Persistent Müllerian Duct Syndrome with Supernumerary Testicles Due to a Novel Homozygous Variant in the AMHR2 Gene and Literature Review
Abstract
1. Introduction
2. Case Report
2.1. At Age 4 Years
2.2. At Age 5 Years
2.3. At Age 9–12 Years
2.4. At Age 13 Years
2.5. Genetic Testing
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Josso, N.; Rey, R.A. What Does AMH Tell Us in Pediatric Disorders of Sex Development? Front. Endocrinol. 2020, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Renton, C.J.C. A Case of Polyorchidism with Intersex. J. Urol. 1975, 113, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.N.; Bhansali, M.S.; Tongaonkar, H.B.; Kamat, M.R.; Borges, A.M. Carcinoma in the Third Testis in a Case of Polyorchidism and Persistent Müllerian Structure Syndrome. Eur. Urol. 1992, 22, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Chirita-Emandi, A.; Andreescu, N.; Zimbru, C.G.; Tutac, P.; Arghirescu, S.; Serban, M.; Puiu, M. Challenges in Reporting Pathogenic/Potentially Pathogenic Variants in 94 Cancer Predisposing Genes—In Pediatric Patients Screened with NGS Panels. Sci. Rep. 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Tian, H.-J.; Wu, D.-H.; Ru, W.; Wu, D.-W.; Tao, C.; Chen, G.-J.; Yuan, J.-N.; Fu, J.-F.; Tang, D.-X. Surgical Management and Molecular Diagnosis of Persistent Müllerian Duct Syndrome in Chinese Patients. Asian J. Androl. 2022, 24, 78. [Google Scholar] [CrossRef]
- Silveri, M.; Zaccara, A.; Cappa, M. A Simplified Management of Transverse Testicular Ectopia in Patients with Persistent Mullerian Duct Syndrome. Urol. J. 2020, 18, 237–239. [Google Scholar] [CrossRef]
- Tosca, L.; Giltay, J.C.; Bouvattier, C.; Klijn, A.J.; Bouligand, J.; Lambert, A.S.; Lecerf, L.; Josso, N.; Tachdjian, G.; Picard, J.Y. Persistent Müllerian Duct Syndrome Due to Anti-Müllerian Hormone Receptor 2 Microdeletions: A Diagnostic Challenge. Hum. Reprod. 2020, 35, 999–1003. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, S.-O.; Yu, H.S.; Kim, S.-S.; Kwon, D.D. Transverse Testicular Ectopia Associated with Persistent Müllerian Duct Syndrome Treated by Transseptal Orchiopexy. Medicine 2018, 97, e13305. [Google Scholar] [CrossRef]
- Saleem, M.; Ather, U.; Mirza, B.; Iqbal, S.; Sheikh, A.; Shaukat, M.; Sheikh, M.T.; Ahmad, F.; Rehan, T. Persistent Mullerian Duct Syndrome: A 24-Year Experience. J. Pediatr. Surg. 2016, 51, 1721–1724. [Google Scholar] [CrossRef]
- Bowen, D.K.; Matulewicz, R.S.; Gong, E.M. Preservation of Müllerian Structures with Laparoscopic Management of Intra-Abdominal Testes in Persistent Müllerian Duct Syndrome. J. Pediatr. Urol. 2016, 12, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Moriya, K.; Ishizu, K.; Tajima, T. Two Heterozygous Mutations of the AMH Gene in a Japanese Patient with Persistent Müllerian Duct Syndrome. J. Pediatr. Endocrinol. Metab. 2014, 27, 1223–1226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ju, X.; Li, Z.; Zhang, C.; Qin, C.; Shao, P.; Li, J.; Li, P.; Cao, Q.; Zhang, W.; Wang, Z.; et al. Clinical Aspects and Molecular Genetics of Persistent Müllerian Duct Syndrome Associated with Transverse Testicular Ectopia: Report of Three Cases. Urol. Int. 2013, 90, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Zhapa, E.; Castagnetti, M.; Alaggio, R.; Talenti, E.; Rigamonti, W. Testicular Fusion in a Patient with Transverse Testicular Ectopia and Persistent Mullerian Duct Syndrome. Urology 2010, 76, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Wuerstle, M.; Lesser, T.; Hurwitz, R.; Applebaum, H.; Lee, S.L. Persistent Mullerian Duct Syndrome and Transverse Testicular Ectopia: Embryology, Presentation, and Management. J. Pediatr. Surg. 2007, 42, 2116–2119. [Google Scholar] [CrossRef]
