Validation of Diagnostic Accuracy and Disease Severity Correlation of Chest Computed Tomography Severity Scores in Patients with COVID-19 Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. CT Scanning
2.3. Image Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pattern Distribution
3.3. Reproducibility and Diagnostic Accuracy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Revel, M.P.; Parkar, A.P.; Prosch, H.; Silva, M.; Sverzellati, N.; Gleeson, F.; Brady, A.; European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department-advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur. Radiol. 2020, 30, 4903–4909. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.P.; Boussouar, S.; de Margerie-Mellon, C.; Saab, I.; Lapotre, T.; Mompoint, D.; Chassagnon, G.; Milon, A.; Lederlin, M.; Bennani, S.; et al. Study of Thoracic CT in COVID-19: The STOIC Project. Radiology 2021, 301, E361–E370. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur. Radiol. 2020, 30, 4407–4416. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhong, Z.; Zhao, W.; Zheng, C.; Wang, F.; Liu, J. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology 2020, 296, E41–E45. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Huang, G.; Gong, T.; Wang, G.; Wang, J.; Guo, X.; Cai, E.; Li, S.; Li, X.; Yu, Y.; Lin, L. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores from Sequential Chest CT. AJR Am. J. Roentgenol. 2020, 215, 367–373. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Liu, H.; Zhen, Y.; Zhang, X.; Xiong, Q.; Luo, Y.; Gao, C.; Zeng, W. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol. Cardiothorac. Imaging 2020, 2, e200047. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, T.; Wang, Y.; Xia, L. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur. Radiol. 2020, 30, 5446–5454. [Google Scholar] [CrossRef]
- Yuan, M.; Yin, W.; Tao, Z.; Tan, W.; Hu, Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE 2020, 15, e0230548. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Carotti, M.; Tardella, M.; Borgheresi, A.; Agostini, A.; Minorati, D.; Di Carlo, M.; Galli, M.; Giovagnoni, A.; Sarzi-Puttini, P. The role of a chest computed tomography severity score in coronavirus disease 2019 pneumonia. Medicine 2020, 99, e22433. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission; National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. 2020, 133, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Prokop, M.; van Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Investig. Radiol. 2020, 55, 327–331. [Google Scholar] [CrossRef]
