Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrollment and Clinical Study of Individuals
2.2. Genetic Examination
2.3. Construction of Gene-Expressing Plasmids
2.4. Cellular Transfection and Dual-Reporter Gene Measurement
2.5. Statistical Analysis
3. Results
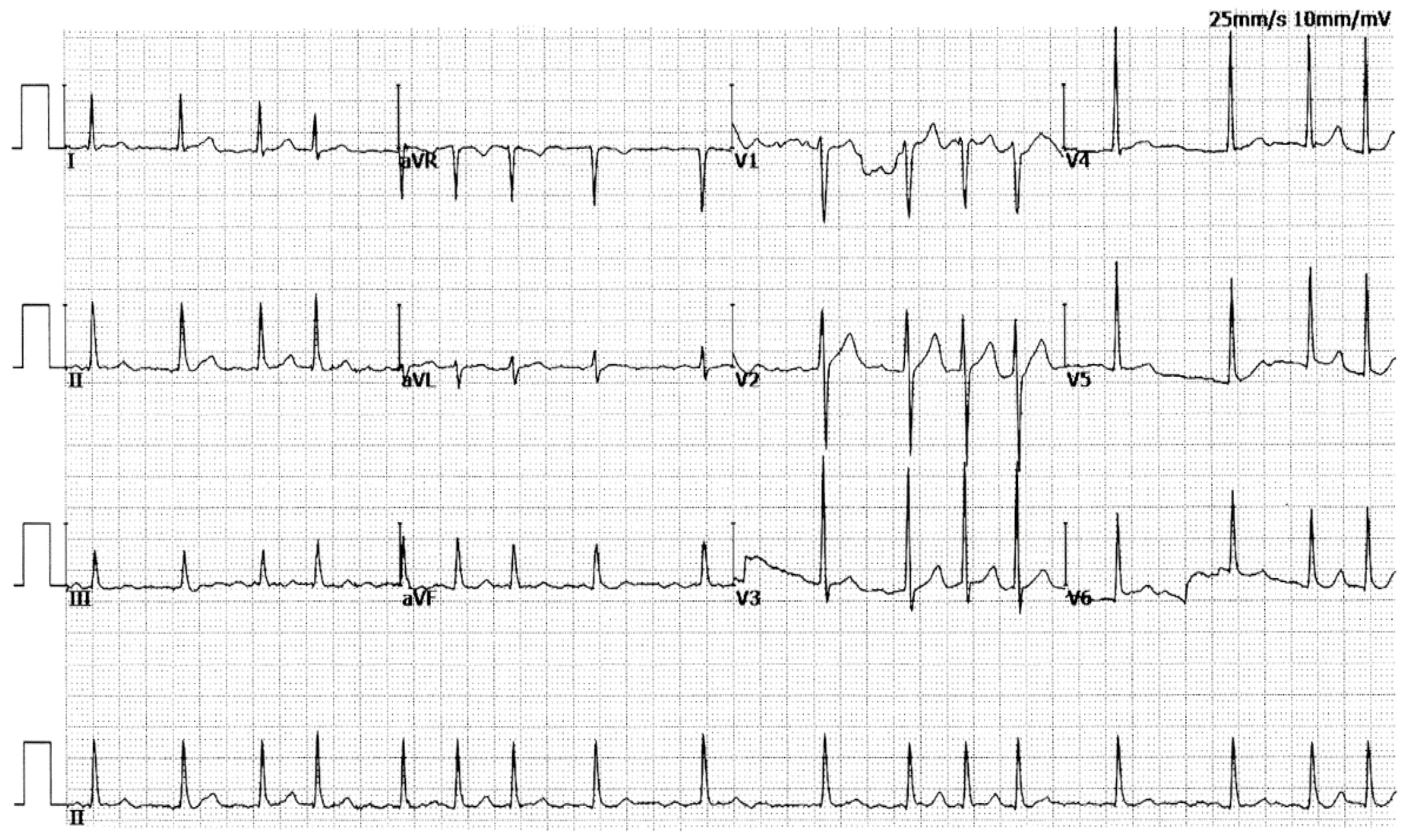
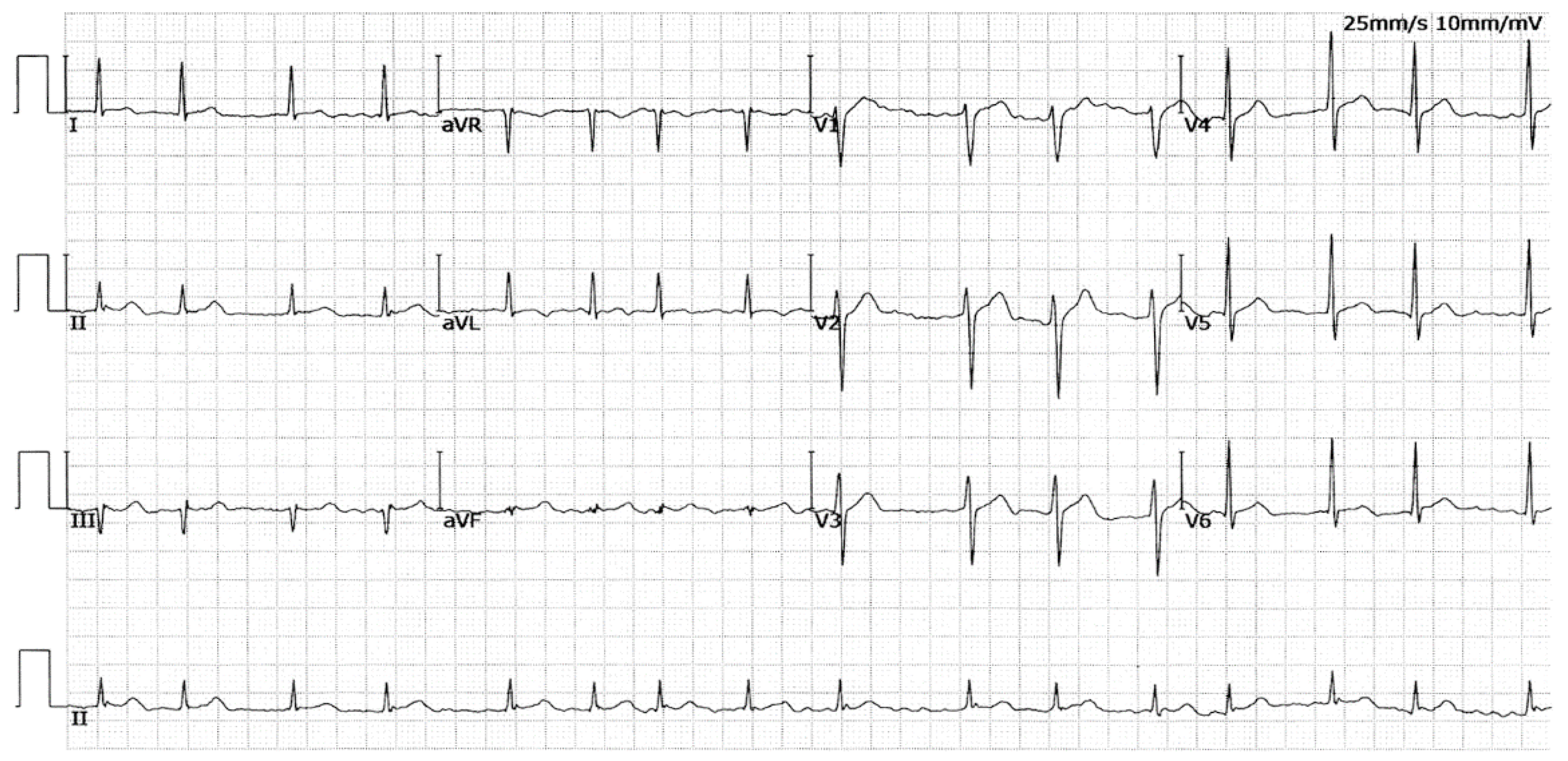
3.1. Baseline Demographic and Clinical Profiles of the Pedigree with AF and Other Study Participants
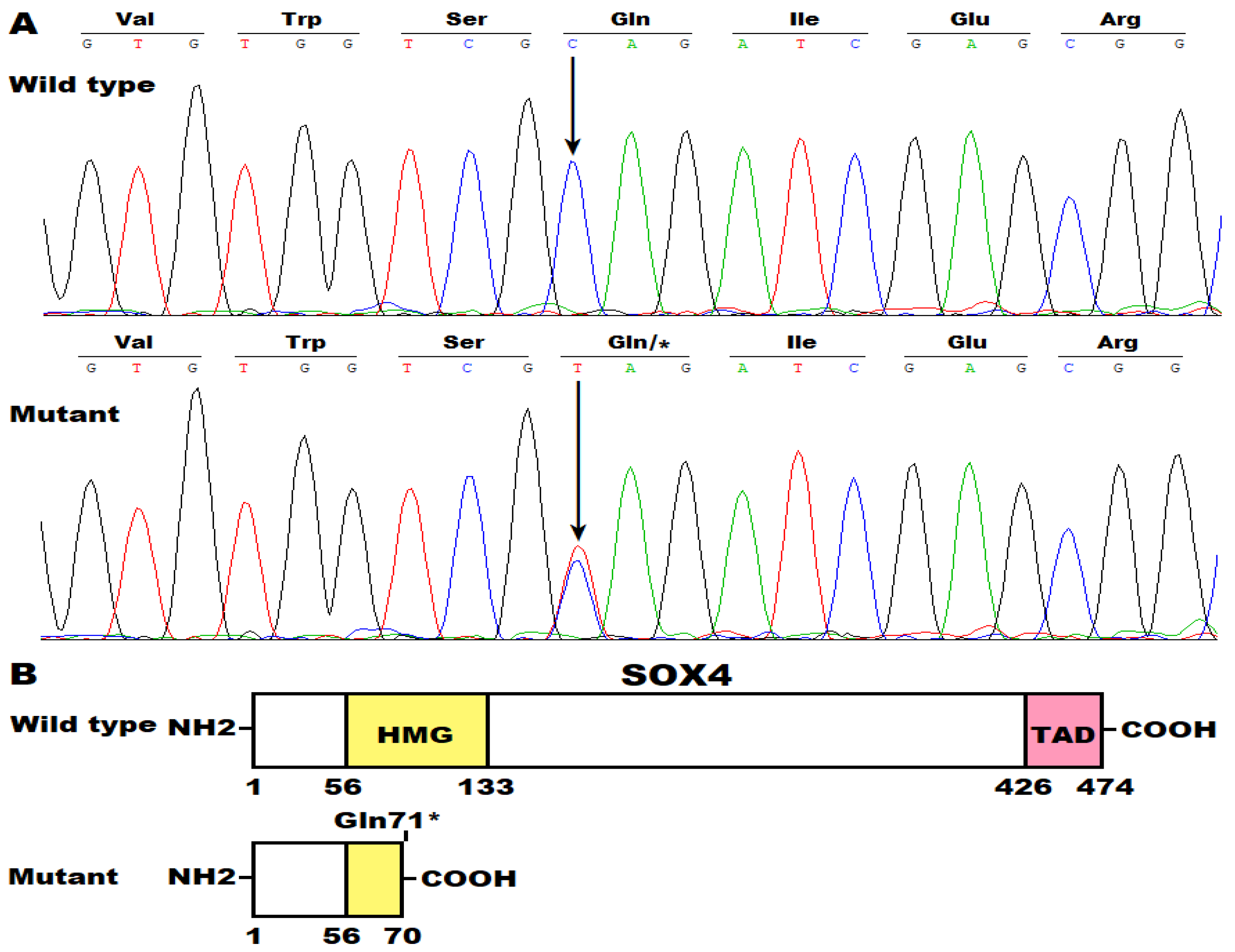
3.2. Discovery of Two New AF-Causative Mutations in SOX4
3.3. Inability of Gln71*- or Trp97*-Mutant SOX4 to Transcriptionally Activate GJA1
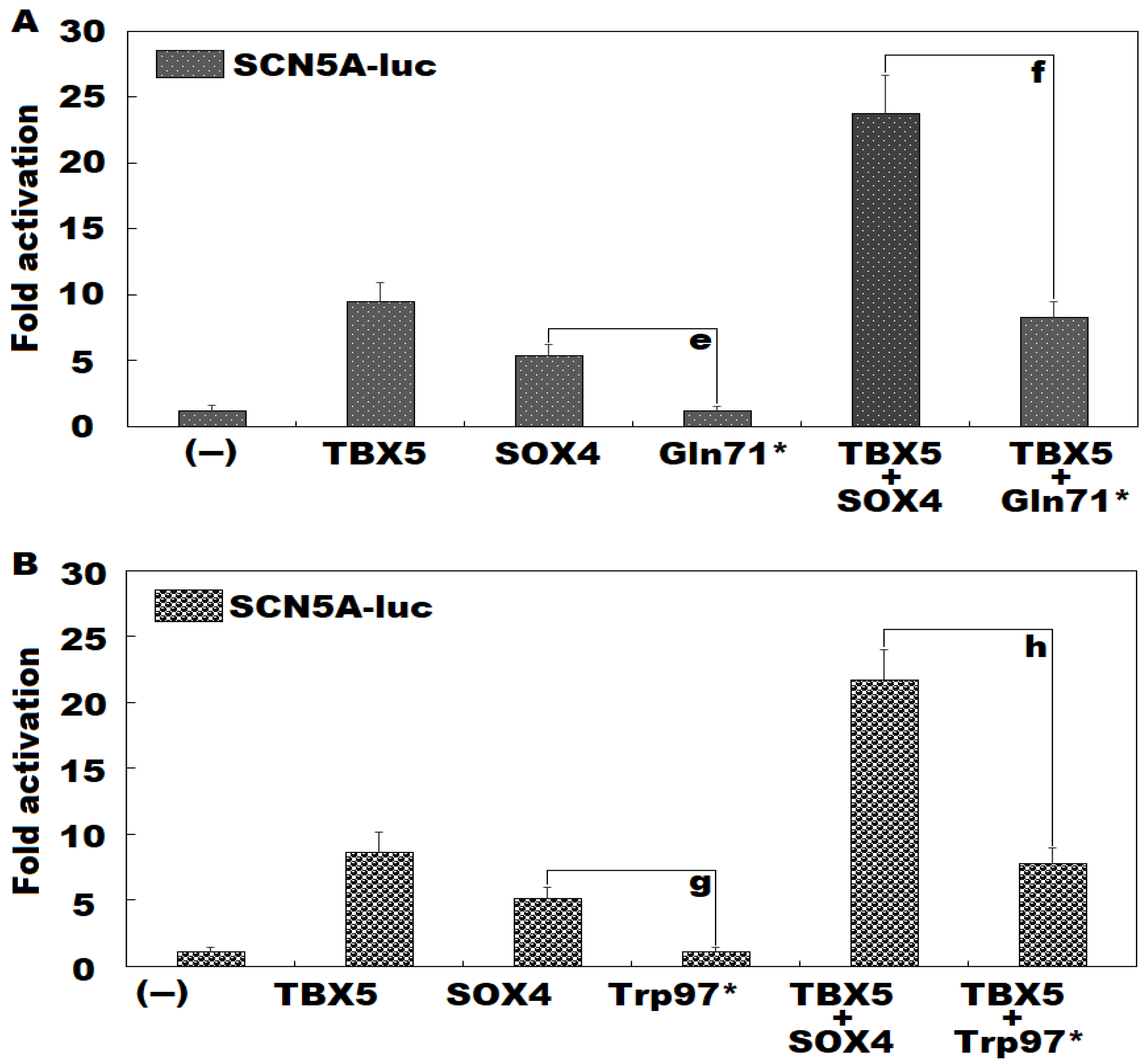
3.4. Failure of Gln71*- or Trp97*-Mutant SOX4 to Transactivate SCN5A Alone or in Synergy with TBX5
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.M.; Damrauer, S.M.; Levin, M.G. Genetics of atrial fibrillation. Curr. Opin. Cardiol. 2023, 38, 162–168. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Imberti, J.F.; Bonini, N.; Tosetti, A.; Mei, D.A.; Gerra, L.; Malavasi, V.L.; Mazza, A.; Lip, G.Y.H.; Boriani, G. Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation. Biology 2022, 11, 443. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.S.; Park, H.W.; Choi, E.K.; Park, J.K.; Kim, J.B.; Kang, K.W.; Shim, J.; Joung, B.; Park, K.M. Clinical Outcomes of Rhythm Control Strategies for Asymptomatic Atrial Fibrillation According to the Quality-of-Life Score: The CODE-AF (Comparison Study of Drugs for Symptom Control and Complication Prevention of Atrial Fibrillation) Registry. J. Am. Heart Assoc. 2022, 11, e025956. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, X.Y.; Long, D.Y.; Jiang, C.X.; Sang, C.H.; Tang, R.B.; Li, S.N.; Wang, W.; Guo, X.Y.; Ning, M.; et al. Asymptomatic atrial fibrillation among hospitalized patients: Clinical correlates and in-hospital outcomes in Improving Care for Cardiovascular Disease in China-trial Fibrillation. Europace 2023, 25, euad272. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Bennett, R.; Macle, L. Assessment and management of asymptomatic atrial fibrillation. Heart 2024, 110, 675–682. [Google Scholar] [CrossRef]
- Wazni, O.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Quality of life after the initial treatment of atrial fibrillation with cryoablation versus drug therapy. Heart Rhythm 2022, 19, 197–205. [Google Scholar] [CrossRef]
- Seki, Y.; Fujisawa, T.; Ikemura, N.; Ibe, S.; Tsuzuki, I.; Hashimoto, K.; Yamashita, T.; Miyama, H.; Niimi, N.; Suzuki, M.; et al. Catheter ablation improves outcomes and quality of life in Japanese patients with early-stage atrial fibrillation: A retrospective cohort study. Heart Rhythm 2022, 19, 1076–1083. [Google Scholar] [CrossRef]
- Walfridsson, U.; Hassel Jönsson, A.; Karlsson, L.O.; Liuba, I.; Almroth, H.; Sandgren, E.; Walfridsson, H.; Charitakis, E. Symptoms and health-related quality of life 5 years after catheter ablation of atrial fibrillation. Clin. Cardiol. 2022, 45, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, E.P.; Li, Y.; Silverstein, A.P.; Russo, A.M.; Poole, J.E.; Daniels, M.R.; Al-Khalidi, H.R.; Lee, K.L.; Bahnson, T.D.; Anstrom, K.J.; et al. Effects of Ablation Versus Drug Therapy on Quality of Life by Sex in Atrial Fibrillation: Results from the CABANA Trial. J. Am. Heart Assoc. 2023, 12, e027871. [Google Scholar] [CrossRef] [PubMed]
- Särnholm, J.; Skúladóttir, H.; Rück, C.; Axelsson, E.; Bonnert, M.; Bragesjö, M.; Venkateshvaran, A.; Ólafsdóttir, E.; Pedersen, S.S.; Ljótsson, B.; et al. Cognitive Behavioral Therapy Improves Quality of Life in Patients with Symptomatic Paroxysmal Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 82, 46–56. [Google Scholar] [CrossRef]
- Teppo, K.; Airaksinen, K.E.J.; Jaakkola, J.; Halminen, O.; Salmela, B.; Kouki, E.; Haukka, J.; Putaala, J.; Linna, M.; Aro, A.L.; et al. Ischaemic stroke in women with atrial fibrillation: Temporal trends and clinical implications. Eur. Heart J. 2024, 45, 1819–1827. [Google Scholar] [CrossRef]