- Marjanović, Z.O.; Perović, S.V.; Slavković, A.; Živanović, D.; Đorđević, I. Transverse Testicular Ectopia with and without Persistent Müllerian Duct Syndrome. Int. Urol. Nephrol. 2007, 39, 1167–1171. [Google Scholar] [CrossRef]
- Mouli, K.; Mccarthy, P.; Ray, P.; Ray, V.; Rosenthal, I.M. Persistent Müllerian Duct Syndrome in a Man with Transverse Testicular Ectopia. J. Urol. 1988, 139, 373–375. [Google Scholar] [CrossRef]
- Sloan, W.R.; Walsh, P.C. Familial Persistent Müllerian Duct Syndrome. J. Urol. 1976, 115, 459–461. [Google Scholar] [CrossRef]
- Fang, J.; Gao, G.; Liu, J.; Cai, L.; Cui, Y.; Yang, X. A Novel Mutation of AMHR2 in Two Brothers with Persistent Müllerian Duct Syndrome and Their Intracytoplasmic Sperm Injection Outcome. Mol. Genet. Genom. Med. 2021, 9, e1801. [Google Scholar] [CrossRef]
- Lima, M.; Morabito, A.; Libri, M.; Bertozzi, M.; Dòmini, M.; Lauro, V.; Strano, C.; Messina, P.; Tani, G. Laparoscopic Removal of a Persistent Müllerian Duct in a Male: Case Report. Eur. J. Pediatr. Surg. 2000, 10, 265–269. [Google Scholar] [CrossRef]
- Zhou, W.; Li, S.; Wang, H.; Yin, J.; Liu, X.; Jiang, J.; Zhou, G.; Wen, J. Diagnostic Value of Ultrasound in Children with Transverse Testicular Ectopia. Front. Pediatr. 2022, 10, 914139. [Google Scholar] [CrossRef] [PubMed]
- Dhua, A.; Varshney, A.; Bhatnagar, V. Transverse Testicular Ectopia with a Blind Ending Vas Deferens. Indian J. Urol. 2016, 32, 317. [Google Scholar] [CrossRef] [PubMed]
- Sancar, S.; Ozcakir, E.; Kaya, M. Management of the Patients with Persistent Müllerian Duct Syndrome: Is the Ultimate Goal Testicular Descent? Türk. Urol. Derg./Turk. J. Urol. 2018, 44, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.K.; Thapa, D.K.; Pokhrel, D.; Shah, A.K. Persistent Mullerian Duct Syndrome with Polysplenia and Short Pancreas: A Case Report. J. Nepal Med. Assoc. 2019, 57, 123. [Google Scholar] [CrossRef] [PubMed]
- Farikullah, J.; Ehtisham, S.; Nappo, S.; Patel, L.; Hennayake, S. Persistent Müllerian Duct Syndrome: Lessons Learned from Managing a Series of Eight Patients over a 10-year Period and Review of Literature Regarding Malignant Risk from the Müllerian Remnants. BJU Int. 2012, 110, E1084–E1089. [Google Scholar] [CrossRef]
- Imbeaud, S.; Rey, R.; Berta, P.; Chaussain, J.-L.; Wit, J.-M.; Lustig, R.H.; Picard, J.-Y.; Josso, N. Testicular Degeneration in Three Patients with the Persistent Müllerian Duct Syndrome. Eur. J. Pediatr. 1995, 154, 187–190. [Google Scholar] [CrossRef]
- Scott, J.E.S. The Hutson Hypothesis A Clinical Study. Br. J. Urol. 1987, 60, 74–76. [Google Scholar] [CrossRef]
- Shah, D.S.; Shah, U.S.; Kumaresan, N. Persistent Mullerian Duct Syndrome: Rare Presentation in an Elderly Man. BMJ Case Rep. 2020, 13, e234890. [Google Scholar] [CrossRef]
- Aw, L.D.; Zain, M.M.; Esteves, S.C.; Humaidan, P. Persistent Mullerian Duct Syndrome: A Rare Entity with a Rare Presentation in Need of Multidisciplinary Management. Int. Braz. J. Urol. 2016, 42, 1237–1243. [Google Scholar] [CrossRef]
- Østergren, P.; Juul, A.; Azawi, N.H. Disorders of Sex Development Presenting as Unilateral Cryptorchidism. Scand. J. Urol. 2013, 47, 433–436. [Google Scholar] [CrossRef]
- Demir, O.; Kizer, O.; Sen, V.; Esen, A.A. Persistent Mullerian Duct Syndrome in Adult Men Diagnosed Using Laparoscopy. Urology 2011, 78, 566. [Google Scholar] [CrossRef] [PubMed]
- Beyribey, S.; Çetinkaya, M.; Adsan, Ö.; Memis, A.; Öztürk, B. Persistent Müllerian Duct Syndrome. Scand. J. Urol. Nephrol. 1993, 27, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chen, J.-H.; Lu, H.-F.; Shen, W.-C. Persistent Müllerian Duct Syndrome with Seminoma. Am. J. Roentgenol. 2000, 174, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Imbeaud, S. A 27 Base-Pair Deletion of the Anti-Mullerian Type II Receptor Gene Is the Most Common Cause of the Persistent Mullerian Duct Syndrome. Hum. Mol. Genet. 1996, 5, 1269–1277. [Google Scholar] [CrossRef]
