Feasibility of Peroral Cholangioscopy in the Initial Endoscopic Retrograde Cholangiopancreatography for Malignant Biliary Strictures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
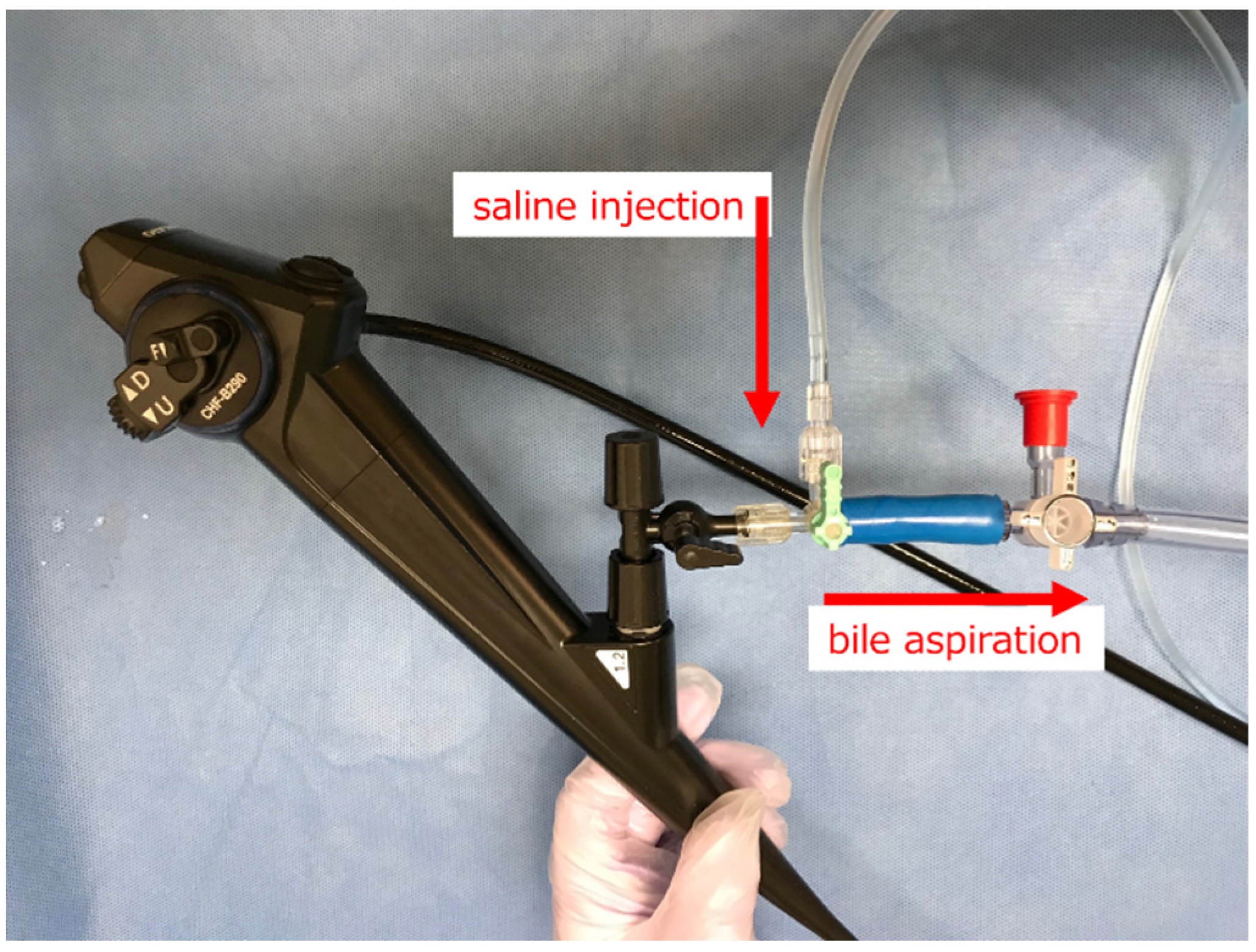
2.2. Original Irrigation System
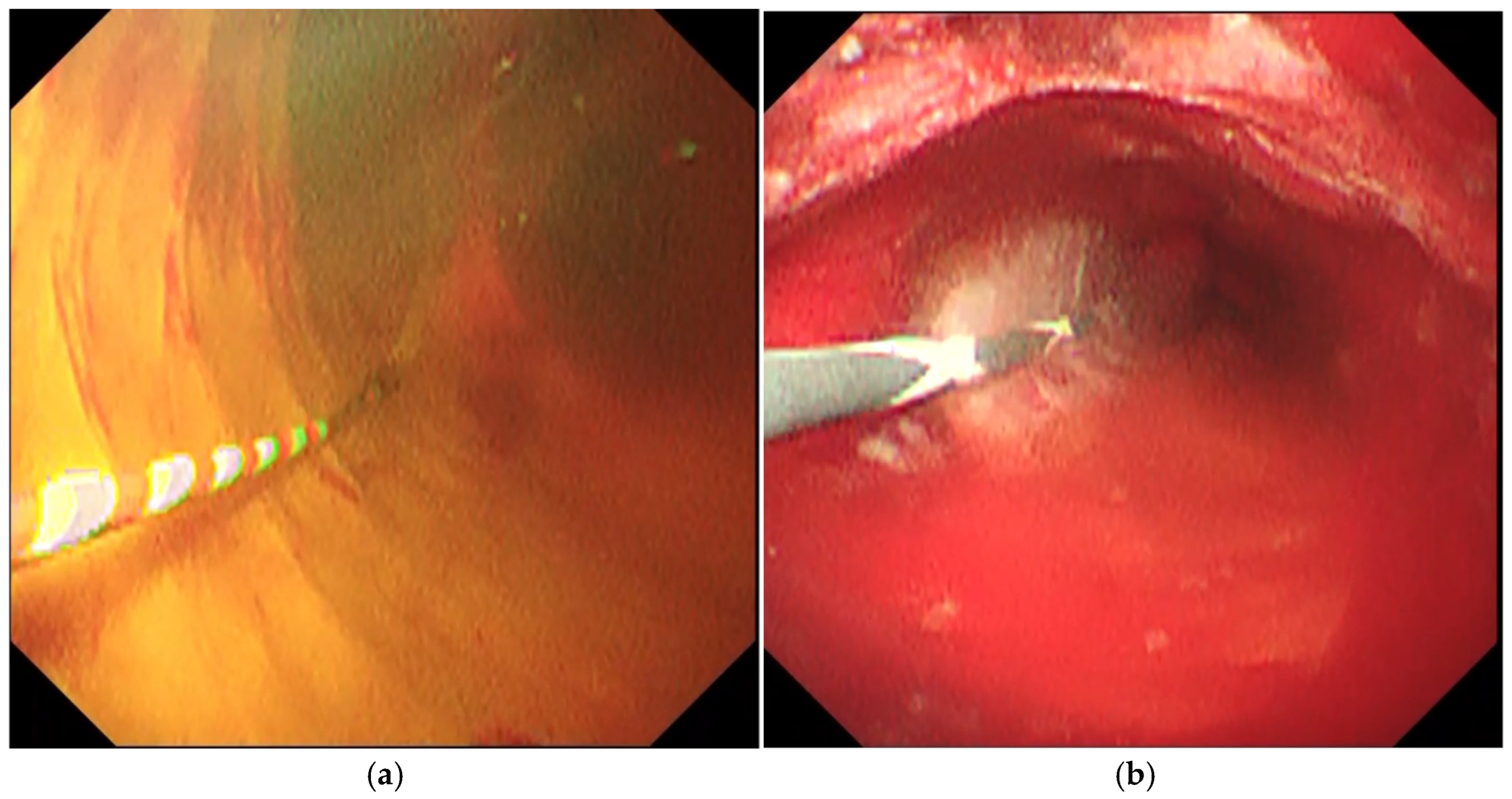
2.3. Procedures
2.4. Definitions and Outcomes
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Patients
3.2. Procedures and Outcomes
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerges, C.; Beyna, T.; Tang, R.S.Y.; Bahin, F.; Lau, J.Y.W.; van Geenen, E.; Neuhaus, H.; Nageshwar Reddy, D.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 91, 1105–1113. [Google Scholar] [CrossRef]
- Shah, R.J.; Raijman, I.; Brauer, B.; Gumustop, B.; Pleskow, D.K. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy 2017, 49, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Turowski, F.; Hugle, U.; Dormann, A.; Bechtler, M.; Jakobs, R.; Gottschalk, U.; Notzel, E.; Hartmann, D.; Lorenz, A.; Kolligs, F.; et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS: Results of a multicenter retrospective cohort study. Surg. Endosc. 2018, 32, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Imanishi, M.; Kurisu, Y.; Onda, S.; Sano, T.; Takagi, W.; Okuda, A.; Miyano, A.; Amano, M.; Nishioka, N.; et al. Prospective evaluation of digital single-operator cholangioscope for diagnostic and therapeutic procedures (with videos). Dig. Endosc. 2017, 29, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Koshita, S.; Ogawa, T.; Masu, K.; Kusunose, H.; Sakai, T.; Murabayashi, T.; Haegawa, S.; Kozakai, F.; Yonamine, K.; et al. Peroral cholangioscopy by SpyGlass DS versus CHF-B260 for evaluation of the lateral spread of extrahepatic cholangiocarcinoma. Endosc. Int. Open 2018, 6, E1349–E1354. [Google Scholar] [CrossRef]
- Ishida, Y.; Itoi, T.; Okabe, Y. Types of Peroral Cholangioscopy: How to Choose the Most Suitable Type of Cholangioscopy. Curr. Treat. Options Gastroenterol. 2016, 14, 210–219. [Google Scholar] [CrossRef]
- Ishii, T.; Kaneko, T.; Murakami, A.; Ueda, M.; Sugimori, K.; Kawana, I.; Maeda, S. New image-enhanced cholangioscopy for the diagnosis of cholangiocarcinoma. Endoscopy 2023, 55, E139–E140. [Google Scholar] [CrossRef]