- Chao, T.F.; Potpara, T.S.; Lip, G.Y.H. Atrial fibrillation: Stroke prevention. Lancet Reg. Health Eur. 2024, 37, 100797. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, O.P.; Martinez-Majander, N.; Broman, J.; Mannismäki, L.; Aro, A.; Curtze, S.; Pakarinen, S.; Lehto, M.; Putaala, J. Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis. J. Clin. Med. 2023, 13, 30. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Proietti, M.; Potpara, T.; Mansour, M.; Savelieva, I.; Tse, H.F.; Goette, A.; Camm, A.J.; Blomstrom-Lundqvist, C.; Gupta, D.; et al. Atrial fibrillation and stroke prevention: 25 years of research at EP Europace journal. Europace 2023, 25, euad226. [Google Scholar] [CrossRef]
- Elsheikh, S.; Hill, A.; Irving, G.; Lip, G.Y.H.; Abdul-Rahim, A.H. Atrial fibrillation and stroke: State-of-the-art and future directions. Curr. Probl. Cardiol. 2024, 49, 102181. [Google Scholar] [CrossRef] [PubMed]
- Kogelschatz, B.; Zenger, B.; Steinberg, B.A.; Ranjan, R.; Jared Bunch, T. Atrial fibrillation and the risk of early-onset dementia and cognitive decline: An updated review. Trends Cardiovasc. Med. 2024, 34, 236–241. [Google Scholar] [CrossRef]
- Giannone, M.E.; Filippini, T.; Whelton, P.K.; Chiari, A.; Vitolo, M.; Boriani, G.; Vinceti, M. Atrial Fibrillation and the Risk of Early-Onset Dementia: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e025653. [Google Scholar] [CrossRef]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Yee Chen, L.; et al. Atrial Fibrillation and Dementia: A Report From the AF-SCREEN International Collaboration. Circulation 2022, 145, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.; Conen, D. Mechanisms and Clinical Manifestations of Cognitive Decline in Atrial Fibrillation Patients: Potential Implications for Preventing Dementia. Can. J. Cardiol. 2023, 39, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Varrias, D.; Saralidze, T.; Borkowski, P.; Pargaonkar, S.; Spanos, M.; Bazoukis, G.; Kokkinidis, D. Atrial Fibrillation and Dementia: Pathophysiological Mechanisms and Clinical Implications. Biomolecules 2024, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- van der Burgh, A.C.; Geurts, S.; Ikram, M.A.; Hoorn, E.J.; Kavousi, M.; Chaker, L. Bidirectional Association Between Kidney Function and Atrial Fibrillation: A Population-Based Cohort Study. J. Am. Heart Assoc. 2022, 11, e025303. [Google Scholar] [CrossRef]
- Öztürk, B.; Göçer, K.; Aksu, E.; Doğan, K. Impaired Renal Vein Flow in Atrial Fibrillation: A Potential Risk for Renal Dysfunction. Med. Sci. Monit. 2023, 29, e941435. [Google Scholar] [CrossRef]
- Venier, S.; Vaxelaire, N.; Jacon, P.; Carabelli, A.; Desbiolles, A.; Garban, F.; Defaye, P. Severe acute kidney injury related to haemolysis after pulsed field ablation for atrial fibrillation. Europace 2023, 26, euad371. [Google Scholar] [CrossRef]
- Yue, B.; Hou, Q.; Bredehorst, J.; Han, Q.; Zhang, B.; Zhang, C.; Zhang, J.; Chen, S.; Wu, S.; Li, K. Atrial fibrillation increases the risk of new-onset myocardial infarction amongst working-age population: A propensity-matched study. Herz 2023, 48, 408–412. [Google Scholar] [CrossRef]
- Wu, J.; Hou, Q.; Han, Q.; Mao, R.; Yue, B.; Yu, J.; Chen, S.; Wu, S.; Li, K. Atrial fibrillation is an independent risk factor for new-onset myocardial infarction: A prospective study. Acta Cardiol. 2023, 78, 341–348. [Google Scholar] [CrossRef]
- Frederiksen, T.C.; Benjamin, E.J.; Trinquart, L.; Lin, H.; Dahm, C.C.; Christiansen, M.K.; Jensen, H.K.; Preis, S.R.; Kornej, J. Bidirectional Association Between Atrial Fibrillation and Myocardial Infarction, and Relation to Mortality in the Framingham Heart Study. J. Am. Heart Assoc. 2024, 13, e032226. [Google Scholar] [CrossRef]
- Gabarin, M.; Hornik-Lurie, T.; Minha, S.; Omelchenko, A.; Barashi, R.; Arow, Z.; Assali, A.; Pereg, D. CHA(2)DS(2)-VASc Score, Mortality and Acute Myocardial Infarction in Patients with Nonvalvular Atrial Fibrillation. Am. J. Cardiol. 2022, 180, 24–28. [Google Scholar] [CrossRef]