- Adamsbaum, C.; Rolland, Y.; Josso, N.; Kalifa, G. Radiological Findings in Three Cases of Persistent Müllerian Duct Syndrome. Pediatr. Radiol. 1993, 23, 55–56. [Google Scholar] [CrossRef]
- Sheehan, S.J.; Tobbia, I.N.; Ismail, M.A.; Kelly, D.G.; Duff, F.A. Persistent Müllerian Duct Syndrome: Review and Report of 3 Cases. Br. J. Urol. 1985, 57, 548–551. [Google Scholar] [CrossRef]
- Barad, A.K. Persistent Mullerian Duct Syndrome with Embryonal Cell Carcinoma along with Ectopic Cross Fused Kidney. J. Clin. Diagn. Res. 2016, 10, PD07. [Google Scholar] [CrossRef]
- Thiel, D.D.; Erhard, M.J. Uterine Adenosarcoma in a Boy with Persistent Müllerian Duct Syndrome: First Reported Case. J. Pediatr. Surg. 2005, 40, e29–e31. [Google Scholar] [CrossRef]
- Cansaran, S. Management of Transverse Testicular Ectopia with Persistent Mullerian Duct Syndrome. North Clin. Istanb. 2018, 5, 357–360. [Google Scholar] [CrossRef]
- Urioste, M.; Rodríguez, J.I.; Barcia, J.M.; Martín, M.; Escribá, R.; Pardo, M.; Camino, J.; Frías, M.L.M. Persistence of Müllerian Derivatives, Lymphangiectasis, Hepatic Failure, Postaxial Polydactyly, Renal and Craniofacial Anomalies. Am. J. Med. Genet. 1993, 47, 494–503. [Google Scholar] [CrossRef]
- Pappis, C.; Constantinides, C.; Chiotis, D.; Dacou-Voutetakis, C. Persistent Müllerian Duct Structures in Cryptorchid Male Infants: Surgical Dilemmas. J. Pediatr. Surg. 1979, 14, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Barriola, M.; Jimenez, J.; Gutierrez-Hoyos, A.; Tovar, J. Cryptorchidism and Persistence of Müllerian Remnants in a Normal Male. Eur. J. Pediatr. Surg. 1981, 33, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Dimasis, N.; Koukourikis, P.; Klampatsas, A.; Xirou, P.; Sountoulides, P. A Unique Case of Aggressive Uterine Cancer in a 45-Year-Old Man with Persistent Müllerian Duct Syndrome. Arch. Esp. Urol. 2019, 72, 435–438. [Google Scholar] [PubMed]
- Hossaini, D.; Wahdat, M.M.; Aklaqi, A.; Haidary, M. A Rare Case Report of Orchiopexy and Hysterectomy in an Afghan Boy with Persistent Müllerian Duct Syndrome. Int. J. Surg. Case Rep. 2024, 115, 109235. [Google Scholar] [CrossRef] [PubMed]
- van Haelst, M.M.; Hoogeboom, J.; Galjaard, R.H.; Kleijer, W.J.; den Hollander, N.S.; de Krijger, R.R.; Hennekam, R.C.M.; Niermeijer, M.F. Lymphangiectasia with Persistent Müllerian Derivatives: Confirmation of Autosomal Recessive Urioste Syndrome. Am. J. Med. Genet. 2001, 104, 65–68. [Google Scholar] [CrossRef]
- Bellini, C.; Bonioli, E.; Josso, N.; Belville, C.; Mazzella, M.; Costabel, S.; Sementa, A.R.; Marino, C.E.; Tomà, P.; Hennekam, R.C.M.; et al. Persistence of Müllerian Derivatives and Intestinal Lymphangiectasis in Two Newborn Brothers: Confirmation of the Urioste Syndrome. Am. J. Med. Genet. 2001, 104, 69–74. [Google Scholar] [CrossRef]
- Cass, D.T.; Hutson, J. Association of Hirschsprung’s Disease and Müllerian Inhibiting Substance Deficiency. J. Pediatr. Surg. 1992, 27, 1596–1599. [Google Scholar] [CrossRef]
- Cass, D. Aganglionosis: Associated Anomalies. J. Paediatr. Child Health 1990, 26, 351–354. [Google Scholar] [CrossRef]
- Picard, J.-Y.; Morin, G.; Devouassoux-Shisheboran, M.; Van der Smagt, J.; Klosowski, S.; Pienkowski, C.; Pierre-Renoult, P.; Masson, C.; Bole, C.; Josso, N. Persistent Müllerian Duct Syndrome Associated with Genetic Defects in the Regulatory Subunit of Myosin Phosphatase. Hum. Reprod. 2022, 37, 2952–2959. [Google Scholar] [CrossRef]
- Klosowski, S.; Abriak, A.; Morisot, C.; Bayart, H.; Belville, C.; Thelliez, P.; Gottrand, F.; Croquette, M.; Deroubaix, P. Atrésie Jejunale et Syndrome de Persistance Des Dérivés Müllériens. Arch. Pédiatr. 1997, 4, 1264–1265. [Google Scholar] [CrossRef]