- Pan, Y.; Guan, H. Imaging changes in patients with 2019-nCov. Eur. Radiol. 2020, 30, 3612–3613. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 2020, 30, 4381–4389. [Google Scholar] [CrossRef]
- Hani, C.; Trieu, N.H.; Saab, I.; Dangeard, S.; Bennani, S.; Chassagnon, G.; Revel, M.P. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn. Interv. Imaging 2020, 101, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Ben, S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J. Med. Virol. 2021, 93, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sirajuddin, A.; Zhang, X.; Liu, G.; Teng, Z.; Zhao, S.; Lu, M. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur. Radiol. 2020, 30, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Takahashi, H.; Ibe, T.; Ishii, H.; Kurata, Y.; Ishizuka, Y.; Hamamoto, Y. Comparison of semiquantitative chest CT scoring systems to estimate severity in coronavirus disease 2019 (COVID-19) pneumonia. Eur. Radiol. 2022, 32, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Elmokadem, A.H.; Mounir, A.M.; Ramadan, Z.A.; Elsedeiq, M.; Saleh, G.A. Comparison of chest CT severity scoring systems for COVID-19. Eur. Radiol. 2022, 32, 3501–3512. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, W.A.; Hanai, B.; Kim, P.; Anthony, T.; Rivera, Z. Radiological Finding of Crazy-Paving Pattern in COVID-19 Pneumonia. Cureus 2022, 14, e26107. [Google Scholar] [CrossRef] [PubMed]
- De Wever, W.; Meersschaert, J.; Coolen, J.; Verbeken, E.; Verschakelen, J.A. The crazy-paving pattern: A radiological-pathological correlation. Insights Imaging 2011, 2, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Agustina Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.-K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Mardani, R.; Ahmadi Vasmehjani, A.; Zali, F.; Gholami, A.; Mousavi Nasab, S.D.; Kaghazian, H.; Kaviani, M.; Ahmadi, N. Laboratory Parameters in Detection of COVID-19 Patients with Positive RT-PCR; A Diagnostic Accuracy Study. Arch. Acad. Emerg. Med. 2020, 8, e43. [Google Scholar] [PubMed]
- Ayinbuomwan, S.A.; Mokogwu, N.; Akoria, O.A.; Okwara, B.U.; Omuemu, C.E.; Obaseki, D.E. Arterial Oxygen Saturation and other Clinical Predictors of Survival in Patients with COVID-19: A Review of Cases in a Tertiary Care Hospital in Nigeria. West Afr. J. Med. 2021, 38, 109–113. [Google Scholar] [PubMed]

| All (N = 218) | Mild Disease (N = 105) | Severe Disease (N = 113) | p Value | |
|---|---|---|---|---|
| Mean ± SD | ||||
| Age (years) | 62 ± 15 | 55 ± 15 | 68 ± 12 | <0.001 * |
| Length of stay in hospital (days) | 15 ± 9 | 13 ± 6 | 17 ± 10 | 0.001 * |
| N (%) | ||||
| Gender | ||||
| Male | 138 (63.3) | 62 (28.4) | 76 (34.9) | 0.208 |
| Female | 80 (36.7) | 43 (19.7) | 37 (16.9) | |
| Symptoms | ||||