- Cismaru, G.; Negru, A.G. Atrial Fibrillation and Heart Failure. Life 2024, 14, 572. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratnam, J.P.; Elliott, A.D.; Mishima, R.S.; Kadhim, K.; McNamee, O.; Kuklik, P.; Emami, M.; Malik, V.; Fitzgerald, J.L.; Gallagher, C.; et al. Identification of Subclinical Heart Failure with Preserved Ejection Fraction in Patients with Symptomatic Atrial Fibrillation. JACC Heart Fail. 2023, 11, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Bergonti, M.; Ascione, C.; Marcon, L.; Pambrun, T.; Della Rocca, D.G.; Ferrero, T.G.; Pannone, L.; Kühne, M.; Compagnucci, P.; Bonomi, A.; et al. Left ventricular functional recovery after atrial fibrillation catheter ablation in heart failure: A prediction model. Eur. Heart J. 2023, 44, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.M.; Bisson, A.; Bodin, A.; Herbert, J.; Lip, G.Y.H.; Fauchier, L. Atrial Fibrillation and the Risk of Ventricular Arrhythmias and Cardiac Arrest: A Nationwide Population-Based Study. J. Clin. Med. 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.M.; Bisson, A.; Bentounes, S.A.; Bodin, A.; Herbert, J.; Lip, G.Y.H.; Fauchier, L. Ventricular arrhythmias and cardiac arrest in atrial fibrillation patients with pacemakers and implantable cardioverter-defibrillators. Eur. J. Intern. Med. 2023, 115, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Lenhoff, H.; Järnbert-Petersson, H.; Darpo, B.; Tornvall, P.; Frick, M. Mortality and ventricular arrhythmias in patients on d,l-sotalol for rhythm control of atrial fibrillation: A nationwide cohort study. Heart Rhythm 2023, 20, 1473–1480. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, Y.Y.; Han, K.D.; Min, K.; Choi, H.Y.; Shim, J.; Choi, J.I.; Kim, Y.H. Atrial fibrillation is associated with increased risk of lethal ventricular arrhythmias. Sci. Rep. 2021, 11, 18111. [Google Scholar] [CrossRef]
- de Terwangne, C.; Lelubre, C.; Hanotier, P.; de Meester, A.; Descamps, O.; Duray, C.; Pannone, L.; Chierchia, G.B.; de Asmundis, C.; Nokerman, H.; et al. Prevalence and Impact of Atrial Fibrillation on Intra-Hospital Mortality in Patients Aged 75 Years. Am. J. Cardiol. 2022, 177, 40–47. [Google Scholar] [CrossRef]
- Wu, J.; Nadarajah, R.; Nakao, Y.M.; Nakao, K.; Wilkinson, C.; Cowan, J.C.; Camm, A.J.; Gale, C.P. Temporal trends of cause-specific mortality after diagnosis of atrial fibrillation. Eur. Heart J. 2023, 44, 4422–4431. [Google Scholar] [CrossRef]
- Obeid, M.J.; Zhou, J.; Sale, A.J.; Longacre, C.; Zeitler, E.P.; Andrade, J.; Mittal, S.; Piccini, J.P. Early mortality after inpatient versus outpatient catheter ablation in patients with atrial fibrillation. Heart Rhythm 2023, 20, 833–841. [Google Scholar] [CrossRef]
- Zuin, M.; Bertini, M.; Vitali, F.; Turakhia, M.; Boriani, G. Heart Failure-Related Death in Subjects with Atrial Fibrillation in the United States, 1999 to 2020. J. Am. Heart Assoc. 2024, 13, e033897. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Hammill, B.G.; Sinner, M.F.; Hernandez, A.F.; Walkey, A.J.; Benjamin, E.J.; Curtis, L.H.; Heckbert, S.R. Clinical course of atrial fibrillation in older adults: The importance of cardiovascular events beyond stroke. Eur. Heart J. 2014, 35, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.S.; Li, Y.; Cowper, P.A.; Anstrom, K.J.; Piccini, J.P.; Poole, J.E.; Daniels, M.R.; Monahan, K.H.; Davidson-Ray, L.; Bahnson, T.D.; et al. Cost-Effectiveness of Catheter Ablation Versus Antiarrhythmic Drug Therapy in Atrial Fibrillation: The CABANA Randomized Clinical Trial. Circulation 2022, 146, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Denman, R.; Hay, K.; Kaambwa, B.; Ganesan, A.; Ranasinghe, I. Excess Bed Days and Hospitalization Costs Associated with 30-Day Complications Following Catheter Ablation of Atrial Fibrillation. J. Am. Heart Assoc. 2023, 12, e030236. [Google Scholar] [CrossRef]
- Di, J.; Wei, Y.; Zhang, G.; Yue, Y.; Sun, S. Comparison of clinical effects and costs among dabigatran etexilate, rivaroxaban and warfarin in elderly patients with atrial fibrillation. Am. J. Transl. Res. 2023, 15, 3639–3646. [Google Scholar]
- Peigh, G.; Zhou, J.; Rosemas, S.C.; Roberts, A.I.; Longacre, C.; Nayak, T.; Schwab, G.; Soderlund, D.; Passman, R.S. Impact of Atrial Fibrillation Burden on Health Care Costs and Utilization. JACC Clin. Electrophysiol. 2024, 10, 718–730. [Google Scholar] [CrossRef]