- Bugrul, F.; Abali, Z.Y.; Kirkgoz, T.; Cerit, K.K.; Canmemis, A.; Turan, S.; Tugtepe, H.; Picard, J.-Y.; Bereket, A.; Guran, T. Persistent Müllerian Duct Syndrome: A Rare But Important Etiology of Inguinal Hernia and Cryptorchidism. Sex. Dev. 2019, 13, 264–270. [Google Scholar] [CrossRef]
- Josso, N.; Fekete, C.; Cachin, O.; Nezelof, C.; Rappaport, R. Persistence of müllerian ducts in male pseudohermaphroditism, and its relationship to cryptorchidism. Clin. Endocrinol. 1983, 19, 247–258. [Google Scholar] [CrossRef]
- Dadheech, D. A Rare Case Report of Inguinal Hernia with Persistent Mullerian Duct and Klinefelter Syndrome. J. Clin. Diagn. Res. 2016, 10, PD28. [Google Scholar] [CrossRef]
- Rehman, A.; Hasan, Z.; Amanat, S.; Shaukat, T.; Saeed, A.; Jamil, K.; Zaidi, A.; Akram, M. Combined Persistent Mullerian Duct Syndrome, Transverse Testicular Ectopia and Mosaic Klinefelter’s Syndrome. J. Coll. Physicians Surg. Pak. 2008, 18, 375–377. [Google Scholar]
- Delaney, D.P.; Kolon, T.F.; Zderic, S.A. Persistent Mullerian Duct Syndrome Associated with 47,XXY Genotype. J. Urol. 2004, 171, 852–853. [Google Scholar] [CrossRef]
- Panesar, N.S.; Yeung, V.T.F.; Chan, J.C.N.; Shek, C.C.; Nicholls, M.G.; Cockram, C.S. 17α-Hydroxylase Deficiency with Persistence of Müllerian Ducts in a Genotypic Male and Paradoxical Aldosterone Secretion. Postgrad. Med. J. 1993, 69, 159–162. [Google Scholar] [CrossRef][Green Version]
- Van Maldergem, L.; Bachy, A.; Feldman, D.; Bouillon, R.; Maassen, J.; Dreyer, M.; Rey, R.; Holm, C.; Gillerot, Y. Syndrome of Lipoatrophic Diabetes, Vitamin D Resistant Rickets, and Persistent Müllerian Ducts in a Turkish Boy Born to Consanguineous Parents. Am. J. Med. Genet. 1996, 64, 506–513. [Google Scholar] [CrossRef]
- Nilson, O. Hernia Uteri Inguinalis Beim Manne. Acta Chir. Scand. 1939, 83, 231–249. [Google Scholar]
- Hutson, J.M.; Baker, M.L. A Hypothesis to Explain Abnormal Gonadal Descent in Persistent Mllerian Duct Syndrome. Pediatr. Surg. Int. 1994, 9, 542–543. [Google Scholar] [CrossRef]
- Picard, J.-Y.; Cate, R.L.; Racine, C.; Josso, N. The Persistent Müllerian Duct Syndrome: An Update Based Upon a Personal Experience of 157 Cases. Sex. Dev. 2017, 11, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Grover, S.R.; O’connell, M.A.; Bouty, A.; Hanna, C.A. An Integrated Approach to Management Disorders/Differences of Sex Development, 2nd ed.; Springer: Singapore, 2020. [Google Scholar]
- Shenoy, K.; Dama, S.; Makam, R. Persistent Müllerian Duct Syndrome: A Surgical Surprise and Management during Laparoscopic Transabdominal Pre-Peritoneal Repair. J. Minim. Access Surg. 2023, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Chua, I.; Samnakay, N. Persistent Müllerian Duct Syndrome: Understanding the Challenges. Case Rep. Urol. 2022, 2022, 2643833. [Google Scholar] [CrossRef] [PubMed]
- Cools, M.; Nordenström, A.; Robeva, R.; Hall, J.; Westerveld, P.; Flück, C.; Köhler, B.; Berra, M.; Springer, A.; Schweizer, K.; et al. Caring for Individuals with a Difference of Sex Development (DSD): A Consensus Statement. Nat. Rev. Endocrinol. 2018, 14, 415–429. [Google Scholar] [CrossRef] [PubMed]
- León, N.Y.; Reyes, A.P.; Harley, V.R. A Clinical Algorithm to Diagnose Differences of Sex Development. Lancet Diabetes Endocrinol. 2019, 7, 560–574. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Achermann, J.; Alderson, J.; Crouch, N.S.; Elford, S.; Hughes, I.A.; Krone, N.; McGowan, R.; Mushtaq, T.; O’Toole, S.; et al. Society for Endocrinology UK Guidance on the Initial Evaluation of a Suspected Difference or Disorder of Sex Development (Revised 2021). Clin. Endocrinol. 2021, 95, 818–840. [Google Scholar] [CrossRef]
- Wherrett, D.K. Approach to the Infant with a Suspected Disorder of Sex Development. Pediatr. Clin. N. Am. 2015, 62, 983–999. [Google Scholar] [CrossRef]