| Fever | 187 (85.8) | 92 (42.2) | 95 (43.6) | 0.453 |
| Cough | 177 (81.2) | 83 (38.0) | 94 (43.2) | 0.434 |
| Myalgia/fatigue | 149 (68.3) | 74 (33.9) | 75 (34.4) | 0.515 |
| Headache | 70 (32.1) | 39 (17.9) | 31 (14.2) | 0.125 |
| Dyspnea | 92 (42.2) | 31 (14.2) | 61 (27.9) | <0.001 * |
| Diarrhea | 57 (26.1) | 37 (16.9) | 20 (9.2) | 0.003 * |
| Chest pain | 20 (22.5) | 12 (13.5) | 8 (9.0) | 0.871 |
| Nausea/vomiting | 41 (18.8) | 28 (12.8) | 13 (5.9) | 0.004 * |
| Hyposmia/anosmia | 41 (18.8) | 30 (13.8) | 11 (5.1) | <0.001 * |
| Dysgeusia | 44 (20.2) | 29 (13.3) | 15 (6.9) | 0.008 * |
| Comorbidities | ||||
| Hypertension | 122 (55.9) | 47 (21.6) | 75 (34.4) | 0.001 * |
| Diabetes | 40 (18.3) | 16 (7.3) | 24 (11.0) | 0.252 |
| Malignancy | 27 (12.4) | 13 (5.9) | 14 (6.4) | 0.998 |
| Asthma | 16 (7.3) | 11 (5.0) | 5 (2.3) | 0.086 |
| COPD | 16 (7.3) | 3 (1.4) | 13 (5.9) | 0.014 * |
| Smoking | 10 (4.6) | 5 (2.3) | 5 (2.3) | 0.905 |
| Osteoporosis | 6 (2.7) | 2 (0.9) | 4 (1.8) | 0.460 |
| Laboratory Findings | All (N = 218) | Mild Disease (N = 105) | Severe Disease (N = 113) | p Value |
|---|---|---|---|---|
| Mean ± SD | ||||
| Temperature (°C) | 37.5 ± 0.9 | 37.4 ± 0.8 | 37.7 ± 0.9 | 0.039 * |
| SpO2 (%) | 92.8 ± 5.5 | 95.9 ± 1.8 | 90.9 ± 6.3 | <0.001 * |
| PaO2 (kPa) | 10.6 ± 2.5 | 11.7 ± 2.3 | 8.4 ± 0.9 | <0.001 * |
| CRP (mg/L) | 82.5 ± 72.2 | 55.0 ± 52.3 | 110.2 ± 87.5 | <0.001 * |
| D-dimer (mg/L) | 2.3 ± 5.4 | 2.0 ± 2.8 | 3.5 ± 7.3 | 0.099 |
| LDH (U/L) | 286.8 ± 128.0 | 251.4 ± 98.0 | 318.0 ± 124.8 | <0.001 * |
| Erythrocytes (1012/L) | 4.57 ± 0.63 | 4.65 ± 0.58 | 4.46 ± 0.69 | 0.165 |
| Thrombocytes (109/L) | 228.0 ± 96.14 | 216.40 ± 77.65 | 238.74 ± 108.84 | 0.090 |
| Leukocytes (109/L) | 7.44 ± 3.99 | 6.51 ± 2.65 | 8.29 ± 4.76 | 0.001 * |
| Neutrophils (109/L) | 5.54 ± 3.87 | 4.31 ± 2.32 | 6.66 ± 4.60 | <0.001 * |
| Lymphocytes (109/L) | 1.15 ± 0.63 | 1.45 ±0.68 | 0.88 ± 0.44 | <0.001 * |
| Eosinophils (109/L) | 0.06 ± 0.29 | 0.10 ± 0.42 | 0.02 ± 0.06 | 0.079 |
| Procalcitonin (µg/L) | 0.43 ± 1.57 | 0.28 ± 0.47 | 0.49 ± 1.88 | 0.504 |
| Lung Pattern | All | Mild Disease | Severe Disease | p Value |
|---|---|---|---|---|
| N (%) | ||||
| GGO | 187 (85.8) | 89 (40.8) | 98 (44.9) | 0.687 |
| Mixed † | 102 (46.8) | 52 (23.8) | 50 (22.9) | 0.435 |
| Consolidation | 151 (69.2) | 65 (29.8) | 86 (39.5) | 0.023 * |
| Air bronchogram | 112 (52.8) | 47 (22.1) | 65 (30.7) | 0.108 |
| Interlobular septal thickening | 138 (63.3) | 60 (27.5) | 78 (35.8) | 0.062 |
| Crazy-paving pattern | 114 (52.3) | 48 (22.0) | 66 (30.3) | 0.060 |
| Pleural thickening | 52 (23.8) | 27 (12.4) | 25 (11.4) | 0.534 |
| Nodules | 28 (12.8) | 10 (4.6) | 18 (8.2) | 0.158 |