- Buja, A.; Rebba, V.; Montecchio, L.; Renzo, G.; Baldo, V.; Cocchio, S.; Ferri, N.; Migliore, F.; Zorzi, A.; Collins, B.; et al. The Cost of Atrial Fibrillation: A Systematic Review. Value Health 2024, 27, 527–541. [Google Scholar] [CrossRef]
- Elliott, A.D.; Middeldorp, M.E.; Van Gelder, I.C.; Albert, C.M.; Sanders, P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat. Rev. Cardiol. 2023, 20, 404–417. [Google Scholar] [CrossRef]
- Vinciguerra, M.; Dobrev, D.; Nattel, S. Atrial fibrillation: Pathophysiology, genetic and epigenetic mechanisms. Lancet Reg. Health Eur. 2024, 37, 100785. [Google Scholar] [CrossRef]
- Young, L.J.; Antwi-Boasiako, S.; Ferrall, J.; Wold, L.E.; Mohler, P.J.; El Refaey, M. Genetic and non-genetic risk factors associated with atrial fibrillation. Life Sci. 2022, 299, 120529. [Google Scholar] [CrossRef]
- Wren, G.; Davies, W. Sex-linked genetic mechanisms and atrial fibrillation risk. Eur. J. Med. Genet. 2022, 65, 104459. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.; Anagnostopoulos, I.; Kousta, M.; Vergopoulos, S.; Deftereos, S.; Vassilikos, V. Alcohol Consumption and the Risk of Incident Atrial Fibrillation: A Meta-Analysis. Diagnostics 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Boursiquot, B.C.; Bellettiere, J.; LaMonte, M.J.; LaCroix, A.Z.; Perez, M.V. Sedentary Behavior and Atrial Fibrillation in Older Women: The OPACH Study. J. Am. Heart Assoc. 2022, 11, e023833. [Google Scholar] [CrossRef]
- Ding, M.; Viet, N.N.; Gigante, B.; Lind, V.; Hammar, N.; Modig, K. Elevated Uric Acid Is Associated with New-Onset Atrial Fibrillation: Results from the Swedish AMORIS Cohort. J. Am. Heart Assoc. 2023, 12, e027089. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Chu, X.; Wang, M. Meta-analysis of the correlation between recurrence of atrial fibrillation and serum uric acid level after radiofrequency ablation. Am. J. Transl. Res. 2022, 14, 8793–8799. [Google Scholar] [PubMed]
- Xu, Z.; Qian, L.; Zhang, L.; Gao, Y.; Huang, S. Predictive value of NT-proBNP, procalcitonin and CVP in patients with new-onset postoperative atrial fibrillation after cardiac surgery. Am. J. Transl. Res. 2022, 14, 3481–3487. [Google Scholar]
- Hulsmans, M.; Schloss, M.J.; Lee, I.H.; Bapat, A.; Iwamoto, Y.; Vinegoni, C.; Paccalet, A.; Yamazoe, M.; Grune, J.; Pabel, S.; et al. Recruited macrophages elicit atrial fibrillation. Science 2023, 381, 231–239. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, Q. Expression and predictive value of NLRP3 in patients with atrial fibrillation and stroke. Am. J. Transl. Res. 2022, 14, 3104–3112. [Google Scholar]
- Malagù, M.; Marchini, F.; Fiorio, A.; Sirugo, P.; Clò, S.; Mari, E.; Gamberini, M.R.; Rapezzi, C.; Bertini, M. Atrial Fibrillation in β-Thalassemia: Overview of Mechanism, Significance and Clinical Management. Biology 2022, 11, 148. [Google Scholar] [CrossRef]
- Vanchiere, C.; Thirumal, R.; Hendrani, A.; Dherange, P.; Bennett, A.; Shi, R.; Gopinathannair, R.; Olshansky, B.; Smith, D.L.; Dominic, P. Association Between Atrial Fibrillation and Occupational Exposure in Firefighters Based on Self-Reported Survey Data. J. Am. Heart Assoc. 2022, 11, e022543. [Google Scholar] [CrossRef]
- van Wijk, S.W.; Su, W.; Wijdeveld, L.F.J.M.; Ramos, K.S.; Brundel, B.J.J.M. Cytoskeletal Protein Variants Driving Atrial Fibrillation: Potential Mechanisms of Action. Cells 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.P.; Zhang, G.F.; Guo, Y.H.; Sun, Y.M.; Wang, J.; Li, N.; Qiu, X.B.; Xu, Y.J.; Yang, Y.Q. A novel PRRX1 loss-of-function variation contributing to familial atrial fibrillation and congenital patent ductus arteriosus. Genet. Mol. Biol. 2022, 45, e20210378. [Google Scholar] [CrossRef] [PubMed]
- Vad, O.B.; Yan, Y.; Denti, F.; Ahlberg, G.; Refsgaard, L.; Bomholtz, S.H.; Santos, J.L.; Rasmussen, S.; Haunsø, S.; Svendsen, J.H.; et al. Whole-Exome Sequencing Implicates Neuronal Calcium Channel with Familial Atrial Fibrillation. Front. Genet. 2022, 13, 806429. [Google Scholar] [CrossRef]