- Özdemir, M.; Kavak, R.P.; Yalcinkaya, I.; Guresci, K. Ovotesticular Disorder of Sex Development: An Unusual Presentation. J. Clin. Imaging Sci. 2019, 9, 34. [Google Scholar] [CrossRef]
- Kanakatti Shankar, R.; Dowlut-McElroy, T.; Dauber, A.; Gomez-Lobo, V. Clinical Utility of Anti-Mullerian Hormone in Pediatrics. J. Clin. Endocrinol. Metab. 2022, 107, 309–323. [Google Scholar] [CrossRef]
- Unal, E.; Haspolat, Y.K.; Yıldırım, R.; Tekin, S.; Demir, V.; Onay, H. A Novel Mutation of AMHR2 in Two Siblings with Persistent Müllerian Duct Syndrome. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 387. [Google Scholar] [CrossRef]
- Brunello, F.G.; Rey, R.A. AMH and AMHR2 Involvement in Congenital Disorders of Sex Development. Sex. Dev. 2022, 16, 138–146. [Google Scholar] [CrossRef]
- Chen, H.; Lin, P.; Yuan, X.; Chen, R. Two Novel AMHR2 Gene Variants in Monozygotic Twins with Persistent Müllerian Duct Syndrome: A Case Report and Functional Study. Mol. Genet. Genom. Med. 2022, 10, e1999. [Google Scholar] [CrossRef]
- Imbeaud, S.; Faure, E.; Lamarre, I.; Mattéi, M.-G.; di Clemente, N.; Tizard, R.; Carré-Eusèbe, D.; Belville, C.; Tragethon, L.; Tonkin, C.; et al. Insensitivity to Anti–Müllerian Hormone Due to a Mutation in the Human Anti–Müllerian Hormone Receptor. Nat. Genet. 1995, 11, 382–388. [Google Scholar] [CrossRef]
- Fernández-Cancio, M.; Viswanath, N.; Puzhankara, R.; Valiyaprambil Pavithran, P.; Mora-Palma, C.; Camats, N.; Audí, L.; Benito-Sanz, S. A Novel Homozygous AMRH2 Gene Mutation in a Patient with Persistent Müllerian Duct Syndrome. Sex. Dev. 2019, 13, 87–91. [Google Scholar] [CrossRef]
- Belville, C.; Marechal, J.-D.; Pennetier, S.; Carmillo, P.; Masgrau, L.; Messika-Zeitoun, L.; Galey, J.; Machado, G.; Treton, D.; Gonzales, J.; et al. Natural Mutations of the Anti-Mullerian Hormone Type II Receptor Found in Persistent Mullerian Duct Syndrome Affect Ligand Binding, Signal Transduction and Cellular Transport. Hum. Mol. Genet. 2009, 18, 3002–3013. [Google Scholar] [CrossRef]
- Howard, J.A.; Hart, K.N.; Thompson, T.B. Molecular Mechanisms of AMH Signaling. Front. Endocrinol. 2022, 13, 927824. [Google Scholar] [CrossRef]
- Hughes, L.A.; McKay-Bounford, K.; Webb, E.A.; Dasani, P.; Clokie, S.; Chandran, H.; McCarthy, L.; Mohamed, Z.; Kirk, J.M.W.; Krone, N.P.; et al. Next Generation Sequencing (NGS) to Improve the Diagnosis and Management of Patients with Disorders of Sex Development (DSD). Endocr. Connect. 2019, 8, 100–110. [Google Scholar] [CrossRef]
- Carachi, R.; Helmi, S.; Doss, E. Clinical Embryology 123; Springer International Publishing AG: Cham, Switzerland, 2019. [Google Scholar]
- Pliszka, A.; Wawrzyniak, A.; Walocha, J.; Musiał, A.; Bonczar, M.; Ostrowski, T.; Polguj, M.; Wysiadecki, G.; Clarke, E.; Tubbs, R.S.; et al. Embryological Basis of Polyorchidism Including Classification, Reproductive Potential, and Risk of Malignancy: A Review. Clin. Anat. 2023, 37, 405–412. [Google Scholar] [CrossRef]
- Picard, J.-Y.; Josso, N. Persistent Müllerian Duct Syndrome: An Update. Reprod. Fertil. Dev. 2019, 31, 1240. [Google Scholar] [CrossRef]
- Philips, M.R.; Menon, A.R.; Kumar, G.R.; Malik, K.; Chandrasekaran, S.; Ramaswamy, T.; Narayanaswamy, K.; Raja, A. Testicular Malignancy in Persistent Mullerian Duct Syndrome: Experience from an Apex Cancer Center with Review of Literature. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 258.e1–258.e6. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.B.; Aldhowayan, A.; Almuhanna, M.M.; Alghamdi, A.M. Persistent Mullerian Duct Syndrome (PMDS): Case Report and Review of Literature. Urol. Case Rep. 2022, 42, 102031. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Fattal, A.; Ouerdane, Y.; Alsuliman, T.; Kanjawi, O. A 35-Year-Old Father with Persistent Mullerian Duct Syndrome and Seminoma of the Right Undescended Testis: A Rare Case Report. Surg. Case Rep. 2021, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Modi, J.; Modi, D.; Bachani, L. Acute Urinary Retention Caused by Seminoma in a Case of Persistent Mullerian Duct Syndrome. Indian J. Pathol. Microbiol. 2015, 58, 83. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.; Dangmali, D.; Vishwasrao, S.; Puranik, S. Persistent Mullerian Duct Syndrome with Testicular Seminoma in Transverse Testicular Ectopia. J. Hum. Reprod. Sci. 2018, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; Tartas, S.; Lazareth, A.; Jaouen, A.; Tamarelle, B.; You, B.; Maillet, D. Pure Seminoma and Concurrent Aggressive Lymphoma: Case Report of a Patient With Persistent Müllerian Duct Syndrome. Clin. Genitourin. Cancer 2019, 17, e369–e371. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-Y.; Tsai, J.-W.; Wang, H.-P.; Sung, Y.-Z.; Chang, L.-C. Hernia Uterine Inguinale and Seminoma in Persistent Müllerian Duct Syndrome. Int. J. Surg. Pathol. 2010, 18, 440–442. [Google Scholar] [CrossRef]
- Feki, J.; Ennouri, S.; Frikha, R.; Keskes, L.; Boudawara, T.; Kammoun, H.; Rebai, T.; Slimen, M.H.; Khanfir, A. Germ Cell Tumors Revealing a Familial Persistent Müllerian Duct Syndrome. Gulf J. Oncol. 2022, 1, 71–73. [Google Scholar]
- Gul, U.J.; Hussain Zaidi, S.A.; Medhat, N.; Ahmad, D.; Khawaja, F.G. Persistent Mullerian Duct Syndrome. J. Ayub Med. Coll. Abbottabad 2021, 33 (Suppl. S1), S818–S822. [Google Scholar]
- Mohapatra, M.; Subramanya, Y. Persistent Müllerian Duct Syndrome of Mixed Anatomical Variant (Combined Male and Female Type) with Mixed Germ Cell Tumor of Left Intra-Abdominal Testis. Indian J. Pathol. Microbiol. 2016, 59, 212. [Google Scholar] [CrossRef]
- Al Harbi, T.Z.; Azzam, K.A.; Azzam, A.; Amin, T.; Bakshi, N. Incidentally Discovered Persistent Müllerian Duct Syndrome in a 45-Year-Old Male Presenting with Germ Cell Tumor and Bilateral Cryptorchidism: A Rare Case Report and Review of the Literature. Int. J. Surg. Case Rep. 2018, 43, 41–44. [Google Scholar] [CrossRef]
- Manassero, F.; Cuttano, M.G.; Morelli, G.; Salinitri, G.; Spurio, M.; Selli, C. Mixed Germ Cell Tumor after Bilateral Orchidopexy in Persistent Müllerian Duct Syndrome with Transverse Testicular Ectopia. Urol. Int. 2004, 73, 81–83. [Google Scholar] [CrossRef]
- Acero, M.G.; Moreno, O.; Gutiérrez, A.; Sánchez, C.; Cataño, J.G.; Suárez-Obando, F.; Rojas, A. Novel Homozygous Mutation in a Colombian Patient with Persistent Müllerian Duct Syndrome: Expanded Phenotype. Int. Braz. J. Urol. 2019, 45, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S.; Eren, H.; Acehan, T.; Bedir, R.; Gündoğdu, H.; Yüksel, A.O. A Rare Form of Persistent Mullerian Duct Syndrome: Transverse Testicular Ectopia with Germ Cell Testis Cancer and Hernia Uteri Inguinalis. Andrologia 2021, 53, e14229. [Google Scholar] [CrossRef]
- Samet, A.; Mseddi, M.A.; Bahloul, R.; Rebai, N.; Bahloul, A.; Slimène, M.H. Cancer on Cryptorchid Testis Revealing a Persistent Müllerian Duct Syndrome: A Rare Case. Urol. Case Rep. 2019, 26, 100977. [Google Scholar] [CrossRef]
- Kolon, T.F.; Herndon, C.D.A.; Baker, L.A.; Baskin, L.S.; Baxter, C.G.; Cheng, E.Y.; Diaz, M.; Lee, P.A.; Seashore, C.J.; Tasian, G.E.; et al. Evaluation and Treatment of Cryptorchidism: AUA Guideline. J. Urol. 2014, 192, 337–345. [Google Scholar] [CrossRef]
- Shinmura, Y.; Yokoi, T.; Tsutsui, Y. A Case of Clear Cell Adenocarcinoma of the Müllerian Duct in Persistent Müllerian Duct Syndrome. Am. J. Surg. Pathol. 2002, 26, 1231–1234. [Google Scholar] [CrossRef]
- Kovachev, S.M.; Nikolov, S.D.; Mihova, A.P. Uterine Leiomyoma in a Man with Persistent Müllerian Duct Syndrome and Seminoma. Isr. Med. Assoc. J. 2014, 16, 735–737. [Google Scholar]