- Ishii, T.; Kaneko, T.; Murakami, A.; Enomoto, M.; Sugimori, K.; Kawana, I.; Maeda, S. Cholangioscopy in IgG4-related sclerosing cholangitis using texture and color enhancement imaging and red dichromatic imaging. Endoscopy 2023, 55, E1019–E1020. [Google Scholar] [CrossRef]
- Lau, W.Y.; Fan, S.T.; Yip, W.C.; Poon, G.P.; Wong, K.K. Optimal irrigation pressures in operative choledochoscopy. Aust. N. Z. J. Surg. 1988, 58, 63–66. [Google Scholar] [CrossRef]
- Thosani, N.; Zubarik, R.S.; Kochar, R.; Kothari, S.; Sardana, N.; Nguyen, T.; Banerjee, S. Prospective evaluation of bacteremia rates and infectious complications among patients undergoing single-operator choledochoscopy during ERCP. Endoscopy 2016, 48, 424–431. [Google Scholar] [CrossRef]
- Sethi, A.; Chen, Y.K.; Austin, G.L.; Brown, W.R.; Brauer, B.C.; Fukami, N.N.; Khan, A.H.; Shah, R.J. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: A single-center experience. Gastrointest. Endosc. 2011, 73, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mandai, K.; Uno, K.; Yasuda, K. Gastrointestinal: Plastic stent-induced polyp-like lesion in the bile duct. J. Gastroenterol. Hepatol. 2020, 35, 2031. [Google Scholar] [CrossRef] [PubMed]
- Caillol, F.; Bories, E.; Poizat, F.; Pesenti, C.; Esterni, B.; Monges, G.; Giovannini, M. Endomicroscopy in bile duct: Inflammation interferes with pCLE applied in the bile duct: A prospective study of 54 patients. United Eur. Gastroenterol. J. 2013, 1, 120–127. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.B.; van der Heide, F.; Ter Steege, R.W.F.; Koornstra, J.J.; Buddingh, K.T.; Gouw, A.S.H.; Weersma, R.K. Limited diagnostic accuracy and clinical impact of single-operator peroral cholangioscopy for indeterminate biliary strictures. Endoscopy 2020, 52, 107–114. [Google Scholar] [CrossRef]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Wakabayashi, G.; Kozaka, K.; Endo, I.; Deziel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef]
- Liao, W.C.; Angsuwatcharakon, P.; Isayama, H.; Dhir, V.; Devereaux, B.; Khor, C.J.; Ponnudurai, R.; Lakhtakia, S.; Lee, D.K.; Ratanachu-Ek, T.; et al. International consensus recommendations for difficult biliary access. Gastrointest. Endosc. 2017, 85, 295–304. [Google Scholar] [CrossRef]