- Malakootian, M.; Jalilian, M.; Kalayinia, S.; Hosseini Moghadam, M.; Heidarali, M.; Haghjoo, M. Whole-exome sequencing reveals a rare missense variant in DTNA in an Iranian pedigree with early-onset atrial fibrillation. BMC Cardiovasc. Disord. 2022, 22, 37. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yang, Y.Q. Atrial Fibrillation: Focus on Myocardial Connexins and Gap Junctions. Biology 2022, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Jameson, H.S.; Hanley, A.; Hill, M.C.; Xiao, L.; Ye, J.; Bapat, A.; Ronzier, E.; Hall, A.W.; Hucker, W.J.; Clauss, S.; et al. Loss of the Atrial Fibrillation-Related Gene, Zfhx3, Results in Atrial Dilation and Arrhythmias. Circ. Res. 2023, 133, 313–329. [Google Scholar] [CrossRef]
- Vad, O.B.; Angeli, E.; Liss, M.; Ahlberg, G.; Andreasen, L.; Christophersen, I.E.; Hansen, C.C.; Møller, S.; Hellsten, Y.; Haunsoe, S.; et al. Loss of Cardiac Splicing Regulator RBM20 Is Associated with Early-Onset Atrial Fibrillation. JACC Basic Transl. Sci. 2023, 9, 163–180. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.J.; Guo, X.J.; Wu, S.H.; Jiang, W.F.; Zhang, D.L.; Wang, K.W.; Li, L.; Sun, Y.M.; Xu, Y.J.; et al. Discovery of TBX20 as a Novel Gene Underlying Atrial Fibrillation. Biology 2023, 12, 1186. [Google Scholar] [CrossRef]
- O’Reilly, M.; Sommerfeld, L.C.; O’Shea, C.; Broadway-Stringer, S.; Andaleeb, S.; Reyat, J.S.; Kabir, S.N.; Stastny, D.; Malinova, A.; Delbue, D.; et al. Familial atrial fibrillation mutation M1875T-SCN5A increases early sodium current and dampens the effect of flecainide. Europace 2023, 25, 1152–1161. [Google Scholar] [CrossRef]
- Chalazan, B.; Freeth, E.; Mohajeri, A.; Ramanathan, K.; Bennett, M.; Walia, J.; Halperin, L.; Roston, T.; Lazarte, J.; Hegele, R.A.; et al. Genetic testing in monogenic early-onset atrial fibrillation. Eur. J. Hum. Genet. 2023, 31, 769–775. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.L.; Liu, Z.B.; Chen, C.; Ren, X.; Luo, A.T.; Ma, J.H.; Antzelevitch, C.; Barajas-Martínez, H.; Hu, D. Underlying mechanism of atrial fibrillation-associated Nppa-I137T mutation and cardiac effect of potential drug therapy. Heart Rhythm 2024, 21, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Manuel, A.I.; Macías, Á.; Cruz, F.M.; Gutiérrez, L.K.; Martínez, F.; González-Guerra, A.; Martínez Carrascoso, I.; Bermúdez-Jimenez, F.J.; Sánchez-Pérez, P.; Vera-Pedrosa, M.L.; et al. The Kir2.1E299V mutation increases atrial fibrillation vulnerability while protecting the ventricles against arrhythmias in a mouse model of short QT syndrome type 3. Cardiovasc. Res. 2024, 120, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Ito, K.; Ito, M.; Zou, Z.; Kubota, M.; Nomura, S.; Matsunaga, H.; Koyama, S.; Ieki, H.; Akiyama, M.; et al. Cross-ancestry genome-wide analysis of atrial fibrillation unveils disease biology and enables cardioembolic risk prediction. Nat. Genet. 2023, 55, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, J.; Ye, W.G.; Liu, X.Y.; Li, L.; Qiu, X.B.; Chen, H.; Xu, Y.J.; Yang, Y.Q.; Bai, D.; et al. Discovery of GJC1 (Cx45) as a New Gene Underlying Congenital Heart Disease and Arrhythmias. Biology 2023, 12, 346. [Google Scholar] [CrossRef]
- Gu, J.N.; Yang, C.X.; Ding, Y.Y.; Qiao, Q.; Di, R.M.; Sun, Y.M.; Wang, J.; Yang, L.; Xu, Y.J.; Yang, Y.Q. Identification of BMP10 as a Novel Gene Contributing to Dilated Cardiomyopathy. Diagnostics 2023, 13, 242. [Google Scholar] [CrossRef]
- Huang, R.T.; Guo, Y.H.; Yang, C.X.; Gu, J.N.; Qiu, X.B.; Shi, H.Y.; Xu, Y.J.; Xue, S.; Yang, Y.Q. SOX7 loss-of-function variation as a cause of familial congenital heart disease. Am. J. Transl. Res. 2022, 14, 1672–1684. [Google Scholar]
- Guo, Y.H.; Wang, J.; Guo, X.J.; Gao, R.F.; Yang, C.X.; Li, L.; Sun, Y.M.; Qiu, X.B.; Xu, Y.J.; Yang, Y.Q. KLF13 Loss-of-Function Mutations Underlying Familial Dilated Cardiomyopathy. J. Am. Heart Assoc. 2022, 11, e027578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Qiu, X.B.; Yuan, F.; Wang, J.; Zhao, C.M.; Li, R.G.; Xu, L.; Xu, Y.J.; Shi, H.Y.; Hou, X.M.; et al. TBX5 loss-of-function mutation contributes to familial dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2015, 459, 166–171. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Qiao, X.H.; Xu, Y.J.; Liu, X.Y.; Huang, R.T.; Xue, S.; Qiu, H.Y.; Yang, Y.Q. SMAD1 Loss-of-Function Variant Responsible for Congenital Heart Disease. Biomed. Res. Int. 2022, 2022, 9916325. [Google Scholar] [CrossRef]
