positive Macrophages in Primary Tumor Tissues Are Unfavorable for the Survival of Patients With Nasopharyngeal Carcinoma. Technol. Cancer Res. Treat. 2019, 18, 153303381987480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L. Prognostic Significance of Tumor-Associated Macrophages in Patients with Nasopharyngeal Carcinoma. Medicine 2020, 99, e21999. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ke, L.; Lv, X.; Ling, Y.; Lu, J.; Liang, H.; Qiu, W.; Huang, X.; Liu, G.; Li, W.; et al. The Prognostic Significance of Carcinoma-Associated Fibroblasts and Tumor-Associated Macrophages in Nasopharyngeal Carcinoma. Cancer Manag. Res. 2018, 10, 1935–1946. [Google Scholar] [CrossRef]
- Deng, R.; Lu, J.; Liu, X.; Peng, X.-H.; Wang, J.; Li, X.-P. PD-L1 Expression Is Highly Associated with Tumor-Associated Macrophage Infiltration in Nasopharyngeal Carcinoma. Cancer Manag. Res. 2020, 12, 11585–11596. [Google Scholar] [CrossRef]




| Variables | CD47 Expression | CD68 Expression | CD163 Expression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative/Low | High | p * | Negative/Low | High | p * | Negative/Low | high | p * | ||
| n, (%) | n, (%) | n, (%) | n, (%) | n, (%) | n, (%) | |||||
| Age | <55 | 14 (48.3) | 5 (20) | 0.029 | 10 (40) | 9 (31) | 0.343 | 12 (52.2) | 7 (22.6) | 0.025 |
| ≥55 | 15 (51.7) | 20 (80) | 15 (60) | 20 (69) | 11 (47.8) | 24 (77.4) | ||||
| Sex | Male | 21 (72.4) | 22 (88) | 0.14 | 20 (80) | 23 (79.3) | 0.61 | 19 (82.6) | 24 (77.4) | 0.454 |
| Female | 8 (27.6) | 3 (12) | 5 (68) | 6 (20.7) | 4 (17.4) | 7 (22.6) | ||||
| ECOG PS | 0–1 | 29 (100) | 20 (80) | 0.017 | 24 (96) | 25 (86.2) | 0.225 | 22 (95.7) | 27 (87.1) | 0.283 |
| 2 | 0 (0) | 5 (20) | 1 (4) | 4 (13.8) | 1 (4.3) | 4 (12.9) | ||||
| Smoking status | No | 10 (34.5) | 10 (40) | 0.445 | 9 (36) | 11 (37.9) | 0.555 | 7 (30.4) | 13 (41.9) | 0.282 |
| Yes | 19 (65.5) | 15 (60) | 16 (64) | 18 (62.1) | 16 (69.6) | 18 (58.1) | ||||
| Comorbidity | No | 21 (72.4) | 16 (64) | 0.355 | 17 (68) | 20 (69) | 0.585 | 18 (78.3) | 19 (61.3) | 0.151 |
| Yes | 8 (27.6) | 9 (36) | 8 (32) | 9 (31) | 5 (21.7) | 12 (38.7) | ||||
| Alcohol consumption | No | 21 (72.4) | 20 (80) | 0.372 | 19 (76) | 22 (75.9) | 0.622 | 17 (73.9) | 24 (77.4) | 0.506 |
| Yes | 8 (27.6) | 5 (20) | 6 (24) | 7 (24.1) | 6 (26.1) | 7 (22.6) | ||||
| EBV-DNA copy number | Low | 17 (53.1) | 5 (20) | 0.004 | 13 (52) | 9 (31) | 0.099 | 13 (56.5) | 9 (29) | 0.04 |
| High | 12 (46.9) | 20 (80) | 12 (48) | 20 (69) | 10 (43.5) | 22 (71) | ||||
| Clinical stage | II-III | 22 (75.9) | 10 (40) | 0.008 | 20 (80) | 12 (41.4) | 0.004 | 17 (73.9) | 15 (48.4) | 0.053 |