- Cotton, P.B.; Lehman, G.; Vennes, J.; Geenen, J.E.; Russell, R.C.; Meyers, W.C.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef]
- Kanda, Y. Statistical analysis using freely-available “EZR (Easy R)” software. Rinsho. Ketsueki. 2015, 56, 2258–2266. [Google Scholar] [CrossRef]
- Chen, Y.K.; Parsi, M.A.; Binmoeller, K.F.; Hawes, R.H.; Pleskow, D.K.; Slivka, A.; Haluszka, O.; Petersen, B.T.; Sherman, S.; Deviere, J.; et al. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest. Endosc. 2011, 74, 805–814. [Google Scholar] [CrossRef]
- Murabayashi, T.; Ogawa, T.; Koshita, S.; Kanno, Y.; Kusunose, H.; Sakai, T.; Masu, K.; Yonamine, K.; Miyamoto, K.; Kozakai, F.; et al. Peroral Cholangioscopy-guided Electrohydraulic Lithotripsy with a SpyGlass DS Versus a Conventional Digital Cholangioscope for Difficult Bile Duct Stones. Intern. Med. 2020, 59, 1925–1930. [Google Scholar] [CrossRef]
- Wong, J.C.; Tang, R.S.; Teoh, A.Y.; Sung, J.J.; Lau, J.Y. Efficacy and safety of novel digital single-operator peroral cholangioscopy-guided laser lithotripsy for complicated biliary stones. Endosc. Int. Open 2017, 5, E54–E58. [Google Scholar] [CrossRef]
- Bernica, J.; Elhanafi, S.; Kalakota, N.; Jia, Y.; Dodoo, C.; Dwivedi, A.; Sealock, R.J.; Patel, K.; Raijman, I.; Zuckerman, M.J.; et al. Cholangioscopy Is Safe and Feasible in Elderly Patients. Clin. Gastroenterol. Hepatol. 2018, 16, 1293–1299.e2. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.W.; Haider, S.; Chung, M.; Pandey, A.; Smith, I.; Kahaleh, M.; Sauer, B.G. Endoscopic retrograde cholangiopancreatography complications in the era of cholangioscopy: Is there an increased risk? Dig. Liver. Dis. 2012, 44, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Yasuda, I.; Isayama, H.; Tsuyuguchi, T.; Yamaguchi, T.; Kawabe, K.; Okabe, Y.; Hanada, K.; Hayashi, T.; Ohtsuka, T.; et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy in biliopancreatic diseases: Prospective multicenter study in Japan. World J. Gastroenterol. 2016, 22, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Ghersi, S.; Fuccio, L.; Bassi, M.; Fabbri, C.; Cennamo, V. Current status of peroral cholangioscopy in biliary tract diseases. World J. Gastrointest. Endosc. 2015, 7, 510–517. [Google Scholar] [CrossRef]