- Wu, S.H.; Wang, X.H.; Xu, Y.J.; Gu, J.N.; Yang, C.X.; Qiao, Q.; Guo, X.J.; Guo, Y.H.; Qiu, X.B.; Jiang, W.F.; et al. ISL1 loss-of-function variation causes familial atrial fibrillation. Eur. J. Med. Genet. 2020, 63, 104029. [Google Scholar] [CrossRef]
- Abhinav, P.; Zhang, G.F.; Zhao, C.M.; Xu, Y.J.; Wang, J.; Yang, Y.Q. A novel KLF13 mutation underlying congenital patent ductus arteriosus and ventricular septal defect, as well as bicuspid aortic valve. Exp. Ther. Med. 2022, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Xie, M.S.; Yang, C.X.; Huang, R.T.; Xue, S.; Liu, X.Y.; Xu, Y.J.; Yang, Y.Q. Identification of SOX18 as a New Gene Predisposing to Congenital Heart Disease. Diagnostics 2022, 12, 1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.J.; Yang, C.X.; Huang, R.T.; Xue, S.; Yuan, F.; Yang, Y.Q. SMAD4 loss-of-function mutation predisposes to congenital heart disease. Eur. J. Med. Genet. 2023, 66, 104677. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.B.; Li, Y.J.; Liu, X.Y.; Huang, R.T.; Yang, C.X.; Xu, Y.J.; Lv, H.T.; Yang, Y.Q. Discovery of BMP10 as a new gene underpinning congenital heart defects. Am. J. Transl. Res. 2024, 16, 109–125. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.Y.; Yang, C.X.; Zhou, H.M.; Li, Y.J.; Qiu, X.B.; Huang, R.T.; Cen, S.S.; Wang, Y.; Xu, Y.J.; et al. Discovery and functional investigation of BMP4 as a new causative gene for human congenital heart disease. Am. J. Transl. Res. 2024, 16, 2034–2048. [Google Scholar] [CrossRef]
- Abhinav, P.; Li, Y.J.; Huang, R.T.; Liu, X.Y.; Gu, J.N.; Yang, C.X.; Xu, Y.J.; Wang, J.; Yang, Y.Q. Somatic GATA4 mutation contributes to tetralogy of Fallot. Exp. Ther. Med. 2024, 27, 91. [Google Scholar] [CrossRef]
- Grippa, M.; Graziano, C. Landscape of Constitutional SOX4 Variation in Human Disorders. Genes 2024, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.S. SOX4: The unappreciated oncogene. Semin. Cancer Biol. 2020, 67, 57–64. [Google Scholar] [CrossRef]
- Farr, C.J.; Easty, D.J.; Ragoussis, J.; Collignon, J.; Lovell-Badge, R.; Goodfellow, P.N. Characterization and mapping of the human SOX4 gene. Mamm. Genome 1993, 4, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Schilham, M.W.; Oosterwegel, M.A.; Moerer, P.; Ya, J.; de Boer, P.A.; van de Wetering, M.; Verbeek, S.; Lamers, W.H.; Kruisbeek, A.M.; Cumano, A.; et al. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature 1996, 380, 711–714. [Google Scholar] [CrossRef]
- Ya, J.; Schilham, M.W.; de Boer, P.A.; Moorman, A.F.; Clevers, H.; Lamers, W.H. Sox4-deficiency syndrome in mice is an animal model for common trunk. Circ. Res. 1998, 83, 986–994. [Google Scholar] [CrossRef]
- Penzo-Méndez, A.; Dy, P.; Pallavi, B.; Lefebvre, V. Generation of mice harboring a Sox4 conditional null allele. Genesis 2007, 45, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, L.; Zheng, N.; Li, R.; Yang, R.; Li, T.; Li, Y.; Liu, Y.; Luo, H.; Li, X.; et al. Suppression of Sox4 protects against myocardial ischemic injury by reduction of cardiac apoptosis in mice. J. Cell. Physiol. 2021, 236, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Ni, Y.; Thachil, V.; Morley, M.; Moravec, C.S.; Tang, W.H.W. Differential expression of members of SOX family of transcription factors in failing human hearts. Transl. Res. 2022, 242, 66–78. [Google Scholar] [CrossRef]
- Cheng, C.K.; Lin, X.; Pu, Y.; Tse, J.K.Y.; Wang, Y.; Zhang, C.L.; Cao, X.; Lau, C.W.; Huang, J.; He, L.; et al. SOX4 is a novel phenotypic regulator of endothelial cells in atherosclerosis revealed by single-cell analysis. J. Adv. Res. 2023, 43, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Y.; Jie, H.; Lu, W.; Chen, Y.; Xing, X.; Tang, B.; Xu, G.; Sun, J.; Liang, Y. CircHDAC9 regulates myocardial ischemia-reperfusion injury via miR-671-5p/SOX4 signaling axis. Am. J. Med. Sci. 2024, 367, 49–60. [Google Scholar] [CrossRef]