| Lymph node enlargement | 60 (27.5) | 26 (11.9) | 34 (15.6) | 0.378 |
| Pleural effusion | 45 (20.6) | 16 (7.3) | 29 (13.3) | 0.052 |
| Pericardial effusion | 6 (2.8) | 0 (0) | 6 (2.8) | 0.017 * |
| Predominant Lung Pattern | Odds Ratio | 95% CI |
|---|---|---|
| GGO | 1.21 | 0.87–1.48 |
| Mixed † | 1.40 | 1.06–1.86 |
| Consolidation | 2.54 | 1.78–3.61 |
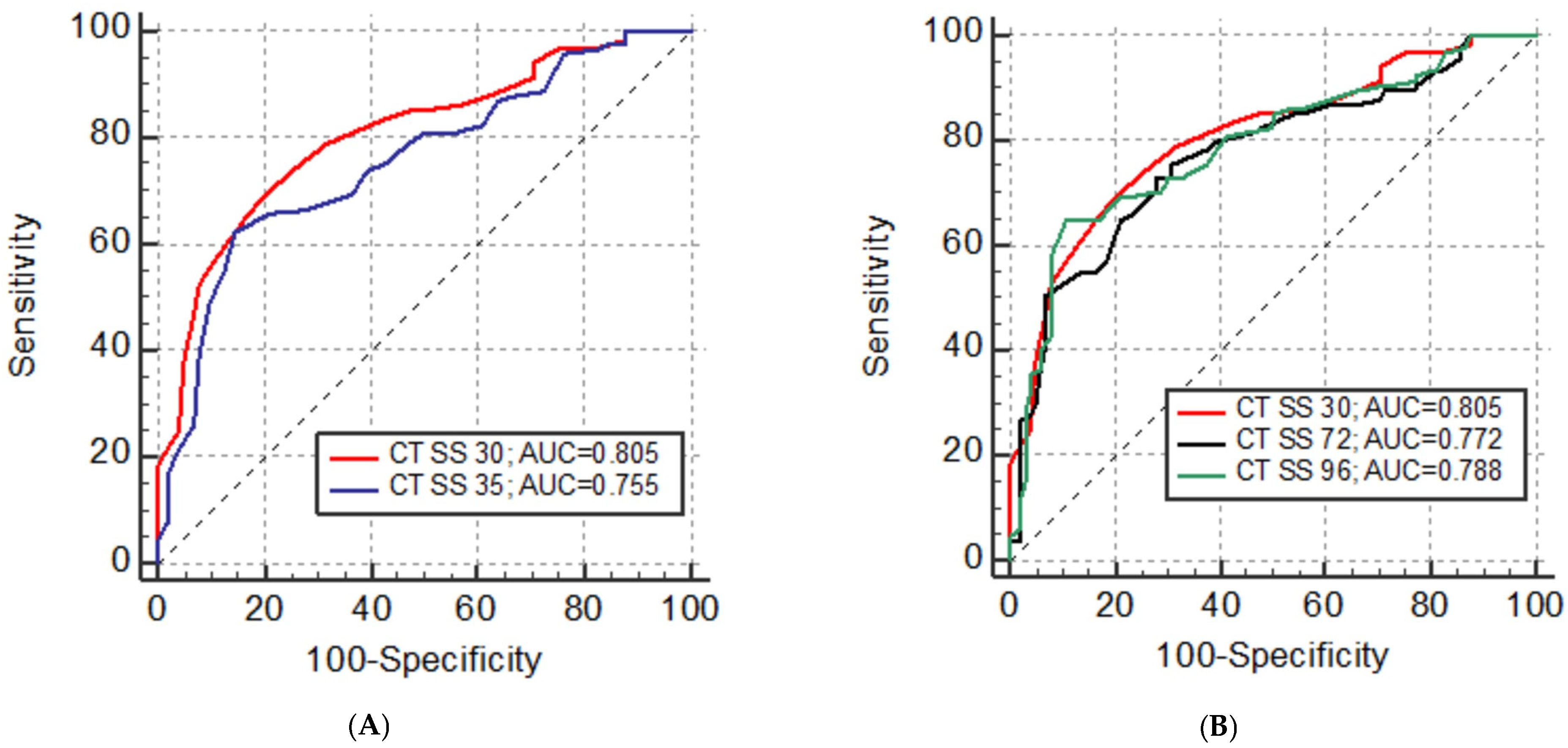
| CT SS | AUC | SE | 95% CI | p | Cut-Off | Sensitivity % | Specifity % |
|---|---|---|---|---|---|---|---|
| CT SS 20 | 0.768 | 0.032 | 0.706 to 0.822 | <0.001 * | >9 | 61.9 | 84.6 |
| CT SS 24 | 0.764 | 0.032 | 0.702 to 0.819 | <0.001 * | >10 | 68.1 | 79.0 |
| CT SS 25 | 0.756 | 0.032 | 0.694 to 0.812 | <0.001 * | >13 | 62.8 | 80.2 |
| CT SS 30 | 0.805 | 0.029 | 0.747 to 0.856 | <0.001 * | >13 | 69.0 | 80.0 |
| CT SS 35 | 0.755 | 0.032 | 0.692 to 0.811 | <0.001 * | >20 | 61.9 | 85.7 |
| CT SS 40 | 0.781 | 0.031 | 0.721 to 0.834 | <0.001 * | >19 | 74.3 | 72.4 |
| CT SS 48 | 0.771 | 0.032 | 0.710 to 0.825 | <0.001 * | >18 | 73.5 | 78.1 |
| CT SS 72 | 0.772 | 0.032 | 0.710 to 0.826 | <0.001 * | >20 | 72.6 | 72.4 |
| CT SS 96 | 0.788 | 0.031 | 0.727 to 0.840 | <0.001 * | >28 | 64.6 | 82.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brumini, I.; Dodig, D.; Žuža, I.; Višković, K.; Mehmedović, A.; Bartolović, N.; Šušak, H.; Cekinović Grbeša, Đ.; Miletić, D. Validation of Diagnostic Accuracy and Disease Severity Correlation of Chest Computed Tomography Severity Scores in Patients with COVID-19 Pneumonia. Diagnostics 2024, 14, 148. https://doi.org/10.3390/diagnostics14020148
Brumini I, Dodig D, Žuža I, Višković K, Mehmedović A, Bartolović N, Šušak H, Cekinović Grbeša Đ, Miletić D. Validation of Diagnostic Accuracy and Disease Severity Correlation of Chest Computed Tomography Severity Scores in Patients with COVID-19 Pneumonia. Diagnostics. 2024; 14(2):148. https://doi.org/10.3390/diagnostics14020148
Chicago/Turabian StyleBrumini, Ivan, Doris Dodig, Iva Žuža, Klaudija Višković, Armin Mehmedović, Nina Bartolović, Helena Šušak, Đurđica Cekinović Grbeša, and Damir Miletić. 2024. "Validation of Diagnostic Accuracy and Disease Severity Correlation of Chest Computed Tomography Severity Scores in Patients with COVID-19 Pneumonia" Diagnostics 14, no. 2: 148. https://doi.org/10.3390/diagnostics14020148
APA StyleBrumini, I., Dodig, D., Žuža, I., Višković, K., Mehmedović, A., Bartolović, N., Šušak, H., Cekinović Grbeša, Đ., & Miletić, D. (2024). Validation of Diagnostic Accuracy and Disease Severity Correlation of Chest Computed Tomography Severity Scores in Patients with COVID-19 Pneumonia. Diagnostics, 14(2), 148. https://doi.org/10.3390/diagnostics14020148