- Woo, Y.S.; Lee, J.K.; Oh, S.H.; Kim, M.J.; Jung, J.G.; Lee, K.H.; Lee, K.T. Role of SpyGlass peroral cholangioscopy in the evaluation of indeterminate biliary lesions. Dig. Dis. Sci. 2014, 59, 2565–2570. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef]
- Angsuwatcharakon, P.; Kulpatcharapong, S.; Moon, J.H.; Ramchandani, M.; Lau, J.; Isayama, H.; Seo, D.W.; Maydeo, A.; Wang, H.P.; Nakai, Y.; et al. Consensus guidelines on the role of cholangioscopy to diagnose indeterminate biliary stricture. HPB 2022, 24, 17–29. [Google Scholar] [CrossRef]
- Navaneethan, U.; Lourdusamy, D.; Gutierrez, N.G.; Zhu, X.; Vargo, J.J.; Parsi, M.A. New approach to decrease post-ERCP adverse events in patients with primary sclerosing cholangitis. Endosc. Int. Open 2017, 5, E710–E717. [Google Scholar] [CrossRef]
- Navaneethan, U.; Lourdusamy, V.; Jegadeesan, R.; Sanaka, M.R.; Vargo, J.J.; Parsi, M.A. Su1626 Bile Aspiration During ERCP Is Associated with Lower Risk of Post-ERCP Cholangitis: A Single Center Prospective Study. Gastrointest. Endosc. 2015, 81, AB357. [Google Scholar] [CrossRef]
- Facciorusso, A.; Ramai, D.; Gkolfakis, P.; Khan, S.R.; Papanikolaou, I.S.; Triantafyllou, K.; Tringali, A.; Chandan, S.; Mohan, B.P.; Adler, D.G. Comparative efficacy of different methods for difficult biliary cannulation in ERCP: Systematic review and network meta-analysis. Gastrointest. Endosc. 2022, 95, 60–71.e12. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Hasan, M.K.; Kommaraju, K.; Zhu, X.; Hebert-Magee, S.; Hawes, R.H.; Vargo, J.J.; Varadarajulu, S.; Parsi, M.A. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: A multicenter clinical experience (with video). Gastrointest. Endosc. 2016, 84, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Onoyama, T.; Takeda, Y.; Kawata, S.; Kurumi, H.; Koda, H.; Yamashita, T.; Hamamoto, W.; Sakamoto, Y.; Matsumoto, K.; Isomoto, H. Adequate tissue acquisition rate of peroral cholangioscopy-guided forceps biopsy. Ann. Transl. Med. 2020, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
| POCS (n = 53) | Non-POCS (n = 94) | p-Value | |||
|---|---|---|---|---|---|
| Age, years, median (range) | 78 | (37–95) | 77 | (51–97) | 0.86 |
| Sex, female, n (%) | 19 | (36) | 44 | (47) | 0.23 |
| Age-adjusted CCI, median (range) | 5 | (1–8) | 5 | (2–9) | 0.62 |