| IVA-IVB | 7 (24.1) | 15 (60) | 5 (20) | 17 (58.6) | 6 (26.1) | 16 (51.6) | ||||
| Disease burden | Locoregional | 29 (100) | 17 (68) | 0.001 | 25 (100) | 21 (72.4) | 0.004 | 23 (100) | 23 (74.2) | 0.008 |
| Oligometastatic | 0 (0) | 8 (32) | 0 (0) | 8 (27.6) | 0 (0) | 8 (25.8) | ||||
| Response to CCRT | PD | 1 (3.4) | 6 (24) | <0.001 | 1 (4) | 6 (20.7) | <0.001 | 1 (4.3) | 6 (19.4) | 0.005 |
| SD | 3 (10.4) | 12 (48) | 1 (4) | 14 (48.3) | 3 (13.1) | 12 (38.7) | ||||
| PR + CR | 25 (86.2) | 7 (28) | 23 (92) | 9 (31) | 19 (82.6) | 13 (41.9) | ||||
| MLR | Low | 28 (96.6) | 7 (28) | <0.001 | 23 (92) | 12 (41.4) | <0.001 | 21 (91.3) | 14 (45.2) | <0.001 |
| High | 1 (3.4) | 18 (72) | 2 (8) | 17 (58.6) | 2 (8.7) | 17 (54.8) | ||||
| MAR | Low | 27 (93.1) | 8 (32) | <0.001 | 21 (84) | 14 (48.3) | 0.006 | 22 (95.7) | 13 (41.9) | <0.001 |
| High | 2 (6.1) | 17 (68) | 4 (16) | 15 (51.7) | 1 (4.3) | 18 (58.1) | ||||
| AUC | 95% CI | Sensitivity | Specificity | p | |
|---|---|---|---|---|---|
| MLR | 0.898 | 0.800–0.995 | 82.6% | 100% | <0.0001 |
| MAR | 0.870 | 0.766–0.973 | 82.6% | 77.4% | <0.0001 |
| CD47 | p | CD68 | p | CD163 | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative/Low | High | Negative/Low | High | Negative/Low | High | |||||||||
| CD68 | 0/low | 23 (79.3) | 2 (8.0) | <0.001 | CD163 | 0/low | 18 (72.0) | 5 (17.2) | <0.001 | CD47 | 0/low | 20 (87.0) | 9 (29.0) | <0.001 |
| high | 6 (20.7) | 23 (92.0) | high | 7 (28.0) | 24 (82.8) | high | 3 (13.0) | 22 (71.0) | ||||||
| CD163 | 0/low | 20 (69.0) | 3 (12.0) | <0.001 | CD47 | 0/low | 23 (92.0) | 6 (20.7) | <0.001 | CD68 | 0/low | 18 (78.3) | 7 (22.6) | <0.001 |
| high | 9 (31.0) | 22 (88.0) | high | 2 (8.0) | 23 (79.3) | high | 5 (21.7) | 24 (77.4) | ||||||
| Overall Survival | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age | 0.07 | 0.01–0.54 | 0.011 | Age | 0.17 | 0.02–1.54 | 0.115 |
| Sex | 0.51 | 0.17–1.53 | 0.228 | Sex | - | - | - |
| Smoking | 1.47 | 0.60–3.57 | 0.398 | Smoking | - | - | - |
| Alcohol | 0.12 | 0.02–0.86 | 0.035 | Alcohol | 0.61 | 0.07–5.28 | 0.652 |
| EBV-DNA | 3.08 | 1.43–10.10 | 0.007 | EBV-DNA | 5.17 | 0.94–28.54 | 0.059 |
| ECOG PS | 3.01 | 1.00–9.50 | 0.050 | ECOG PS | 1.49 | 0.35–6.35 | 0.587 |
| Clinical stage | 2.80 | 1.17–6.71 | 0.021 | Clinical stage | 0.59 | 0.22–1.59 | 0.29 |
| Disease burden | 4.40 | 1.63–11.87 | 0.003 | Disease burden | 2.74 | 0.65–11.48 | 0.169 |
| MLR | 84.93 | 11.06–652.3 | <0.001 | MLR | 187.14 | 6.79–5151.0 | 0.002 |
| MAR | 11.34 | 4.06–31.81 | <0.001 | MAR | 9.76 | 1.50–63.45 | 0.017 |