- Boogerd, C.J.; Wong, L.Y.; van den Boogaard, M.; Bakker, M.L.; Tessadori, F.; Bakkers, J.; ‘t Hoen, P.A.; Moorman, A.F.; Christoffels, V.M.; Barnett, P. Sox4 mediates Tbx3 transcriptional regulation of the gap junction protein Cx43. Cell. Mol. Life Sci. 2011, 68, 3949–3961. [Google Scholar] [CrossRef]
- Arnolds, D.E.; Liu, F.; Fahrenbach, J.P.; Kim, G.H.; Schillinger, K.J.; Smemo, S.; McNally, E.M.; Nobrega, M.A.; Patel, V.V.; Moskowitz, I.P. TBX5 drives Scn5a expression to regulate cardiac conduction system function. J. Clin. Investig. 2012, 122, 2509–2518. [Google Scholar] [CrossRef]
- Nieto-Marín, P.; Tinaquero, D.; Utrilla, R.G.; Cebrián, J.; González-Guerra, A.; Crespo-García, T.; Cámara-Checa, A.; Rubio-Alarcón, M.; Dago, M.; Alfayate, S.; et al. Tbx5 variants disrupt Nav1.5 function differently in patients diagnosed with Brugada or Long QT Syndrome. Cardiovasc. Res. 2022, 118, 1046–1060. [Google Scholar] [CrossRef]
- Nadadur, R.D.; Broman, M.T.; Boukens, B.; Mazurek, S.R.; Yang, X.; van den Boogaard, M.; Bekeny, J.; Gadek, M.; Ward, T.; Zhang, M.; et al. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci. Transl. Med. 2016, 8, 354ra115. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Christoffels, V.M.; Anderson, R.H.; Moorman, A.F. Atrial fibrillation: A developmental point of view. Heart Rhythm 2009, 6, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Postma, A.V.; Dekker, L.R.; Soufan, A.T.; Moorman, A.F. Developmental and genetic aspects of atrial fibrillation. Trends Cardiovasc. Med. 2009, 19, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Macrae, C.A. The developmental basis of adult arrhythmia: Atrial fibrillation as a paradigm. Front. Physiol. 2013, 4, 221. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, F.; Dai, Q.; Dou, J.; Wu, Y.; Zhu, Y. Identification of a novel de novo mutation in SOX4 for syndromic tooth agenesis. Clin. Oral Investig. 2024, 28, 287. [Google Scholar] [CrossRef]
- Flöttmann, R.; Wagner, J.; Kobus, K.; Curry, C.J.; Savarirayan, R.; Nishimura, G.; Yasui, N.; Spranger, J.; Van Esch, H.; Lyons, M.J.; et al. Microdeletions on 6p22.3 Are Associated with Mesomelic Dysplasia Savarirayan Type. J. Med. Genet. 2015, 52, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ladinsky, H.T.; Elizalde, A.; Schickler, R.; Dees, P.B.; Crenshaw, M.L.; Sleasman, J.W. Hypereosinophilic Syndrome and Hemimelia in a Patient with Chromosome 6p22.3 Deletion. Pediatr. Allergy Immunol. 2014, 25, 500–503. [Google Scholar] [CrossRef]
- Trimouille, A.; Barouk-Simonet, E.; Charron, S.; Bouron, J.; Bernhard, J.C.; Lacombe, D.; Fergelot, P.; Rooryck, C. Deletion of the Transcription Factor SOX4 Is Implicated in Syndromic Nephroblastoma. Clin. Genet. 2017, 92, 449–450. [Google Scholar] [CrossRef]
- Gambale, A.; Russo, R.; Andolfo, I.; Quaglietta, L.; De Rosa, G.; Contestabile, V.; De Martino, L.; Genesio, R.; Pignataro, P.; Giglio, S.; et al. Germline Mutations and New Copy Number Variants among 40 Pediatric Cancer Patients Suspected for Genetic Predisposition. Clin. Genet. 2019, 96, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Nedios, S.; Dinov, B.; Seewöster, T.; Lindemann, F.; Richter, S.; Arya, A.; Dagres, N.; Husser, D.; Bollmann, A.; Hindricks, G.; et al. Characteristics of left atrial remodeling in patients with atrial fibrillation and hypertrophic cardiomyopathy in comparison to patients without hypertrophy. Sci. Rep. 2021, 11, 12411. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.J.; Kemme, M.J.B.; Visser, C.L.; Hopman, L.H.G.A.; van Diemen, P.A.; van de Ven, P.M.; Götte, M.J.W.; Danad, I.; Knaapen, P.; van Rossum, A.C.; et al. Left atrial sphericity as a marker of atrial remodeling: Comparison of atrial fibrillation patients and controls. Int. J. Cardiol. 2020, 304, 69–74. [Google Scholar] [CrossRef] [PubMed]
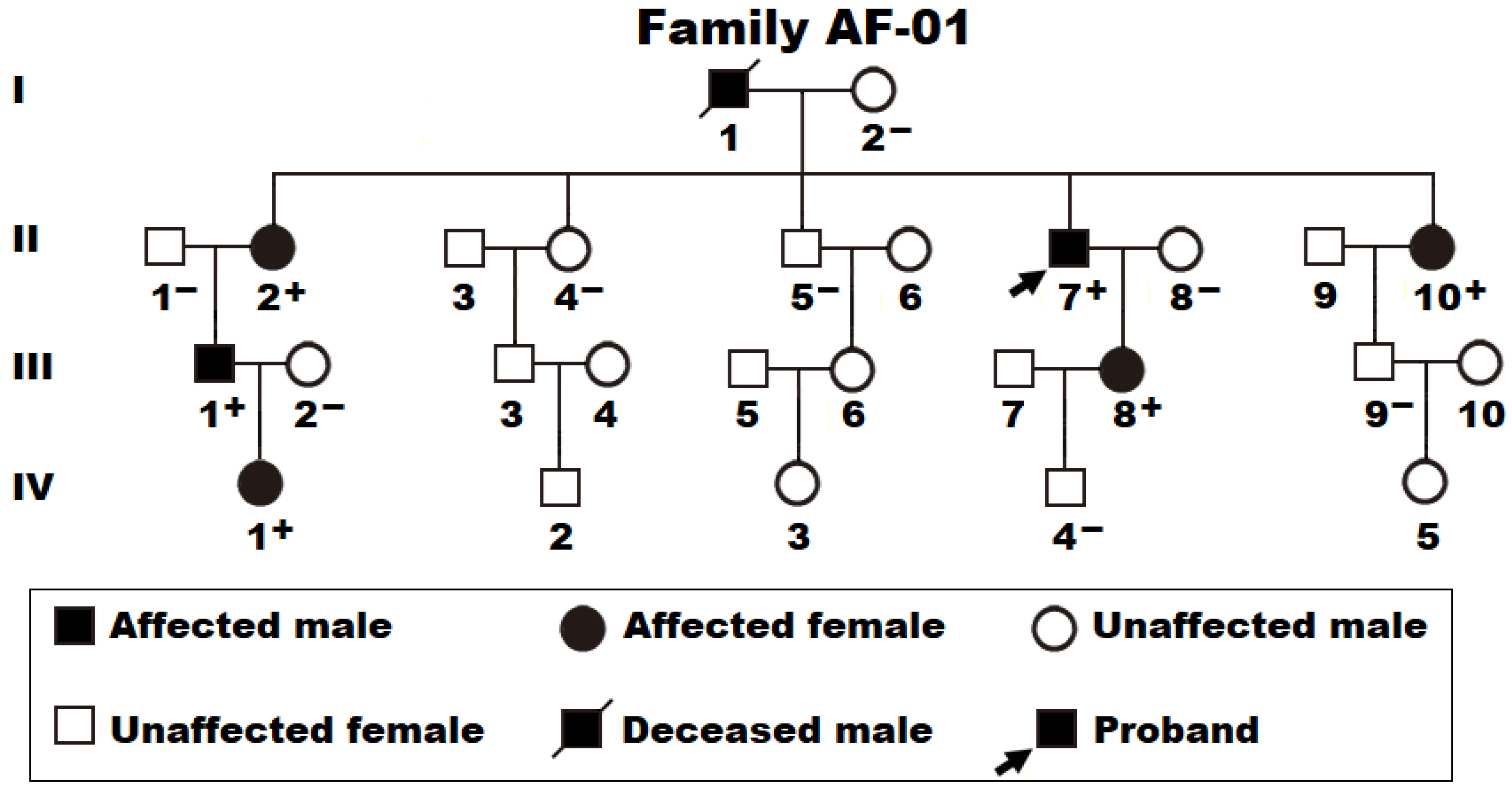




| Subject Information | Cardiac Phenotype | Electrocardiogram | Echocardiogram | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Identity (Family AF-01) | Sex | Age at Initial Diagnosis of AF (years) | Age at Recruitment (years) | AF (Clinical Classification) | Heart Rate (Beats/min) | QRS Interval (ms) | QTc (ms) | LAD (mm) | LVEF (%) |