| ECOG PS, n (%) | 0.22 | ||||
| 0 | 42 | (79) | 67 | (71) | |
| 1 | 9 | (17) | 16 | (17) | |
| 2 | 2 | (3.8) | 9 | (9.6) | |
| 3 | 0 | (0) | 2 | (2.1) | |
| 4 | 0 | (0) | 0 | (0) | |
| Antithrombotic drug, n (%) | 15 | (28) | 16 | (17) | 0.14 |
| Pre-ERCP cholangitis, n (%) | 27 | (51%) | 42 | (45%) | 0.50 |
| Mild | 7 | (13%) | 17 | (18%) | 0.50 |
| Moderate | 20 | (38%) | 25 | (27%) | 0.19 |
| Positive blood cultures, n (%) | 3 | (5.7%) | 4 | (4.3%) | 0.70 |
| Disease, n (%) | |||||
| Pancreatic caner | 28 | (53) | 42 | (45) | 0.39 |
| Biliary tract cancer | 21 | (40) | 43 | (46) | 0.49 |
| Extrahepatic bile duct cancer | 18 | (34) | 30 | (32) | 0.86 |
| Distal | 10 | 21 | |||
| Perihilar | 8 | 9 | |||
| Gallbladder cancer | 1 | (1.9) | 6 | (6.4) | 0.42 |
| Ampullary cancer | 0 | (0) | 6 | (6.4) | 0.09 |
| Intrahepatic cholangiocarcinoma | 2 | (3.8) | 1 | (1.1) | 0.30 |
| Hepatocellular cancer | 1 | (1.9) | 0 | (0) | 0.36 |
| Other malignant disease | 3 | (5.7) | 9 | (9.6) | 0.54 |
| POCS (n = 53) | Non-POCS (n = 94) | p-Value | |||
|---|---|---|---|---|---|
| WBC, /μL, median (range) | 6000 | (3100–30,900) | 5400 | (2700–19,600) | 0.21 |
| Hb, g/dL, median (range) | 12.3 | (6.7–16.8) | 12.0 | (7.9–15.1) | 0.37 |
| Plt, ×104/μL, median (range) | 22.4 | (11.7–49.3) | 24.3 | (5.0–43.4) | 0.85 |
| Cre, mg/dL, median (range) | 0.82 | (0.42–1.91) | 0.74 | (0.41–3.72) | 0.74 |
| Alb, g/dL, median (range) | 3.1 | (1.6–4.5) | 3.2 | (1.8–4.4) | 0.09 |
| T-Bil, mg/dL, median (range) | 7.0 | (0.4–31.2) | 5.5 | (0.4–24.6) | 0.44 |
| AST, U/L, median (range) | 110 | (18–877) | 137 | (16–547) | 0.26 |
| ALT, U/L, median (range) | 137 | (11–1188) | 168 | (12–897) | 0.42 |
| CRP, mg/dL, median (range) | 1.27 | (0.03–20.2) | 1.04 | (0.06–41.7) | 0.94 |
| POCS (n = 53) | Non-POCS (n = 94) | p-Value | |||
|---|---|---|---|---|---|
| Cannulation time, min, median (range) | 17 | (1–48) | 10 | (1–83) | 0.81 |
| Difficult biliary cannulation, n (%) | 34 | (64) | 49 | (52) | 0.17 |
| Procedure time of POCS, min, median (range) | 10 | (1–37) | - | - | - |
| Procedure time of ERCP, min, median (range) | 67 | (37–124) | 50 | (14–110) | <0.01 |
| Endoscopic sphincterotomy, n (%) | 52 | (98) | 65 | (69) | <0.01 |
| Precutting, n (%) | 2 | (3.8) | 11 | (12) | 0.14 |