| CD47 | 19.84 | 4.50–87.57 | <0.001 | CD47 | 0.33 | 0.01–16.36 | 0.574 |
| CD68 | 5.79 | 1.92–17.42 | 0.002 | CD68 | 8.82 | 0.42–184.03 | 0.160 |
| CD163 | 3.31 | 1.21–9.06 | 0.019 | CD163 | 0.34 | 0.03–0.45 | 0.010 |
| Progression-Free Survival | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age | 0.87 | 0.01–0.65 | 0.017 | Age | 0.31 | 0.03–2.84 | 0.299 |
| Sex | 0.51 | 0.17–1.51 | 0.223 | Sex | - | - | - |
| Smoking | 1.30 | 0.54–3.12 | 0.558 | Smoking | - | - | - |
| Alcohol | 0.13 | 0.02–0.96 | 0.046 | Alcohol | 0.49 | 0.04–5.57 | 0.566 |
| EBV-DNA | 3.32 | 1.28–8.62 | 0.014 | EBV-DNA | 1.05 | 0.26–4.28 | 0.941 |
| ECOG PS | 2.89 | 0.96–8.72 | 0.060 | ECOG PS | - | - | - |
| Clinical stage | 2.66 | 1.10–6.40 | 0.029 | Clinical stage | 0.79 | 0.28–2.30 | 0.671 |
| Disease burden | 2.31 | 0.88–6.12 | 0.091 | Disease burden | - | - | - |
| MLR | 22.65 | 16.53–78.64 | <0.001 | MLR | 14.77 | 1.92–113.45 | 0.010 |
| MAR | 7.68 | 2.95–20.03 | <0.001 | MAR | 1.50 | 0.29–7.69 | 0.625 |
| CD47 | 11.80 | 3.86–36.10 | <0.001 | CD47 | 1.85 | 0.12–28.78 | 0.574 |
| CD68 | 5.39 | 1.95–14.90 | 0.001 | CD68 | 2.52 | 0.30–20.95 | 0.394 |
| CD163 | 3.21 | 1.17–8.84 | 0.024 | CD163 | 0.14 | 0.02–1.33 | 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, A.A.; Yuceer, R.O.; Yildirim, S.; Unlu, A.; Kayikcioglu, E.; Kocer, M. The Prognostic Significance of CD47, CD68, and CD163 Expression Levels and Their Relationship with MLR and MAR in Locally Advanced and Oligometastatic Nasopharyngeal Carcinoma. Diagnostics 2024, 14, 2648. https://doi.org/10.3390/diagnostics14232648
Aydin AA, Yuceer RO, Yildirim S, Unlu A, Kayikcioglu E, Kocer M. The Prognostic Significance of CD47, CD68, and CD163 Expression Levels and Their Relationship with MLR and MAR in Locally Advanced and Oligometastatic Nasopharyngeal Carcinoma. Diagnostics. 2024; 14(23):2648. https://doi.org/10.3390/diagnostics14232648
Chicago/Turabian StyleAydin, Asim Armagan, Ramazan Oguz Yuceer, Senay Yildirim, Ahmet Unlu, Erkan Kayikcioglu, and Murat Kocer. 2024. "The Prognostic Significance of CD47, CD68, and CD163 Expression Levels and Their Relationship with MLR and MAR in Locally Advanced and Oligometastatic Nasopharyngeal Carcinoma" Diagnostics 14, no. 23: 2648. https://doi.org/10.3390/diagnostics14232648
APA StyleAydin, A. A., Yuceer, R. O., Yildirim, S., Unlu, A., Kayikcioglu, E., & Kocer, M. (2024). The Prognostic Significance of CD47, CD68, and CD163 Expression Levels and Their Relationship with MLR and MAR in Locally Advanced and Oligometastatic Nasopharyngeal Carcinoma. Diagnostics, 14(23), 2648. https://doi.org/10.3390/diagnostics14232648