- Gagliardi, F.; Lauro, A.; De Anna, L.; Tripodi, D.; Esposito, A.; Forte, F.; Pironi, D.; Lori, E.; Gentile, P.A.; Marino, I.R.; et al. The Risk of Malignant Degeneration of Müllerian Derivatives in PMDS: A Review of the Literature. J. Clin. Med. 2023, 12, 3115. [Google Scholar] [CrossRef]
- Umair, M.; Khan, A.U.; Arruda, J.B.; Lakhani, D.A.; Adelanwa, A.; Hadi, Y.B.; Markovich, B.; Salkini, M.W. Prostatic Adenocarcinoma in a Patient with Persistent Müllerian Duct Syndrome. Urol. Ann. 2022, 14, 398–402. [Google Scholar] [CrossRef]
- McCroskey, Z.; Koen, T.M.; Lim, D.J.; Divatia, M.K.; Shen, S.S.; Ayala, A.G.; Ro, J.Y. Prostatic Adenocarcinoma in the Setting of Persistent Müllerian Duct Syndrome: A Case Report. Hum. Pathol. 2018, 75, 125–131. [Google Scholar] [CrossRef]
- Ren, X.; Wu, D.; Gong, C. Persistent Mullerian Duct Syndrome: A Case Report and Review. Exp. Ther. Med. 2017, 14, 5779–5784. [Google Scholar] [CrossRef]
- Agrawal, A.S.; Kataria, R. Persistent Müllerian Duct Syndrome (PMDS): A Rare Anomaly the General Surgeon Must Know About. Indian J. Surg. 2015, 77, 217–221. [Google Scholar] [CrossRef][Green Version]
- Du, Q.; Qiu, C.; Zhang, L.; Wang, Q.-Y.; Hong, K.; Liu, X.-L. Persistent Müllerian Duct Syndrome in an Assisted Reproductive Patient: A Novel Variant Impairs the Biosynthesis and Secretion of Anti-Müllerian Hormone (AMH). Asian J. Androl. 2023, 25, 534–536. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, R.; Yang, J. Identification of AMH and AMHR2 Variants Led to the Diagnosis of Persistent Müllerian Duct Syndrome in Three Cases. Genes 2022, 13, 159. [Google Scholar] [CrossRef]
- Natarajan, S.; Periasamy, M.; Rangasamy, S.; Mohan, S.; Sundararajan, P. Persistent Mullerian Duct Syndrome: A Single-Center Experience. J. Indian Assoc. Pediatr. Surg. 2018, 23, 203. [Google Scholar] [CrossRef]
- Niazi, S.A.K.; Mukhtar, M.U.; Hassan, R.; Mehmood, Q. Lessons Learned from Five Patients of Persistent Mullerian Duct Syndrome: A Case Series. Int. J. Surg. Case Rep. 2022, 97, 107459. [Google Scholar] [CrossRef]
- Manjunath, B.G.; Shenoy, V.G.; Raj, P. Persistent Müllerian Duct Syndrome: How to Deal with the Müllerian Duct Remnants—A Review. Indian J. Surg. 2010, 72, 16–19. [Google Scholar] [CrossRef]
- Patil, V.; Muktinaini, S.; Patil, R.; Verma, A. Persistent Müllerian Duct Syndrome: A Case Report. Indian J. Surg. 2013, 75, 460–462. [Google Scholar] [CrossRef]
- Mattone, M.C.; Lobo de la Vega, M.V.; Redondo, E.J.; D’Alessandro, P.; Perez Garrido, N.; Galluzzo, M.L.; Costanzo, M.; Zaidman, V.; Lazzati, J.M.; Berensztein, E.; et al. A Surgical and Clinical Approach to Persistent Müllerian Duct Syndrome: Laparoscopic, Histological, and Molecular Findings. Sex. Dev. 2023, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
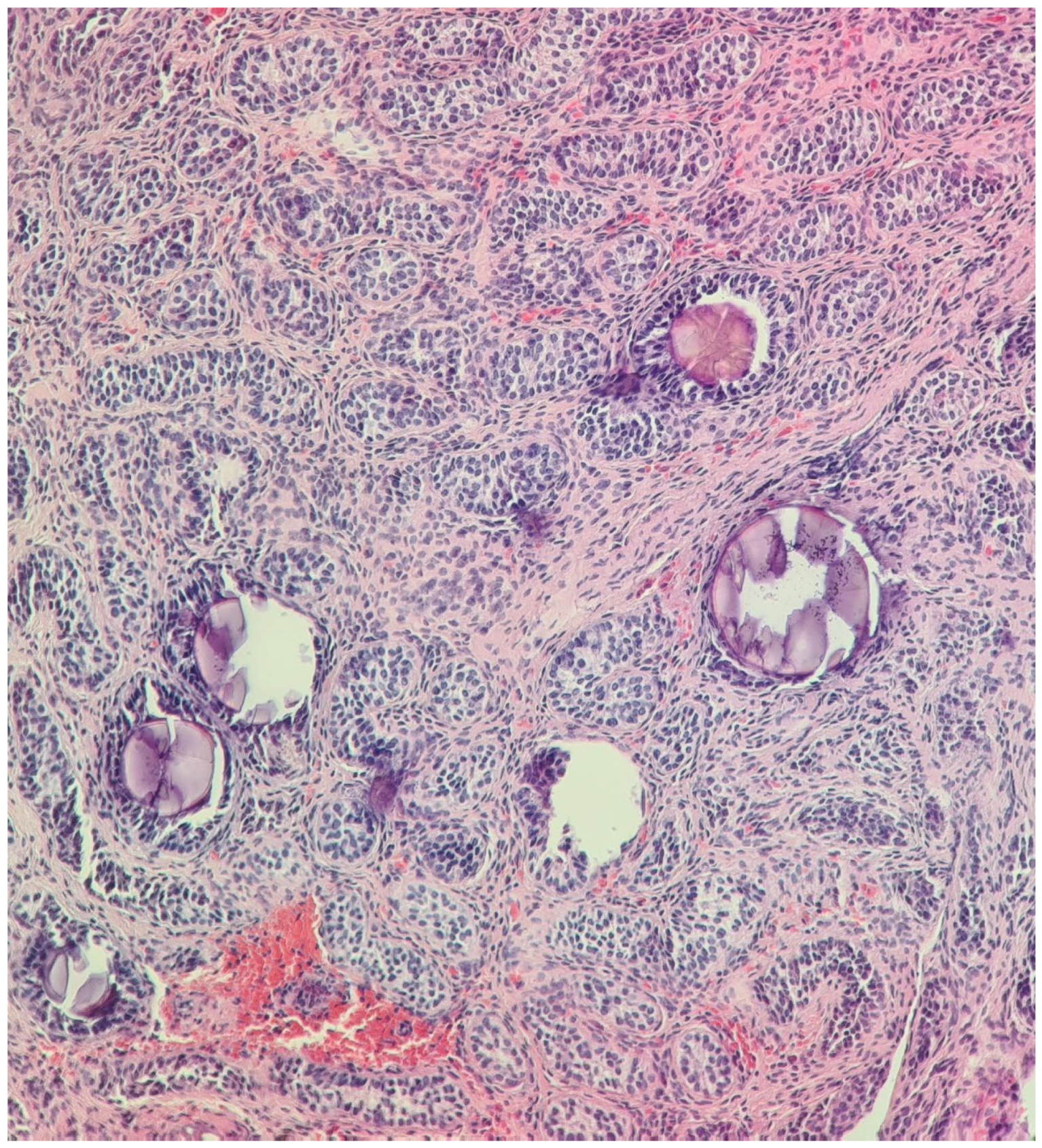
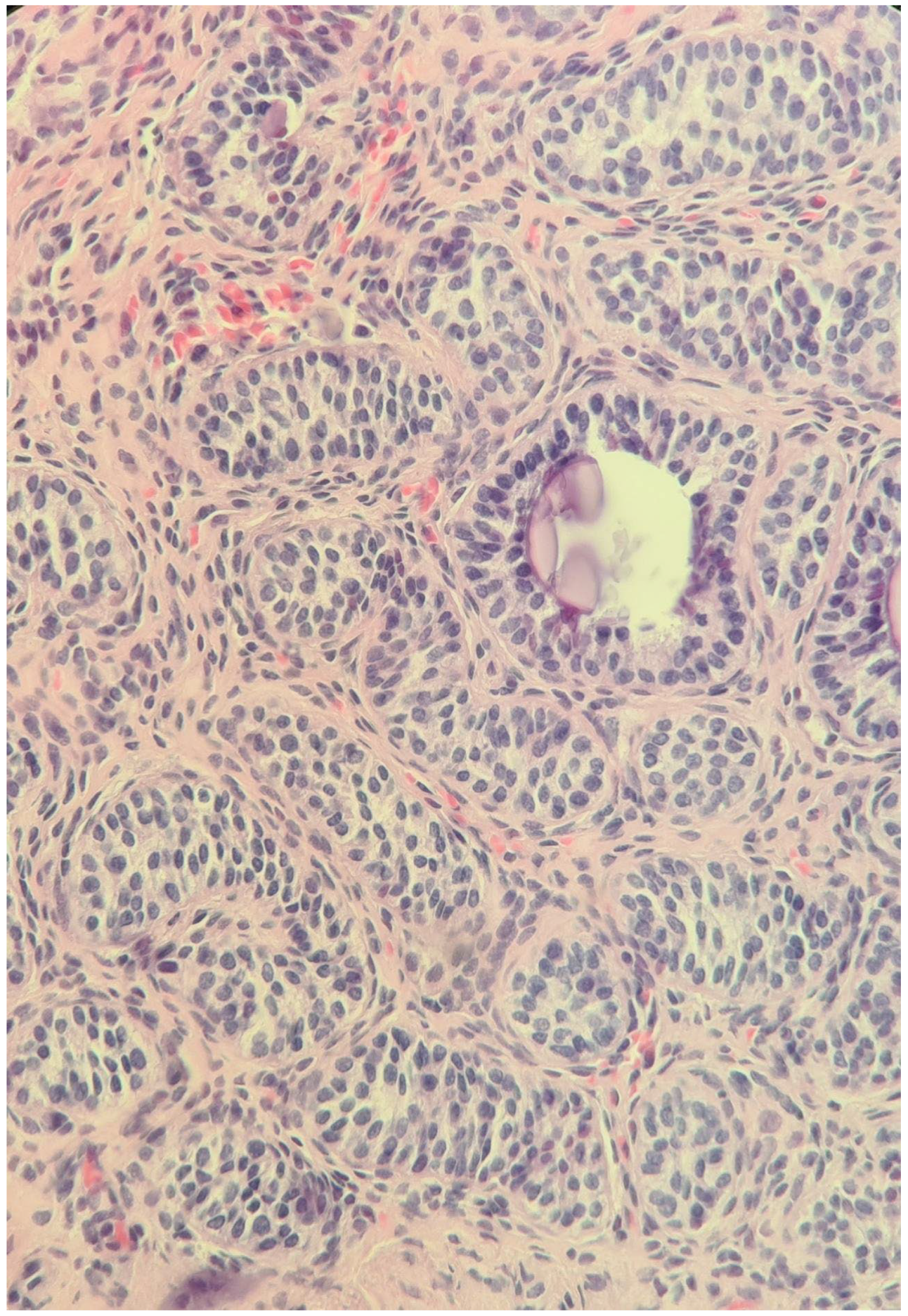
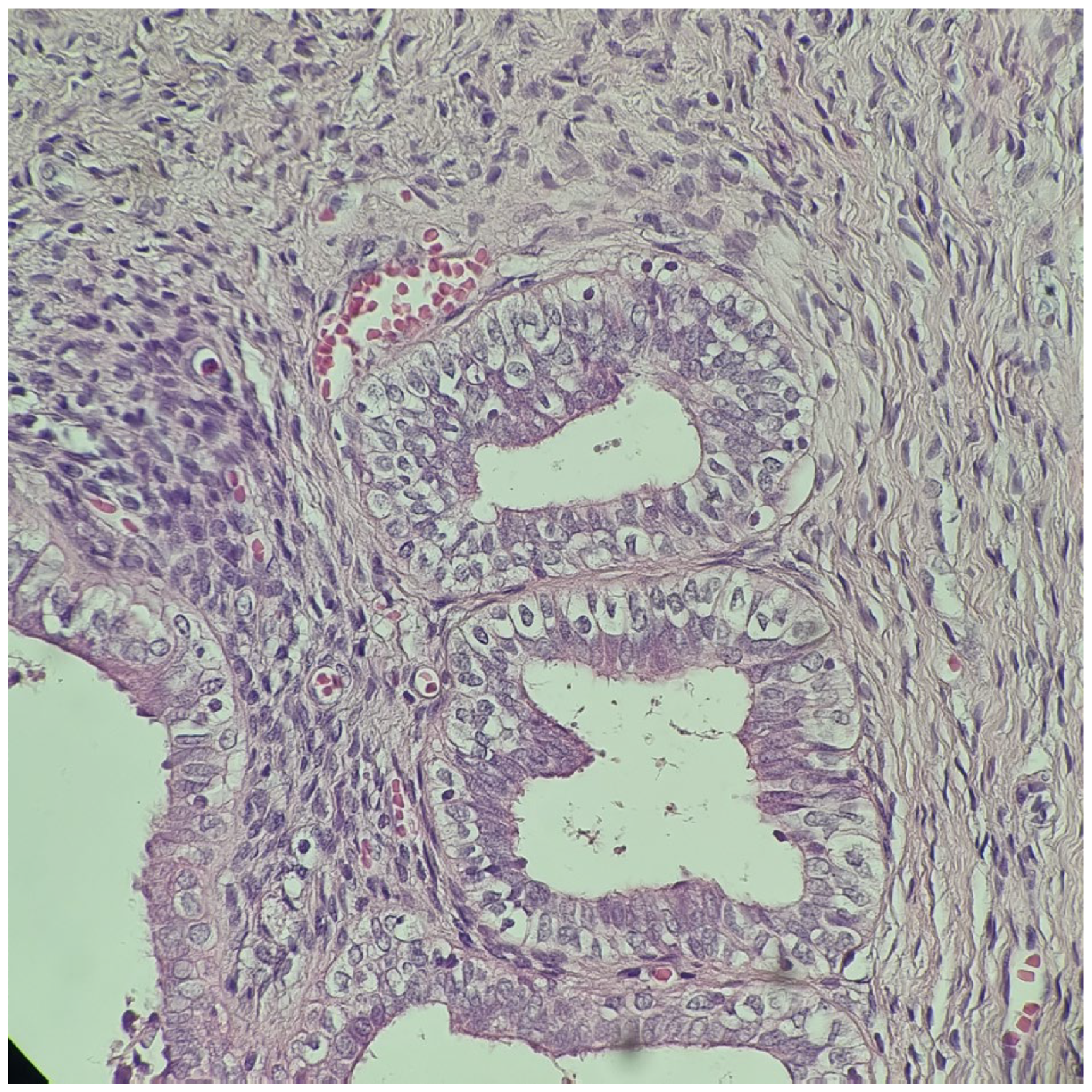
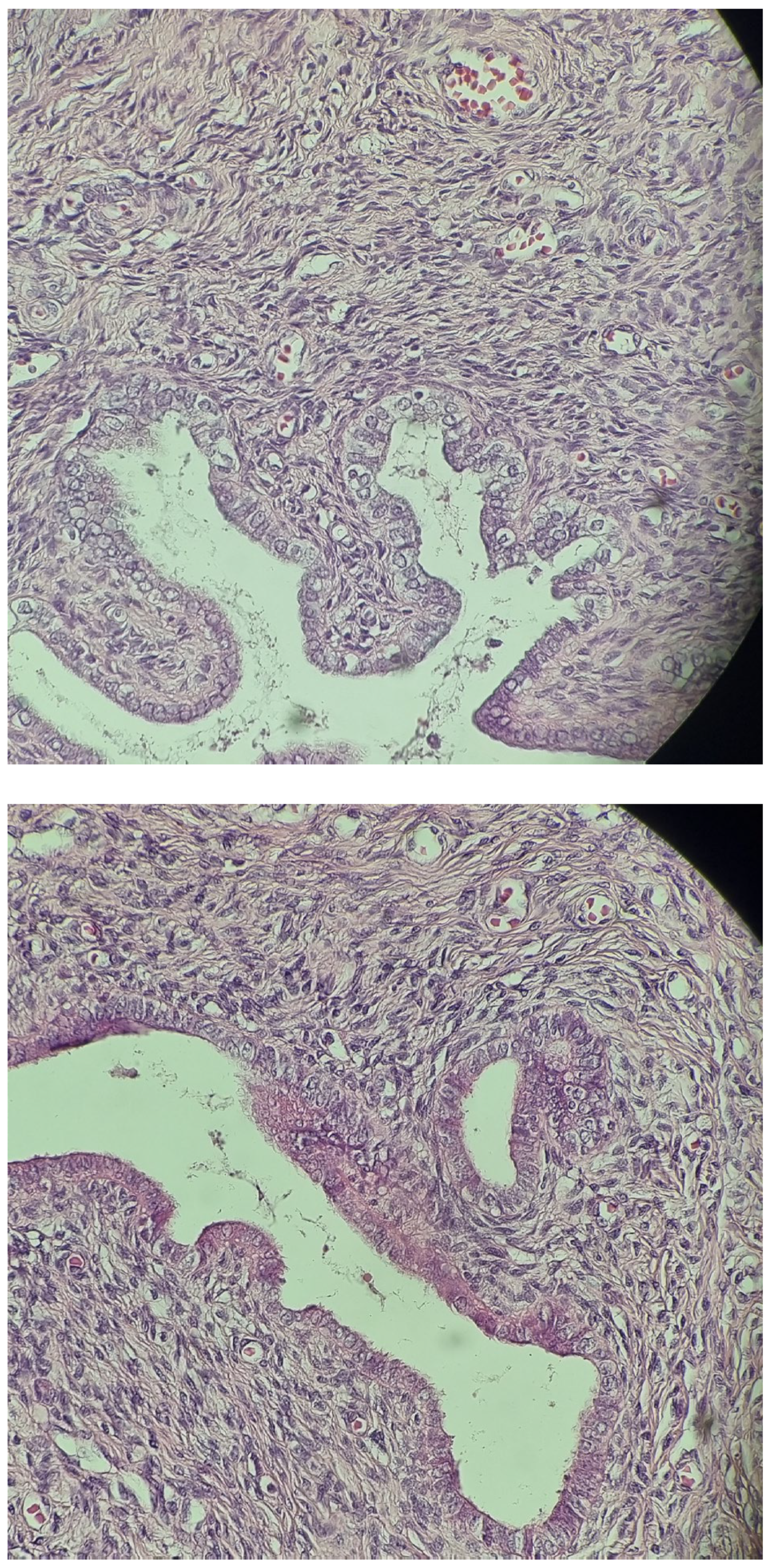
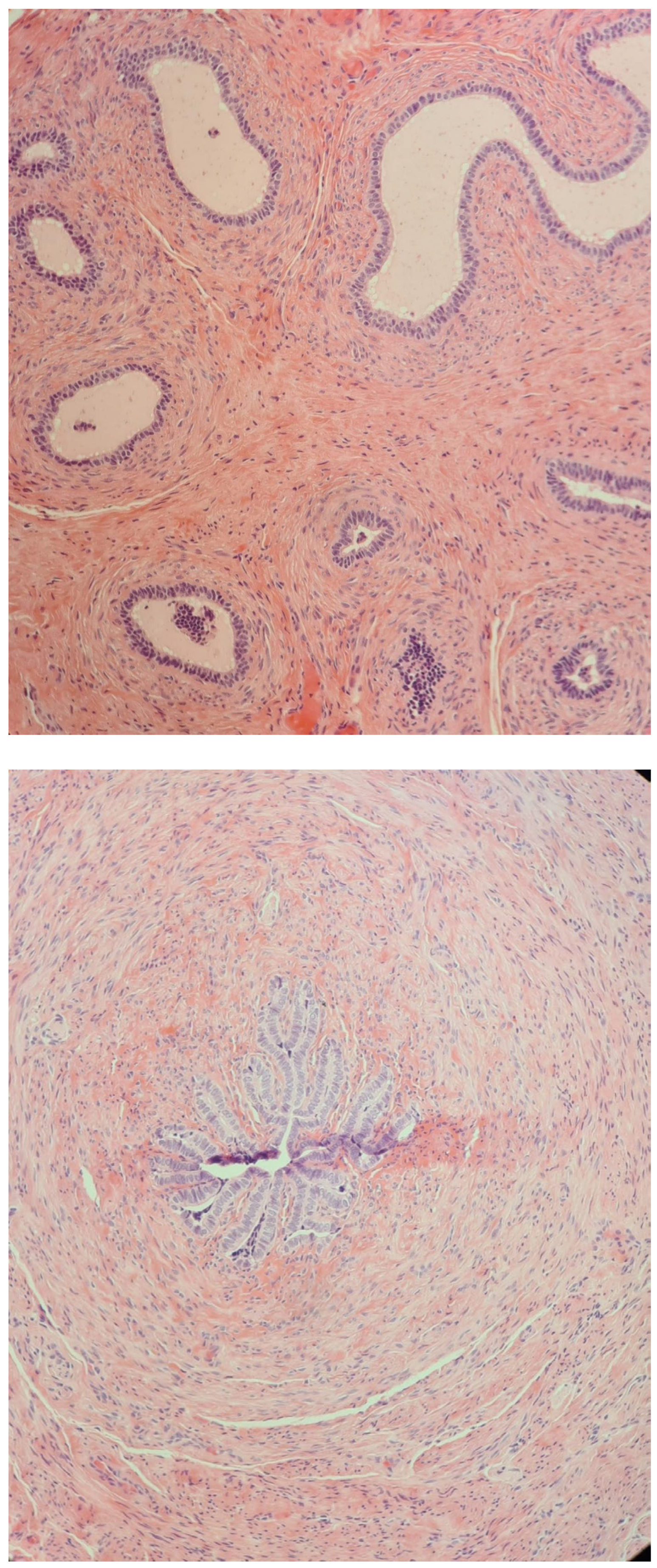
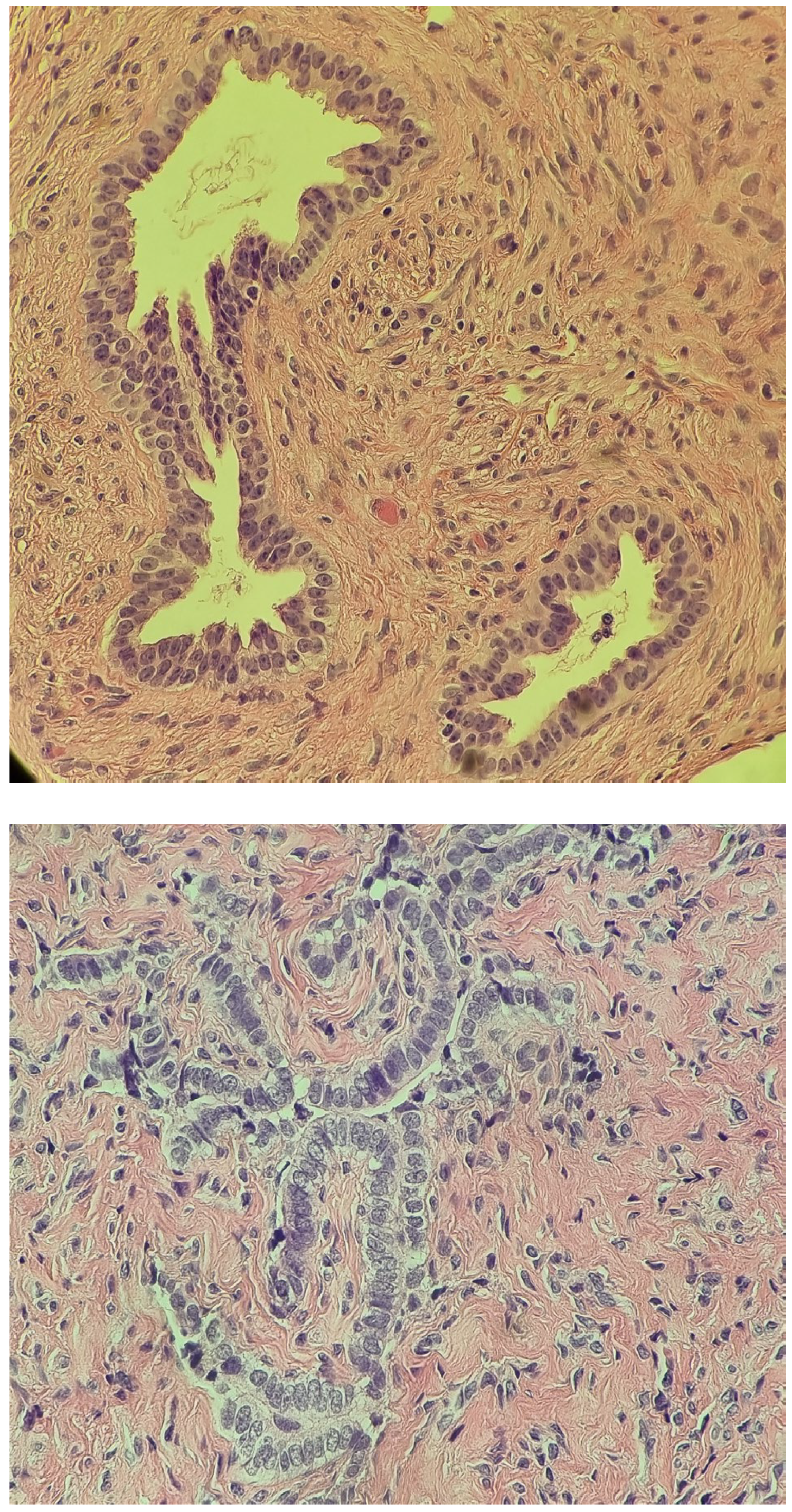
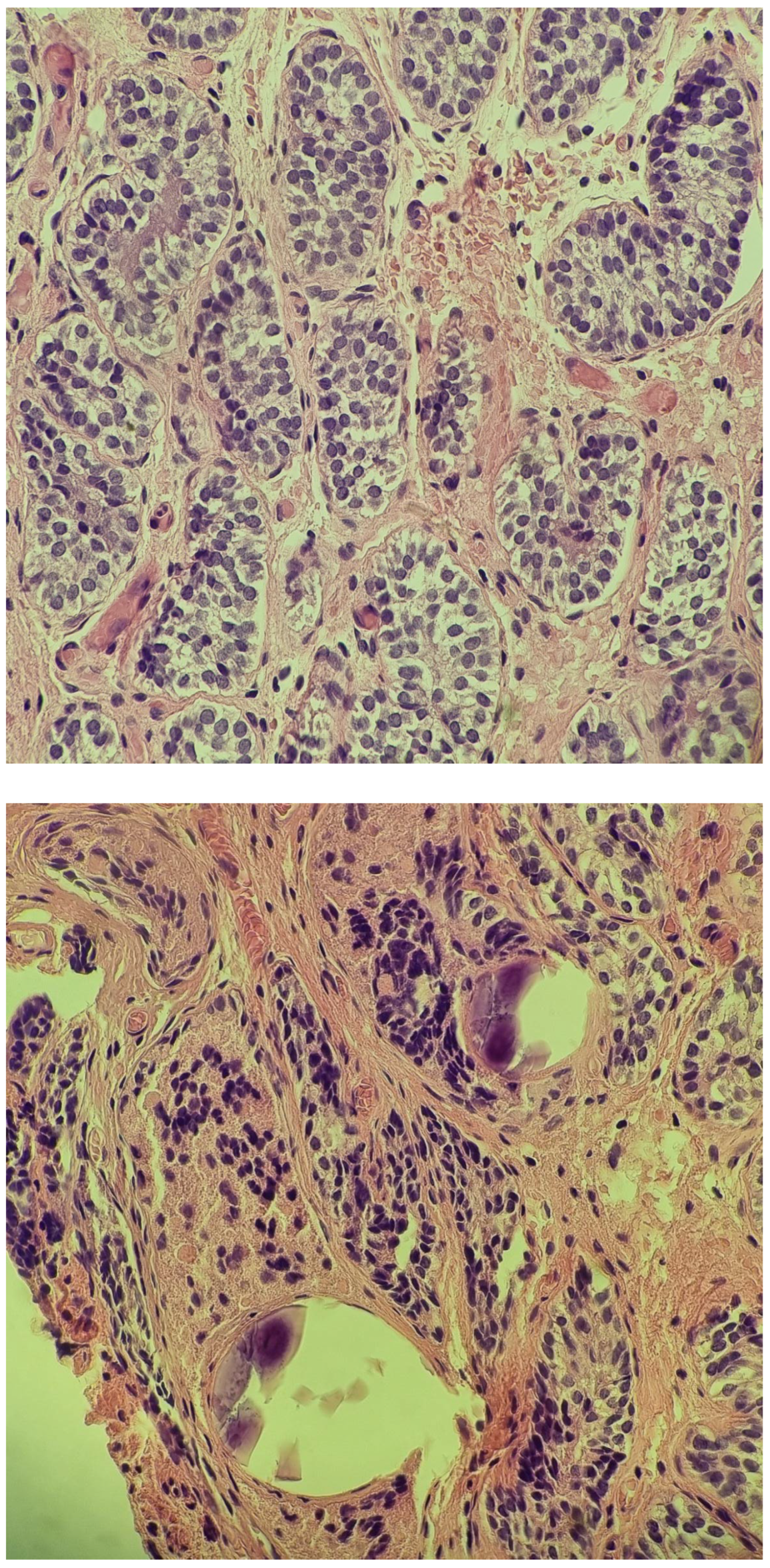
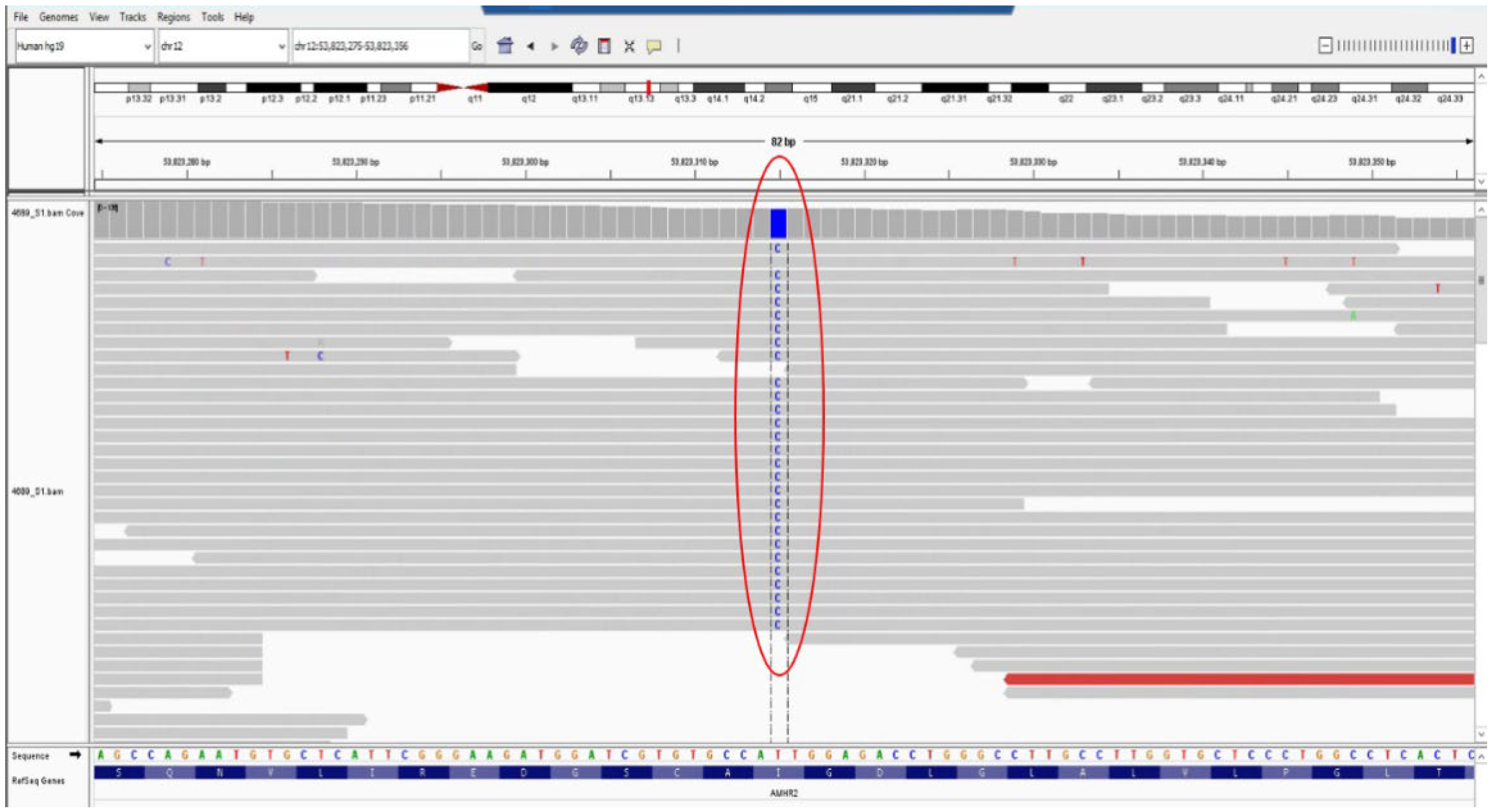
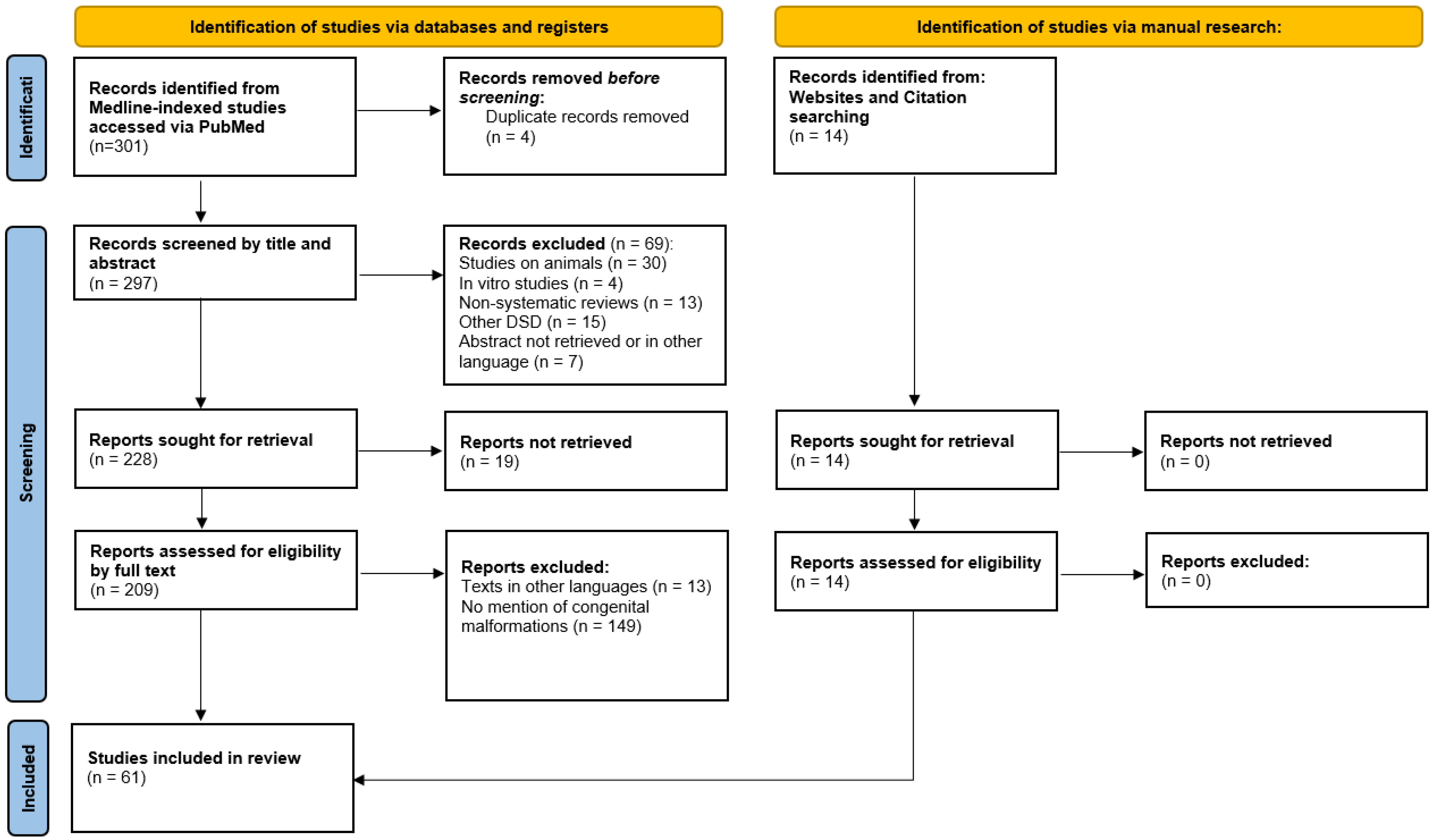
| Malformations Affecting | Cases | Genetic Testing (If Available) | Other Associated Malformations | Observations | References |
|---|---|---|---|---|---|
| Male excretory ducts | |||||
| Vas deferens/epididymis dissociation, agenesia, fusion, duplication, etc. | 29 | Not specific to PMDS, often associated with cryptorchidism | [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22] | ||
| Testes | |||||
| Fused testes | 2 | There is also a case of demonstrated fusion of the testes after birth | [14,23] | ||