| Endoscopic papillary balloon dilation, n (%) | 0 | (0) | 2 | (2.1) | 0.54 |
| Pancreatic duct injection, n (%) | 9 | (17) | 27 | (29) | 0.16 |
| Biliary stent, n (%) | 51 | (96) | 87 | (93) | 0.49 |
| Plastic stent | 51 | (96) | 83 | (88) | |
| Metallic stent | 0 | (0) | 4 | (4.3) | |
| Pancreatic stent, n (%) | 1 | (1.9) | 17 | (18) | <0.01 |
| POCS (n = 53) | Non-POCS (n = 94) | p-Value | |||
|---|---|---|---|---|---|
| Total, n (%) | 5 | (9.4) | 12 | (13) | 0.60 |
| Biliary infection, n (%) | 1 | (1.9) | 5 | (5.3) | 0.42 |
| Cholangitis | 1 | 3 | |||
| Cholecystitis | 0 | 2 | |||
| Mild | 1 | 1 | |||
| Moderate | 0 | 4 | |||
| Severe | 0 | 0 | |||
| Pancreatitis, n (%) | 3 | (5.7) | 6 | (6.4) | 1.00 |
| Mild | 2 | 3 | |||
| Moderate | 1 | 3 | |||
| Severe | 0 | 0 | |||
| Bleeding, n (%) | 1 | (1.9) | 0 | (0) | 0.36 |
| Mild | 0 | ||||
| Moderate | 1 | ||||
| Severe | 0 | ||||
| Perforation, n (%) | 0 | (0) | 1 | (1.1) | 1.00 |
| Mild | 0 | ||||
| Moderate | 0 | ||||
| Severe | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Ishii, T.; Miwa, H.; Sato, T.; Goda, Y.; Irie, K.; Sugimori, K.; Maeda, S. Feasibility of Peroral Cholangioscopy in the Initial Endoscopic Retrograde Cholangiopancreatography for Malignant Biliary Strictures. Diagnostics 2024, 14, 2589. https://doi.org/10.3390/diagnostics14222589
Suzuki Y, Ishii T, Miwa H, Sato T, Goda Y, Irie K, Sugimori K, Maeda S. Feasibility of Peroral Cholangioscopy in the Initial Endoscopic Retrograde Cholangiopancreatography for Malignant Biliary Strictures. Diagnostics. 2024; 14(22):2589. https://doi.org/10.3390/diagnostics14222589
Chicago/Turabian StyleSuzuki, Yuichi, Tomohiro Ishii, Haruo Miwa, Takeshi Sato, Yoshihiro Goda, Kuniyasu Irie, Kazuya Sugimori, and Shin Maeda. 2024. "Feasibility of Peroral Cholangioscopy in the Initial Endoscopic Retrograde Cholangiopancreatography for Malignant Biliary Strictures" Diagnostics 14, no. 22: 2589. https://doi.org/10.3390/diagnostics14222589
APA StyleSuzuki, Y., Ishii, T., Miwa, H., Sato, T., Goda, Y., Irie, K., Sugimori, K., & Maeda, S. (2024). Feasibility of Peroral Cholangioscopy in the Initial Endoscopic Retrograde Cholangiopancreatography for Malignant Biliary Strictures. Diagnostics, 14(22), 2589. https://doi.org/10.3390/diagnostics14222589