| I-1 | M | 25 | 71 * | Permanent | NA | NA | NA | NA | NA |
| II-2 | F | 35 | 65 | LSP | 82 | 102 | 438 | 42 | 58 |
| II-7 | M | 45 | 57 | LSP | 106 | 90 | 416 | 39 | 62 |
| II-10 | F | 41 | 55 | LSP | 95 | 93 | 330 | 38 | 66 |
| III-1 | M | 37 | 42 | LSP | 81 | 98 | 420 | 36 | 63 |
| III-8 | F | 34 | 34 | Persistent | 84 | 88 | 432 | 33 | 67 |
| IV-1 | F | 20 | 20 | Paroxysmal | 102 | 80 | 405 | 31 | 65 |
| Variable | Patient Group (n = 196) | Control Group (n = 238) | p-Value |
|---|---|---|---|
| Gender (female/male) | 88/108 | 107/131 | 0.99000 |
| Age (years) | 56.93 ± 8.48 | 57.11 ± 9.27 | 0.8344 |
| Family history of atrial fibrillation (%) | 35 (18) | 0 (0) | <0.0001 * |
| History of implanted pacemaker (%) | 8 (4) | 0 (0) | 0.0032 * |
| History of ischemic stroke (%) | 11 (6) | 0 (0) | 0.0003 * |
| Body mass index (kg/m2) | 23.97 ± 2.52 | 24.12 ± 2.67 | 0.5506 |
| Total cholesterol (mmol/L) | 3.81 ± 0.73 | 3.78 ± 0.65 | 0.6511 |
| Fasting blood glucose (mmol/L) | 4.58 ± 0.64 | 4.60 ± 0.71 | 0.7603 |
| Triglyceride (mmol/L) | 1.49 ± 0.47 | 1.50 ± 0.51 | 0.8333 |
| Diastolic blood pressure (mmHg) | 82.97 ± 6.58 | 83.05 ± 7.13 | 0.9042 |
| Systolic blood pressure (mmHg) | 125.61 ± 9.73 | 126.23 ± 10.28 | 0.5222 |
| Left atrial diameter (mm) | 38.75 ± 7.61 | 36.04 ± 6.83 | 0.0001 * |
| Resting heart rate (beats/min) | 76.02 ± 14.51 | 75.84 ± 10.33 | 0.8804 |
| Left ventricular ejection fraction (%) | 62.78 ± 7.09 | 63.04 ± 6.82 | 0.6980 |
| History of smoking (%) | 9 (5) | 11 (5) | 0.9882 |
| History of alcohol consumption (%) | 18 (9) | 23 (10) | 0.8648 |
| Chr | Position (GRCh37) | Ref | Alt | Gene | Variation |
|---|---|---|---|---|---|
| 1 | 67,185,057 | A | G | SGIP1 | NM_032291.4: c.1711A>G; p.(Asn571Asp) |
| 1 | 210,267,717 | C | T | SYT14 | NM_001146261.4: c.628C>T; p.(Pro210Ser) |
| 2 | 109,289,367 | A | G | LIMS1 | NM_001193485.3: c.562A>G; p.(Lys188Glu) |
| 2 | 191,375,262 | A | T | NEMP2 | NM_001142645.2: c.955A>T; p.(Lys319*) |
| 3 | 71,064,787 | A | C | FOXP1 | NM_032682.6: c.887A>C; p.(His296Pro) |
| 4 | 139,980,574 | G | A | ELF2 | NM_201999.3: c.1311G>A; p.(Gly437Arg) |
| 5 | 127,686,668 | A | C | FBN2 | NM_001999.4: c.2704A>C; p.(Ile902Leu) |
| 6 | 21,594,976 | C | T | SOX4 | NM_003107.3: c.211C>T; p.(Gln71*) |
| 7 | 64,292,159 | C | A | ZNF138 | NM_006524.4: c.461C>A; p.(Ser154*) |
| 10 | 31,803,533 | A | T | ZEB1 | NM_001128128.3: c.639A>T; p.(Arg217Ser) |
| 11 | 40,137,306 | A | T | LRRC4C | NM_020929.3: c.537A>T; p.(Leu179Phe) |
| 15 | 30,010,872 | A | C | TJP1 | NM_003257.5: c.3474A>C; p.(Glu1158Asp) |
| 18 | 52,946,829 | C | G | TCF4 | NM_001083962.2: c.608C>G; p.(Ser203Cys) |
| Coding Region | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Amplicon (bp) |
|---|---|---|---|
| Part 1 | CTCTCTTTACCCACCTCCGC | GACCTTGTCTCCCTTCTCCC | 643 |
| Part 2 | GCCCAGGAAGAAGGTGAAGT | GCGCCCTCCTCCTCGTACAG | 603 |
| Part 3 | TGGCGGAGAAGAAGGTGAAG | TCGTCTGTCCTTTTCGTTTCT | 647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.-F.; Sun, Y.-M.; Qiu, X.-B.; Wu, S.-H.; Ding, Y.-Y.; Li, N.; Yang, C.-X.; Xu, Y.-J.; Jiang, T.-B.; Yang, Y.-Q. Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation. Diagnostics 2024, 14, 2376. https://doi.org/10.3390/diagnostics14212376
Jiang W-F, Sun Y-M, Qiu X-B, Wu S-H, Ding Y-Y, Li N, Yang C-X, Xu Y-J, Jiang T-B, Yang Y-Q. Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation. Diagnostics. 2024; 14(21):2376. https://doi.org/10.3390/diagnostics14212376
Chicago/Turabian StyleJiang, Wei-Feng, Yu-Min Sun, Xing-Biao Qiu, Shao-Hui Wu, Yuan-Yuan Ding, Ning Li, Chen-Xi Yang, Ying-Jia Xu, Ting-Bo Jiang, and Yi-Qing Yang. 2024. "Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation" Diagnostics 14, no. 21: 2376. https://doi.org/10.3390/diagnostics14212376
APA StyleJiang, W.-F., Sun, Y.-M., Qiu, X.-B., Wu, S.-H., Ding, Y.-Y., Li, N., Yang, C.-X., Xu, Y.-J., Jiang, T.-B., & Yang, Y.-Q. (2024). Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation. Diagnostics, 14(21), 2376. https://doi.org/10.3390/diagnostics14212376