| Testicular regression syndrome (TRS) | 9 | Late regression without hypospadias | We excluded cases with a history of testicular torsion, inguinal hernia incarceration, or surgery in the inguinal–scrotal region | [20,24,25,26,27,28,29,30,31,32,33,34,35,36] | |
| Polyorchidism | 3 | AMHR2 mutation in the index case. No genetic testing in other cases. | [2,3] | ||
| Urinary system | |||||
| Crossed fused renal ectopia | 1 | [37] | |||
| Multicystic kidney | 1 | [38] | |||
| Kidney ectasia | 1 | [39] | |||
| Kidney hypoplasia | 1 | Intestinal lymphangiectasia, prenatal growth deficiency, hypertrophied alveolar ridges, redundant nuchal skin, and hepatomegaly | [40] | ||
| Hydronephrosis | 3 | Intestinal lymphangiectasia (2), pulmonary lymphangiectasia (1), prenatal growth deficiency (2), hypertrophied alveolar ridges (2), redundant nuchal skin (1), postaxial polydactyly (1), and hepatomegaly (2) | [40,41] | ||
| Vesico-uretral reflux | 1 | [42] | |||
| Ureteral duplication | 1 | [43] | |||
| Gastrointestinal tract | |||||
| Intestinal lymphangiectasia/lymphangiomyxoma | 8 | Prenatal growth deficiency (3), hypertrophied alveolar ridges (3), redundant nuchal skin (4), postaxial polydactyly (2), renal anomalies (2—hydronephrosis and 1—renal hypoplasia), and hepatomegaly (4) | [40,44,45,46] | ||
| Pulmonary lymphangiectasia (2) | |||||
| Hirschsprung disease | 2 | [47,48] | |||
| Esophageal atresia | 1 | Idiopathic PMDS (PPP1R12A) | [49] | ||
| Jejunal atresia | 1 | [50] | |||
| Ileal atresia | 3 | Idiopathic PMDS (PPP1R12A) | [49] | ||
| Spleen | |||||
| Polysplenia | 1 | Short pancreas (no body or tail) and unilateral TRS | [24] | ||
| Vascular system | |||||
| Pulmonary artery stenosis | 1 | Facial dysmorphism | [51] | ||
| Mesenteric vein abnormality | 1 | Trisomy 7 | Glaucoma | [52] | |
| Nervous system | |||||
| Cerebellar ataxia and optic nerve atrophy | 1 | [52] | |||
| Mental retardation | 1 | Monosomy 11 | Glaucoma and aniridia | [52] | |
| Sex chromosome abnormalities | |||||
| Klinefelter syndrome | 3 | 47XXY (2), 46XY/47XXY (1) | [53,54,55] | ||
| Adrenal glands | |||||
| Congenital adrenal hyperplasia | 2 | 17 alpha-Hydroxylase deficiency (1) and 21 alpha-Hydroxylase deficiency (1) | [25,56] | ||
| Metabolic abnormalities | |||||
| Lipoatrophic diabetes and vitamin D-resistant rickets | 1 | Normal AMH gene | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cima, L.N.; Grosu, I.; Draghici, I.M.; Enculescu, A.C.; Chirita-Emandi, A.; Andreescu, N.; Puiu, M.; Barbu, C.G.; Fica, S. Persistent Müllerian Duct Syndrome with Supernumerary Testicles Due to a Novel Homozygous Variant in the AMHR2 Gene and Literature Review. Diagnostics 2024, 14, 2621. https://doi.org/10.3390/diagnostics14232621
Cima LN, Grosu I, Draghici IM, Enculescu AC, Chirita-Emandi A, Andreescu N, Puiu M, Barbu CG, Fica S. Persistent Müllerian Duct Syndrome with Supernumerary Testicles Due to a Novel Homozygous Variant in the AMHR2 Gene and Literature Review. Diagnostics. 2024; 14(23):2621. https://doi.org/10.3390/diagnostics14232621
Chicago/Turabian StyleCima, Luminita Nicoleta, Iustina Grosu, Isabela Magdalena Draghici, Augustina Cornelia Enculescu, Adela Chirita-Emandi, Nicoleta Andreescu, Maria Puiu, Carmen Gabriela Barbu, and Simona Fica. 2024. "Persistent Müllerian Duct Syndrome with Supernumerary Testicles Due to a Novel Homozygous Variant in the AMHR2 Gene and Literature Review" Diagnostics 14, no. 23: 2621. https://doi.org/10.3390/diagnostics14232621
APA StyleCima, L. N., Grosu, I., Draghici, I. M., Enculescu, A. C., Chirita-Emandi, A., Andreescu, N., Puiu, M., Barbu, C. G., & Fica, S. (2024). Persistent Müllerian Duct Syndrome with Supernumerary Testicles Due to a Novel Homozygous Variant in the AMHR2 Gene and Literature Review. Diagnostics, 14(23), 2621. https://doi.org/10.3390/diagnostics14232621






